Abstract
In the context of the predicted global warming, we investigated whether the feeding activity and the behaviour of three Oniscidean species (Porcellionides pruinosus, Porcellio laevis and Armadillidium tunisiense) would be affected by the relative humidity (RH) decrease. A decreasing gradient of relative humidity (90%, 70% and 50%) was used to study the impact of predicted climate change on the survival rate, litter consumption rate, growth efficiency, faeces production, localization and stress behaviour within woodlice. After 3 weeks of exposure, we found that all species were variously affected by the RH decrease. The species A. tunisiense showed to be the least affected by the increased dry conditions. In driest conditions, woodlice spend less time at foraging inducing a low rate of litter consumption and faeces production. Perhaps, the authors findings could contribute to changes in the decomposition process and then the soil functionality.
Introduction
Global climate change has induced shifts in both temperature and precipitations pattern on a global scale. At the end of the 21st century, climate models predict that global surface temperatures will have risen by 1.4–5.8°C (Williams et al. Citation2003; IPCC Citation2007; Rosenzweig et al. Citation2008). In North Africa, Ruosteenoja et al. (Citation2003) predicted an increase in temperature (up to 9°C) from June to August for the 2070–2099 periods. In the context of climate change, the spatial distribution and phenology of plants and animals were greatly affected (Walther et al. Citation2002; Parmesan & Yohe Citation2003; Root et al. Citation2003; Fonty et al. Citation2009; Hassall et al. Citation2018). Terrestrial arthropod showed to be sensitive to climatic shifts especially temperature and moisture (Maron et al. Citation2015; Lister & Garcia Citation2018; Depeux et al. Citation2023). In fact, they are able to sense and detect even small shifts in temperature and humidity in order to avoid the risk of desiccation (Hassall & Tuck Citation2009). It has been demonstrated that climatic change and especially global warming constitutes a threat for terrestrial arthropod (Johnson & Jones Citation2016) given its disturbing effect on population structure like the decline in abundance and survival (Khadioli et al. Citation2014; Depeux et al. Citation2023).
It is well known that climatic features and the activity of microorganisms and soil fauna such as Oniscidea are the major drivers of the decomposition process. Indeed, in response to microclimatic conditions, fauna can change the soil profile, soil properties and the plant community, which may in turn, affect the microbial activity and then the decomposition rate (Loureiro et al. Citation2006).
The successful adaptation of Oniscidea to terrestrial habitats coincided with the appearance of a lot of morphological and behavioural adaptations allowing the reduction of water loss due to the lack of external waxy layer in the cuticle (Edney Citation1968). Moisture is a key factor limiting the distribution of terrestrial isopods. It is widely acknowledged that terrestrial arthropod such as Oniscidea is highly sensitive to microclimatic conditions, especially humidity and temperature (Chown & Terblanche Citation2007; Lister & Garcia Citation2018). To face microclimatic changes, Oniscidea develop two kinds of responses. On the one hand, functional responses which include behavioural pattern like aggregation, volvation, sheltering and modifying dietary behaviour. On the other hand, a numerical response can occur affecting the life history traits and the population parameters (abundance, growth rate, survival and reproductive success…) (Dixie et al. Citation2015; Hassall et al. Citation2018; Depeux et al. Citation2023).
These adaptations vary according to species and families. For example, in comparison with the families of Oniscidae and Porcellionidae, representatives from the Armadillidiidae family have an impermeable cuticle and a specialised morphology to limit moisture loss and desiccation. In addition, they have well-developed pleopodal lungs or pseudotracheae destined to absorb oxygen in drier habitats (Csonka et al. Citation2013). Given their thick cuticle and ability to volvation, Armadillidiidae is well adapted to the terrestrial habitat, and they are considered among the most specialised Oniscidea in limiting water loss and then desiccation (Schmidt & Wägele Citation2001).
