Abstract
Morphological similarity between an intruder and a native species can hinder the detection of the intruder. The undetected non-native species will thus have more time to affect the local ecosystem and spread. This paper describes an instance of unnoticed introduction of a fish species morphologically resembling a native endemic species. We report on the first established population of the Rhodes minnow, Ladigesocypris ghigii (Actinopterygii: Leuciscidae), endemic to Rhodes Island, which has been recently introduced to a river near Athens, mainland Greece. When it was first noticed, in 2017, the species was misidentified due to its morphological similarity to Pelasgus marathonicus, the only native leuciscid of the streams and rivers around Athens, until it was subject to genetic and detailed morphological examination. Genetic analyses based on both mitochondrial (cytochrome b) and nuclear (recombination activating gene, RAG1) markers unambiguously identified the collected individuals and provided information on the relationships of Ladigesocypris at both the species and generic level. Our investigation supports that L. ghigii was recently established near Athens, and that the population probably originated from Rhodes Island, an eastern Aegean island. In 2021, a high-density thriving mainland population coexisted solely with the native European eel (Anguilla anguilla) along at least 9.7 km of river habitat. It was assessed as being of high invasive risk for the region where it was established by the Aquatic Species Invasiveness Screening Kit (AS-ISK) screening tool. We propose a feasibility study be undertaken to investigate the application of measures that may include actions to exterminate or contain the population. Special attention is required since the species is considered vulnerable in its native range, while the translocated population coexists with native biota within a stream ecosystem of outstanding local conservation value.
Introduction
Freshwaters are among the ecosystems most impacted by biological invasions (Bampfylde et al. Citation2010). At the same time, invasive species are one of the main causes of extinction among native freshwater species (Miller et al. Citation1989; Witte et al. Citation1991) and one of the main threats to them in many areas (Economidis Citation1995; Dextrase & Mandrak Citation2006; Bogutskaya et al. Citation2012). Fish introductions have figured especially prominently on a global scale (Helfman Citation2007). Along with widespread evidence of the negative impacts of freshwater non-native fishes, the necessity to develop a mode to assess the “real threat” of non-native fish introductions arose (Gozlan Citation2008). Since most non-native freshwater fish are often impossible to control once released, assessing the impacts and threats of introductions has garnered high interest in recent years (Vitule et al. Citation2009; Tarkan et al. Citation2017).
Non-native freshwater fish species are often relegated to two distinct categories: aliens (hailing from other countries, other continents or distant biomes) and translocated non-natives (Economidis et al. Citation2000; Copp et al. Citation2005; Koutsikos et al. Citation2019; Boon et al. Citation2020). The latter category involves intra-country human-assisted fish transfers concerning species translocated out of their native range but within the same country or within the same region (Helfman Citation2007; Boon et al. Citation2020). Most freshwater researchers treat non-indigenous translocated species as having the same level of importance as other alien fish species (Helfman Citation2007; Tarkan et al. Citation2017); however, sometimes there is confusion or doubt regarding the geographical origin of the translocated species (e.g. Zogaris & Apostolou Citation2011). Also, members of the public and policy stakeholders may not have the natural history knowledge to consider these seemingly “native fishes” an invasive threat (Vardakas et al. Citation2022). It is well known that some translocated species can become invasive, producing severe deleterious effects on native species or ecosystems (Perdikaris et al. Citation2016; Koutsikos et al. Citation2019).
In this paper, we report on the overlooked translocation and establishment of the Rhodes minnow Ladigesocypris ghigii near Athens, Greece, due to its morphological similarity to a native species – Pelasgus marathonicus, the Marathon minnow. The Rhodes minnow (also known as the Ghizani) is a threatened species endemic to the eastern Aegean Island of Rhodes (Greece), assessed by the International Union for Conservation of Nature (IUCN) as Vulnerable (Crivelli Citation2006). Rhodes has a very different biogeographical history to the mainland European part of Greece, being a part of the wider western section of the Anatolian peninsula, belonging to the Western Anatolia Freshwater Ecoregion (Abell et al. Citation2008). Although there have been many freshwater fish introductions from Asia to Europe (Muñoz-Mas et al. Citation2023), this is the first case of intra-country freshwater fish translocation from Asia to Europe in the Mediterranean region.
Mainland Greece is inhabited by a diverse native freshwater fish fauna, with a high proportion of endemic species (Barbieri et al. Citation2015). The high endemism has resulted from the complex geological history of the area, which affected the evolution of the hydrological network and enabled local speciation during long stretches of geological time (Buj et al. Citation2019; Viñuela Rodríguez et al. Citation2021). In the region, the Leuciscidae (sensu Schönhuth et al. Citation2018) is the most speciose family. Some leuciscid species, e.g. members of the genus Pelasgus, adapted to small streams and wetlands (Kottelat & Barbieri Citation2004), may be particularly susceptible to human-induced threats; this includes Pelasgus marathonicus confined to the Western Aegean Ecoregion in Greece (Kottelat & Freyhof Citation2007). The genetic structure of populations of P. marathonicus from separated drainages, even those located close to each other, may be distinctive and unique, deserving conservation attention at the population level (Viñuela Rodríguez et al. Citation2020; Tsaparis et al. Citation2021; Vlami et al. Citation2021). Since 2017, rumours of small fish found in a stream near Rafina, east of the capital city of Athens (Attika Region), lying in proximity to the native range of P. marathonicus (Viñuela Rodríguez et al. Citation2020; Tsaparis et al. Citation2021), were loosely attributed to this native species (Zogaris Citation2019). SZ and colleagues first photographed the young fish near the town of Rafina in 2017 and identified them tentatively as Pelasgus, not considering the possibility of an introduction of a similar-looking leuciscid minnow. Upon re-inspection a few years later, the unusual case of a translocation of the Rhodes minnows was discovered. This confusion was detrimental; for a short while it was assumed that there was one more population of the locally scarce P. marathonicus near Athens (Hellenic Centre for Marine Research (HCMR) data), and the Rhodes minnow had time to establish a large population at the locality of initial documentation.
Here, we discuss identification details, provide evidence for the establishment of the translocated population, and assess the risk of invasiveness of the species in the region of translocation. We further provide indications and suggestions on how this introduction may have taken place and the relevant conservation implications.
Materials and methods
Sampling and methods for morphological counts and measurements
Since 2017, small leuciscid fish have been observed in a stream near the port town of Rafina (38°01′14″N, 24°00′17″E), a coastal settlement 25 km east of Athens and very close to Athens International Airport, but were considered native Pelasgus marathonicus. In the autumn of 2021, several samples were collected after unusually large specimens for P. marathonicus (around 10 cm) had been photographed and posted on a relevant citizen science Facebook group (see Acknowledgements). Fin-clips of four individuals were collected for analysis and careful photographic comparison immediately suggested that the population was not the assumed native P. marathonicus ().
Figure 1. Two small Leuciscidae species occurring in the vicinity of Rafina in Attika. Adult Pelasgus marathonicus from the Boeotian population, Kifissos River basin (photo courtesy of J. Freyhof) (top), and Ladigesocypris ghigii from the Rafina translocated population; a very large individual: 95 mm in total length (photo by D. Zogaris) (bottom).

Two standardised survey samplings of the fish population along stream reaches were undertaken in April 2022. Collecting was conducted under Hellenic Ministry of Environment licence no. 143634/1894/20-7-2016 within water bodies monitored by HCMR, with the approval of the Ethical Committee. Sampling consisted of electrofishing, following approaches used through the European Union Water Framework Directive (EU WFD). Sampling was conducted at a river stretch demarcated by physical boundary features to minimise fish escape during electrofishing (e.g. riffle areas, changes in instream habitats, natural or artificial barriers). A high-quality battery-powered backpack electrofishing unit was used (Smith Root 24 L DC pulsed, 1.5 kW, 35–100 Hz, max 980 V). At each site, a single pass of a river stretch at least 90 m long was completed (average fishing time was 55 min), providing an initial estimate of population density. Fish were moved into buckets, identified to the species level in the field, counted, and measured to size-class intervals, and most of them were released alive back to the river. Visual inspection from shore (using binoculars) and underwater video using a mounted action camera (GoPro Hero 8) at several other locations confirmed the local distribution of the species in the river.