On the other side, Porcellionidae is able to aggregate to limit water loss. It has been established that aggregation creates a more favourable microclimate that promotes thermal regulation and then limits water loss (Klok & Chown Citation1999). In fact, aggregation behaviour enables isopods to reduce the rate of water loss by forming a shell of higher relative humidity around the aggregate (Broly et al. Citation2013).
Despite the rising attention on the issue of climate change, little is known about the behavioural and functional reactions of soil fauna organisms to global warming (Römbke et al. Citation2011). Oniscidea provide a suitable model to study such tradeoffs in responses to the predicted climate change. Indeed, terrestrial isopods are commonly used as a model of soil macro-decomposers given their physiological, behavioural and ecological sensitivity towards different microclimates conditions (Sutton Citation1980).
This paper focuses only on the exploration of the effect of relative humidity as microclimatic variable on the Onicidea life history traits since the direct impact of increasing temperature on the soil moisture has been already highlighted (Römbke et al. Citation2011). Therefore, in this work, we test the following hypotheses (1): As the relative humidity decreases, consumption rate, growth efficiency, survival and feeding rate of isopods will decrease. (2): Oniscidea genera react differently to face climate change. (3): Do these findings are sufficient to estimate the climate change impact on terrestrial isopods as a model of decomposer community?
Materials and methods
Experimental animals
Specimens used in our experiments came from the laboratory rearing. Herein, animals were maintained at 22 ± 2°C temperature and 16/8 h (Light/Dark) photoperiod. They were kept in plastic containers and fed on a mixture of dried leaves of Morus nigra and Morus alba. Three common species of terrestrial isopods in Tunisia, with different morphological and behavioural traits, were chosen to test the hypotheses mentioned above:
Porcellionides pruinosus (Brandt 1833) (Porcellionidae): Originally native to Asia Minor, this species is considered the most widely distributed worldwide (Vandel Citation1962; Achouri et al. Citation2008). Indeed, it is a cosmopolitan gregarious species characterized by a high ecological plasticity and a large adaptive performance (Achouri et al. Citation2008; Delhoumi et al. Citation2018, Citation2019). P. pruinosus measures between 9 and 12 mm long (Achouri & Charfi-Cheikhrouha Citation2002).
Porcellio laevis (Latreille 1804) (Porcellionidae): It is a cosmopolitan gregarious species originated from the Mediterranean. Several studies showed that P. laevis tolerates a wide array of environmental conditions. This species has a size ranging between 15 and 20 mm (Medini-Bouaziz Citation2002).
Armadillidium tunisiense (Hamaïed & Charfi-Cheikhrouha 2007) (Armadillidiidae): An endemic species in Tunisia with a large repartition under different climatic features. As Armadillidium species, this species is characterized by evolved pleopodal lungs and a more impermeable cuticle apart from the capacity of roll-up. A. tunisiense measures between 7 and 8.6 mm long (Hamaїed-Melki Citation2008).
Experimental setup
The experiments were set up in a shaded air conditioned room with three relative humidity conditions (50%, 70% and 90%) and a constant temperature (22 ± 2°C) during 3 weeks (Dixie et al. Citation2015; Leclercq-Dransart et al. Citation2019). The RH conditions used in this study were chosen in order to simulate those that may occur at litter, interface and on the soil in Tunisia (Dixie et al. Citation2015). About 50% RH reflects a significant decrease in the relative humidity that may be encountered during drought, 90% RH constitutes a favourable relative humidity for arthropod macro-decomposers and 70% RH was utilised as an intermediate microcosm. The RH was adjusted manually and monitored regularly with a thermo-hygrometer.
After checking individuals for pregnancy and to get a performing result, we exclude gravid females and we select only sub-adults from both sexes. In each species, used specimens belong to the same weight range (25–30 mg for P. pruinosus, 60–70 mg for P. laevis and 15–20 mg for A. tunisiense) and the same age stage. In each RH condition, 30 individuals of every species were deposited in 10 plastic boxes (15 cm × 8 cm, height 6 cm). In each box, containing 3–4 cm of sandy sterile soil, dried leaf (mixture of Morus nigra and Morus alba) and a clay pot serving as an artificial refuge, 3 individuals were weighted, marked and deposited. To quantify the microbial decomposition of litter in each RH condition, five boxes, considered as a control, were devoid from isopods.
To estimate the dispersal behaviour, we note the location of each individual (in the leaves, at the interface/on the refuge or in the soil) and its reaction towards microclimate conditions (aggregation, volvation or scattering) at the end of the experiments. Then, specimens, faeces and remaining litter were weighed to the nearest 0.1 mg in order to determine feeding and physiological parameters. Faeces products were weekly collected and weighed to avoid coprophagy and total lysis.
Feeding parameters
To determine the survival rate, the number of surviving individuals recorded at the end of the experiments was expressed by the initial number of isopods.
After 3 weeks, consumption rate (CR) was calculated as the total mg of consumed leaf per mg of isopod weight.
Growth efficiency (GE) was calculated as the difference of isopod weight at the beginning and the end of the experiments per initial isopod mass.
CR = Wl i−Wl f/Wisop i
GE = (Wisop i−Wisop f/Wisop i) × 100
Wl i: initial leaf weight; Wl f: final leaf weight; Wisop i: initial weight of isopod; Wisop f: final weight of isopod.
Statistical analysis
The survival rate, CR, GE and the faeces weight were compared in the three RH conditions using one-way analysis of variance (ANOVA). Hence, a post hoc Tukey test was performed in order to compare all pairs of group means. Comparison of species location and stress behaviour across different RH conditions were performed using a χ2 test. Statistical analyses were applied with the free version MINITAB 17.
Results
Survival
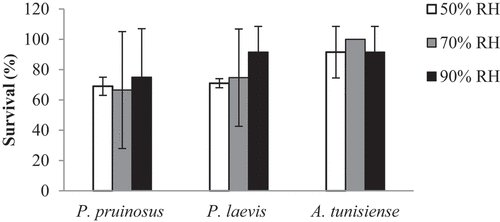
A significant difference in survival was observed between species in the 50% RH (F = 4.17; p = 0.05) with 69%, 71% and 91.5% for P. pruinosus, P. laevis and A. tunisiense, respectively (). P. pruinosus showed a variable survival rate along the different RH conditions (75%, 66.5% and 69% at 90%, 70% and 50% RH respectively). For the species P. laevis, the survival rate decreases proportionally with the RH. We noticed 83.2%, 74.7% and 71% at 90%, 70% and 50% RH respectively. However, A. tunisiense, with the higher rate of survival (91.5%, 100% and 91.5% at 90%, 70% and 50% RH respectively), seems to be the least sensitive to RH variations.
Consumption rate
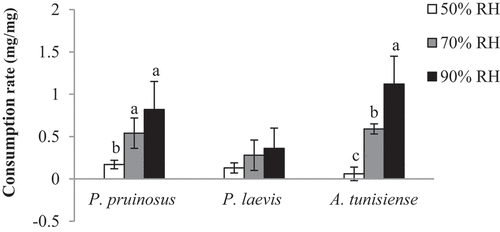
The rate of consumption differed significantly between species at 70% RH (F = 4.51; p = 0.044) but no for the other RH conditions. About species, there were differences of the CR across RH microcosms for P. pruinosus (F = 7.55; p = 0.012) (0.82, 0.54 and 0.17 mg/mg at 90%, 70% and 50% RH respectively) and A. tunisiense (F = 12.2; p < 0.001) (1.12, 0.59 and 0.06 mg/mg at the 90%, 70% and 50% RH respectively). P. laevis showed the lowest CR whatever the microclimate (0.36, 0.28 and 0.13 mg/mg at the 90%, 70% and 50% RH respectively) ().