Ten selected specimens were collected for lab inspection. The fish collected for morphological description were caught by electrofishing, euthanised by over-anaesthetisation and fixed in 10% neutral buffered formalin. Methods for meristic counts and measurements followed Barbieri et al. (Citation2017). Measurements were made point to point, with an electronic calliper, to the nearest 0.1 mm. Lateral line pored scales were counted from the anterior-most pored scale (beginning from the first scale adjacent to the operculum). Midlateral row scales were counted from the anterior-most pored scale to the one at the end of the hypural complex. Scales on the caudal fin itself are indicated by “+” and are presented only in the text. Transverse scales are counted as the number of longitudinal scale rows. The scale row on dorsal and ventral midlines is noted as “1/2”. The last two branched rays articulated on a single pterygiophore in dorsal and anal fin are counted as “11/2”.
DNA laboratory procedure and data analyses
Tissue samples for molecular analysis (fin clips) were preserved in 96% ethanol. Genomic DNA was extracted with the Geneaid Genomic DNA Mini Kit (Tissue) (Geneaid Biotech, Taiwan). Two markers were amplified by polymerase chain reaction (PCR): one mitochondrial, cytochrome b gene (cyt b), and one nuclear marker, recombination activating gene (RAG1). Cyt b has been shown to be a reliable marker for fish phylogeny and species identification (e.g. Freyhof et al. Citation2006; Perea et al. Citation2010; Buj et al. Citation2019; Viñuela Rodríguez et al. Citation2021). RAG1 was chosen as an often-used nuclear marker, useful especially in a higher-level fish phylogeny (e.g. Perea et al. Citation2010; Schönhuth et al. Citation2018; Buj et al. Citation2020). Moreover, cyt b and RAG1 are the most widely used genetic markers for the Leuciscidae (sensu Schönhuth et al. Citation2018). Furthermore, published sequences of these markers of L. ghigiii and other reference species used in phylogenetic reconstructions were available. Using both mitochondrial and nuclear markers is important to detect possible hybridisation events (e.g. Freyhof et al. Citation2005; Perea et al. Citation2016; Buj et al. Citation2020; Viñuela Rodríguez et al. Citation2021). PCRs were performed using PPP Master Mix (Top-Bio, Czech Republic). Cyt b was amplified as described in Šanda et al. (Citation2008), while RAG1 amplification followed Viñuela Rodríguez et al. (Citation2021). Sequencing was performed by Macrogen Inc. (Amsterdam, The Netherlands) using internal primers indicated in Viñuela Rodríguez et al. (Citation2021). Apart from the specimens from Rafina, samples of Ladigesocypris irideus from Turkey were also included in molecular analyses ().
Table I. List of Ladigesocypris specimens, and species used for the genetic analyses. For published sequences, the original publication is indicated. (A) Durand et al. (Citation2002), (B) Freyhof et al. (Citation2006), (C) Perea et al. (Citation2010), (D) Durand et al. (Citation2000), (E) Schönhuth et al. (Citation2018), (F) Viñuela Rodríguez et al. (Citation2021), (G) Benovics et al. (Citation2023), (H) Benovics et al. (Citation2020), (J) Nejat et al. (Citation2023), * - all 32 Pelasgus spp. RAG1 haplotypes (MZ230959–MZ230990) published in Viñuela Rodríguez et al. (Citation2021) were used.
Sequences were visually checked and corrected in CHROMAS v. 2.6.6 (http://technelysium.com.au/wp/chromas/) and aligned in BIOEDIT v. 7.2.6 (Hall Citation1999) and GENEIOUS PRIME v. 2022.1 (Biomatters Ltd.). The newly obtained sequences of individual haplotypes were deposited in GenBank (http://www.ncbi.nlm.nih.gov/Genbank), under the accession numbers OQ859060–OQ859063 (cyt b) and OQ859064–OQ859066 (RAG1).
The dataset was complemented by published cyt b and RAG1 sequences of Ladigesocypris (). To test the phylogenetic placement of Ladigesocypris and possible recent hybridisation with Pelasgus, sequences of selected species of the genera Squalius, Petroleuciscus (i.e. genera closely related to Ladigesocypris) and Pelasgus (i.e. the leuciscid genus that inhabits the nearby rivers) were included in the analyses (). Sequences of the genus Phoxinus (Schönhuth et al. Citation2018) were used as outgroup for phylogenetic analyses.