Growth efficiency
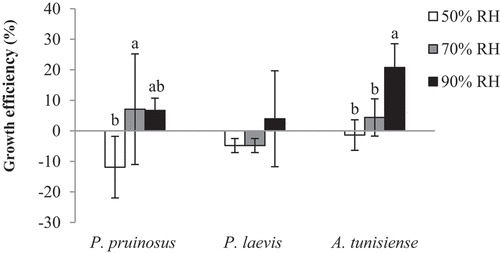
In the driest conditions (50%), GE was negative for all species but not with the same way. Herein, P. pruinosus seems to be the most sensitive to the decreased RH (−11.9%) (). However, GE was positive for all species at 90% RH (6.7%, 3.95% and 20.75% for P. pruinosus, P. laevis and A. tunisiense, respectively). Differently to P. laevis, GE in both species P. pruinosus and A. tunisiense differed significantly between RH conditions (F = 4.17; p = 0.052) and (F = 12.79; p = 0.002), respectively.
Faeces production
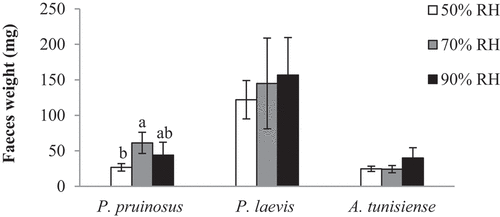
Individuals kept at 50% RH were demonstrated to have a lower weight of faeces. In contrast to P. laevis and A. tunisiense, the mean weight of faeces differed significantly across RH conditions (F = 6.11; p = 0.021) for P. pruinosus (44, 61. 2 and 26.7 mg at the 90%, 70% and 50% RH respectively). For example, the weights of faeces produced by the species A. tunisiense were too close at 50% and 70% but not at 90% RH conditions (24.7, 24.2 and 40 mg respectively). The total amount of faeces produced by the studied woodlice in each RH was clearly higher in the intermediate (70%) and the wettest conditions (90%) ().
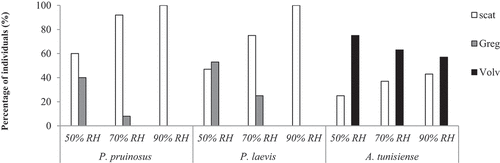
Stress behaviour and location
All species showed a stress related-behaviour especially in the driest conditions (50%). Hence, 40% and 42% of individuals respond with aggregative behaviour for both species P. pruinosus and P. laevis respectively (). The rate of scattered individuals increases proportionally with the increase in the relative humidity for these species. For example, 100% is the percentage of scattered individuals of both species in the wettest conditions. Indeed, the aggregative behaviour within P. pruinosus and P. laevis varied significantly across RH conditions (χ2 = 45.41; p < 0.001) and (χ2 = 44.35; p < 0.001), respectively. Proportionally a significant difference in the proportion of scattered individuals was noticed between RH conditions for P. pruinosus and P. laevis but not for A. tunisiense. In fact, A. tunisiense maintained the same behaviour (volvation) independently to the relative humidity (χ2 = 2.36; p = 0.311).
Figure 5. Stress behaviour (%) of individuals of each species under the three tested RH conditions (Scat: scattering, Greg: aggregation and Volv: volvation).
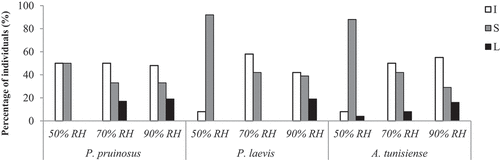
In the driest conditions, isopods tend to excavate into the soil especially P. laevis and A. tunisiense individuals (92% and 88% respectively). However, at 70% and 90% RH, the majority of isopods are located on the soil or on the refuge (). Hence, the studied species were shown to spend more time on the litter in comparison with the driest conditions. Indeed, the number of isopods located on the litter varied significantly between RH conditions especially for P. pruinosus and P. laevis. χ2 test revealed that the RH conditions directly influence the percentage of excavating individuals for P. laevis and A. tunisiense (χ2 = 17.99; p < 0.001) and (χ2 = 22.32; p < 0.001), respectively, but not P. pruinosus (χ2 = 3.46; p = 0.170). Equally, sheltering behaviour was found to be influenced by RH conditions for both species P. laevis and A. tunisiense differently to P. pruinosus.
Discussion
Despite their important role in the ecosystem functioning and the nutrient cycling, studies on Oniscidea ecology are underrepresented in the scientific literature. Herein, a decreasing gradient of relative humidity was assessed to study the physiological and behavioural responses of Oniscidea to face the predicted change of climate variables. We tested the impact of predicted climate shift on litter breakdown and the ecological services provided by primary terrestrial decomposers P. pruinosus, P. laevis and A. tunisiense. These species differ in their degree of specialisation for surviving in dry conditions. In fact, differences in the morphology give these species different abilities to inhabit a wide range of habitats and withstand terrestrial conditions.
A decreasing rate of survival was noticed proportionally with the RH decrease. Similarly, a decrease of 20% of the RH coincided with a significant decrease in both growth rate and survival of four Oniscidea species according to Dixie et al. (Citation2015). A. tunisiense appears the least affected among the three species, a higher rate of survival was recorded regardless the RH conditions. P. pruinosus and P. laevis demonstrate to be more influenced by environmental changes. These differences may be due to the well-developed respiratory system, the thick cuticle and the capacity of volvation within Armadillidiidae members which are classified as the most evolved Oniscidea (Hornung Citation2011). In contrast, the sensitivity toward the driest condition within P. pruinosus and P. laevis may refer to the primitive nature of their respiratory system and the fine cuticle in comparison with A. tunisiense. Despite that P. pruinosus is considered a cosmopolitan species which can resist to higher temperatures and had a constant feeding rate independently to the food source type (Römbke et al. Citation2011), the lowest rate of survival was noticed within this species across different RH conditions. This finding can be explained by the fact that P. pruinosus spends more time in running and moving along a large surface in the field in order to avoid stress variables and for foraging which is not provided in our experimental conditions in which we utilised plastic boxes with a limited surface.
From a global point of view, a lower rate of (consumption rate) CR was found in the driest conditions (50% RH) for all species. For example, the CR of P. pruinosus varied significantly between RH conditions. Indeed, despite that 60% of individuals showed a scattered distribution, a low CR was recorded indicating that this species was affected by the driest conditions. P. laevis showed also a low feeding activity despite its scattered behaviour. The dispersal behaviour of both species especially within P. pruinosus individuals may reflect the running strategy developed to mitigate stress effects.
Indeed, mobile species are able to migrate to areas responding to their ecological requirements which lead to a change in their dispersal pattern (Pearson Citation2006). Therefore, runner isopods are able, via their locomotor activity, to find suitable microhabitat and then avoid desiccation risk (Dailey et al. Citation2009).
In the same line, A. tunisiense showed a lower CR but with a higher survival rate. This finding may be due in part to the strategy adopted by this species in the harsh conditions where it spends more time in volvation in order to limit the water loss and then avoid desiccation. Therefore, a plausible reason for the low CR, especially at 50% RH, is the low intake of food since the low microbial activity in the litter in the case of dryness. Our results showed that P. pruinosus and A. tunisiense were the most effective species on the breakdown process especially in wet microclimates.
Proportionally to the CR, the growth efficiency (GE) of both species P. pruinosus and A. tunisiense varied significantly between microclimates. Indeed, GE was negative for all species in the driest conditions and A. tunisiense appears the least affected. Individuals of Porcellio scaber (Latreille 1804) and Oniscus asellus (Linnaeus 1758) kept at 50% RH showed a negative GE in comparison with those kept at 90% RH (Römbke et al. Citation2011). The acute weight decrease of P. pruinosus at 50% RH may be due to the lack of efficient strategy to face dry conditions. Hence, in the light of the low CR of litter, the running strategy adopted by P. pruinosus requires more energy compared to P. laevis and A. tunisiense.
As the CR and the GE responded to the RH decrease, the production of faeces was lower in the driest conditions. This may affect the dispersion of microorganisms via faecal pellets and then slow down the mineralization process. In accordance, Leclercq-Dransart et al. (Citation2019) found a lower faeces production at RH 50% with four species of terrestrial isopods. Apart from the effect of the low CR of litter, the low number of collected faeces at the driest conditions may refer to the coprophagy behaviour (Delhoumi et al. Citation2019). Indeed, aggregated isopods can use faeces as a food source without the need to spend for longer foraging time.
In the driest conditions, it was noticed that individuals tend to colonize the interface/refuge or into the soil as the case of P. laevis and A. tunisiense. In contrast, under optimum conditions (90% RH), more individuals were observed in the leaves for all species. Regardless the RH conditions, specimens of A. tunisiense were observed in the leaves, it is true with a clear difference especially between 50% and 90% but that finding ensures that this species was the less effected by dryness among the tested species. In fact, the morphological traits of A. tunisiense as the capacity of volvation and the thick cuticle (Hamaїed-Melki Citation2008) allow it to resist to drought for a long time. In the same line, Hassall et al. (Citation2018) justified the interspecific variation of feeding and behaviour within Oniscidea towards climatic shifts by the difference in morphological adaptations to terrestrial life.
P. pruinosus and especially P. laevis showed a gregarious behaviour in the driest conditions. However, under optimum conditions, 100% of individuals of these species showed a scattered distribution. It is well known that species used an aggregative strategy to limit water loss and then desiccation risk due to the primitive structure in comparison with rollers. At wet microcosms, individuals were scattered on the soil, refuge and leaves which increase the CR, GE and faeces production and indirectly litter degradation process. Thus, at dry conditions, the decomposition rate decreases remarkably which may alter the ecosystem functioning. For example, the decrease in the decomposition rate can increase the time of retention of litter in the soil which alters the nutrient cycling in the soil in one hand and raises the fire frequency on the other hand (Loureiro et al. Citation2006).
Conclusion
The decreasing gradient of moisture used in our experimental setup was shown to be stressful for the three studied species which react differently via feeding and behavioural traits. A. tunisiense showed more resistance to dryness due to its evolved morphological traits (thick cuticle and pleopodal lungs) and the ability to volvation compared to P. pruinosus and P. laevis which appear more affected by the decreased relative humidity and indirectly by global warming. The question remains whether these species will be able to continue providing the ecological services necessary for the maintenance of terrestrial ecosystems and whether we could generalise these statements on other Oniscidean species and soil fauna on a large scale in the light of the climate change. Such research on Oniscidean potentially makes them a useful model for the study of a wide array of phenomena including those related to climate change effects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Achouri MS, Charfi-Cheikhrouha F. 2002. Description morphologique et répartition géographique de Porcellionides pruinosus (Brandt, 1833) en Tunisie (Crustacés, Isopode, Oniscoïde). Bulletin de la Société des Sciences Naturelles de Tunisie 28:1–11.
- Achouri MS, Medini-Bouaziz L, Hamaied S, Charfi-Cheikhrouha F. 2008. Diversity of terrestrial isopods at the Oued Laou catchment (North-East of Morocco): Preliminary results. Revue Institut Scientifique Rabat série générale 5:75–79.
- Broly P, Deneubourg JL, Devigne C. 2013. Benefits of aggregation in woodlice: A factor in the terrestrialization process? Insectes sociaux 60(4):419–435. DOI: 10.1007/s00040-013-0313-7.
- Chown SL, Terblanche JS. 2007. Physiological diversity in insects: Ecological and evolutionary contexts. Advances in Insect Physiology 33:50–152.
- Csonka D, Halasy K, Szabó P, Mrak P, Strus J, Hornung E. 2013. Eco-morphological studies on pleopodal lungs and cuticle in Armadillidium species (Crustacea, Isopoda, Oniscidea). Arthropod Structure & Development 42(3):229–235. DOI: 10.1016/j.asd.2013.01.002.
- Dailey TM, Claussen DL, Ladd GB, Buckner ST. 2009. The effects of temperature, desiccation, and body mass on the locomotion of the terrestrial isopod, Porcellio laevis. Comparative Biochemistry and Physiology A 153(2):162–166. DOI: 10.1016/j.cbpa.2009.02.005.
- Delhoumi M, Zaabar W, Ben Rhouma A, Achouri MS. 2018. Effects of agricultural practices and abiotic factors on woodlice diversity across two agroecosystems in Tunisia. Vie Milieu 68:253–261.
- Delhoumi M, Zaabar W, Bouslama MF, Achouri MS. 2019. Effect of symbiont acquisition on growth, survival and fertility of the terrestrial isopod Porcellionides pruinosus (Crustacea, Oniscidea). Invertebrate Reproduction & Development 63(1):60–66. DOI: 10.1080/07924259.2018.1544174.
- Delhoumi M, Zaabar W, Bouslama MF, Zayani D, Achouri MS. 2019. High level of genetic variation in mitochondrial 16S rDNA among populations of Porcellionides pruinosus (Brandt, 1833) (Crustacea: Isopoda: Oniscidea) in Tunisia. Italian Journal of Zoology 86(1):1–8. DOI: 10.1080/24750263.2018.1540669.
- Depeux C, Branger A, Moulignier T, Moreau J, Lemaître JF, Dechaume-Moncharmont FX, Laverre T, Paulhac H, Gaillard JM, Beltran-Bech S. 2023. Deleterious effects of thermal and water stresses on life history and physiology: A case study on woodlouse. Peer Community Journal 3:e7. DOI: 10.24072/pcjournal.228.
- Dixie B, White H, Hassall M. 2015. Effects of microclimate on behavioural and life history traits of terrestrial isopods: Implications for responses to climate change. ZooKeys 515:145–157. DOI: 10.3897/zookeys.515.9399.
- Edney EB. 1968. Transition from water to land in isopod crustaceans. American Zoologist 8(3):309–326. DOI: 10.1093/icb/8.3.309.
- Fonty E, Sarthou S, Larpin D, Ponge JF. 2009. A 10-years decrease in plant species richness on a neotropical inselberg: Detrimental effects of global warming? Global Change Biology 15(10):2360–2374. DOI: 10.1111/j.1365-2486.2009.01923.x.
- Hamaїed-Melki S. 2008. Systématique, répartition géographique et éco-biologie du genre Armadillidium (Brandt, 1833) en Tunisie. Thèse de Doctorat, Université de Tunis. pp. 211.
- Hassall M, Moss A, Dixie B, Gilroy JJ. 2018. Interspecific variation in responses to microclimate by terrestrial isopods: Implications in relation to climate change. ZooKeys 801:5–24. DOI: 10.3897/zookeys.801.24934.
- Hassall M, Tuck HM. 2009. Sheltering behavior of terrestrial isopods in grasslands. Invertebrate Biology 126(1):46–56. DOI: 10.1111/j.1744-7410.2007.00075.x.
- Hornung E. 2011. Evolutionary adaptation of oniscidean isopods to terrestrial life: Structure, physiology and behavior. Terrestrial Arthropod Reviews 4(2):95–130. DOI: 10.1163/187498311X576262.
- IPCC (2007): Climate Change. 2007. The physical science basis. summary for policy maker-contribution of Working group I to the fourth assessment report of the Intergovernmental Panel on climate changes. IPCC, Geneva.
- Johnson SN, Jones TH. 2016. Introduction to global climate change and terrestrial invertebrates. In: Johnson S, Jones T, editors Global climate change and terrestrial invertebrates. Chichester, UK: John Wiley & Sons, Ltd. p. 18.
- Khadioli N, Tonnag ZEH, Muchugu E, Ong’amo G, Achia T, Kipchirchir I, Kroschel J, Le Ru B. 2014. Effect of temperature on the phenology of Chilo partellus (Swinhoe) (Lepidoptera, Crambidae); simulation and visualization of the potential future distribution of C. partellus in Africa under warmer temperatures through the development of life-table parameters. Bulletin of Entomological Research 104(6):809–822. DOI: 10.1017/S0007485314000601.
- Klok CJ, Chown SL. 1999. Assessing the benefits of aggregation: Thermal biology and water relations of anomalous Emperor Moth caterpillars. Functional Ecology 13(3):417–427. DOI: 10.1046/j.1365-2435.1999.00324.x.
- Leclercq-Dransart J, Pernin C, Demuynck S, Grumiauxa F, Lemière S, Leprêtre A. 2019. Isopod physiological and behavioral responses to drier conditions: An experiment with four species in the context of global warming. European Journal of Soil Biology 90:22–30. DOI: 10.1016/j.ejsobi.2018.11.005.
- Lister BC, Garcia A. 2018. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proceedings of the National Academy of Sciences 115(44):10397–10406. DOI: 10.1073/pnas.1722477115.
- Loureiro S, Sampaio A, Brandão A, Nogueira AJA, AMVM S. 2006. Feeding behaviour of the terrestrial isopod Porcellionides pruinosus Brandt, 1833 (Crustacea, Isopoda) in response to changes in food quality and contamination. Science of the Total Environment 369(1–3):119–128. DOI: 10.1016/j.scitotenv.2006.05.023.
- Maron M, McAlpine CA, Watson JEM, Maxwell S, Barnard P, Andersen A. 2015. Climate-induced resource bottlenecks exacerbate species vulnerability: A review. Diversity and Distributions 21(7):731–743. DOI: 10.1111/ddi.12339.
- Medini-Bouaziz L. 2002. Systématique, Biologie et Biogéographie du genre Porcellio en Tunisie (Crustacés, Isopodes Oniscidea). Thèse de Doctorat, Université de Tunis. p.230.
- Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421(6918):37–42. DOI: 10.1038/nature01286.
- Pearson RG. 2006. Climate change and the migration capacity of species. Trends in Ecology & Evolution 21(3):111–113. DOI: 10.1016/j.tree.2005.11.022.
- Römbke T, Römbke J, Russell D. 2011. Effects of temperature increases on the feeding activity of two species of isopods (Porcellio scaber, Porcellionides pruinosus) in laboratory tests. Soil Organisms 83:211–220.
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds A. 2003. Fingerprints of global warming on wild animals and plants. Nature 421(6918):57–60. DOI: 10.1038/nature01333.
- Rosenzweig C, Karoly D, Vicarelli M, Neofotis P, Wu QG, Casassa G, Menzel A, Root TL, Estrella N, Seguin B, Tryjanowski P, Liu CZ, Rawlins S, Imeson A. 2008. Attributing physical and biological impacts to anthropogenic climate change. Nature 453(7193):353–420. DOI: 10.1038/nature06937.
- Ruosteenoja K, Carter TR, Jylhä K, Tuomenvirta H 2003. Future climate in world regions: An intercomparison of model-based projections for the new IPCC emissions scenarios. The Finnish Environment 644, Finnish Environment Institute, Helsinki. p. 83.
- Schmidt C, Wägele JW. 2001. Morphology and evolution of respiratory structures in the pleopod exopodites of terrestrial Isopoda (Crustacea, Isopoda, Oniscidea). Acta Zoologica -Stockholm 82(4):315–330. DOI: 10.1046/j.1463-6395.2001.00092.x.
- Sutton S. 1980. Woodlice. Oxford: Pergamon Press Ltd. p. 144.
- Vandel A. 1962. Faune de France n° 66: Isopodes terrestres, deuxieme partie. Paris: Paul Lechevalier. p. 514.
- Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin JM, Hoegh Gulberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 41(6879):389–395. DOI: 10.1038/416389a.
- Williams SE, Bolitho EE, Fox S. 2003. Climate change in Australian tropical rainforests: An impending environmental catastrophe. Proceeding of the Royal Society B (London), Biological Sciences 270(1527):1887–1892. DOI: 10.1098/rspb.2003.2464.