DNASP v. 6.12.01 (Rozas et al. Citation2017) was used for sorting Ladigesocypris haplotypes () and for phasing the RAG1 nuclear sequences. In the case of one sequence (sample LEGH4), which included two heterozygotic positions, the probability of phasing was not high (<0.7), so we used all four resulting allele variants of the phased sequence to test the relationships of the haplotypes in the analysis of haplotype network reconstruction.
PARTITIONFINDER2 (Lanfear et al. Citation2017) was used to determine the best-fitting nucleotide substitution model for each subset of positions inside codons of the protein coding markers on the basis of the Bayesian information criterion (BIC). The following partitions and models were determined and used for the phylogenetic reconstruction: K80+I+G, TRN+I and GTR+I+G for the first, second and third codon position of cyt b, respectively. For RAG, the model JC+I was applied for the first and second codon position, and SYM+G for the third codon position.
Phylogenetic trees based on individual markers were constructed using Bayesian inference (BI) in MRBAYES v. 3.2.6 (Ronquist et al. Citation2012). For RAG1, unphased data were used. Two independent runs of four Markov chain Monte Carlo (MCMC) simulations were simultaneously run for 1 million generations with sampling trees every 1000 generations. The convergence of the runs was analysed and visualised in TRACER v. 1.7.1 (Rambaut et al. Citation2018). The first 25% of trees obtained were discarded as “burn-in”. Posterior probability indicates branch support. The 50% majority rule consensus trees were visualised using FIGTREE v. 1.4.4 (Rambaut Citation2018) and edited with INKSCAPE v. 0.92.3 (Inkscape Project, https://inkscape.org).
Haplotype networks were constructed with TCS v. 1.21 (Clement et al. Citation2000) applying statistical parsimony estimation (Templeton et al. Citation1992) with a 95% connection limit. For RAG1, phased data were used. Visualisation and editing of haplotype networks were done in the web-based program TCSBU (TCS Beautifier; Santos et al. Citation2016) and INKSCAPE v. 0.92.3 (Inkscape Project, https://inkscape.org).
Risk screening
The Aquatic Species Invasiveness Screening Kit (AS-ISK v2.3.3) (Copp et al. Citation2016), a decision-support tool, was utilised to assess the invasiveness risk of L. ghigii in the Attika Region, an area designated for risk assessment (RA), with the screening carried out by AST and SZ. This tool aligns with the “minimum standards” (Roy et al. Citation2018) outlined in the European Commission Regulation on the prevention and management of invasive alien species, making it a reliable choice for evaluating non-native species. Its effectiveness has been demonstrated in accurately screening potential invasive non-native aquatic organisms in various RA areas worldwide (Vilizzi et al. Citation2021).
The AS-ISK screening protocol consists of 55 questions (Copp et al. Citation2016). The initial 49 questions focus on the Basic Risk Assessment (BRA), examining the biogeographical and biological aspects of the species under assessment. The remaining six questions pertain to the Climate Change Assessment (CCA), requiring the assessor to evaluate how future climate conditions might influence the risks associated with the species’ introduction, establishment, dispersal and impact. Each question in the screening requires a response, a level of confidence in the response, and a justification.
After completing the screening, the species is assigned a BRA score and a BRA+CCA (composite) score, ranging from −20 to 70 and −32 to 82, respectively. Scores below 1 indicate a low risk of invasiveness, while higher scores classify the species as posing either a medium or a high risk. The distinction between medium and high risk levels is determined by a predefined “threshold” value. In this study, the threshold is based on the calibrated BRA score of 15.25 for translocated and traded aquarium freshwater fishes in Greece (Perdikaris et al. Citation2016).
The AS-ISK utilises confidence levels associated with each question-related response, ranked as follows: 1 = low, 2 = medium, 3 = high and 4 = very high. These confidence rankings align with those recommended by the Intergovernmental Panel on Climate Change (IPCC Citation2005). Overall confidence levels (CLTotal), as well as CLBRA and CLCCA, are calculated based on the allocated confidence level for each response across all 55 questions.
Results
Genetics
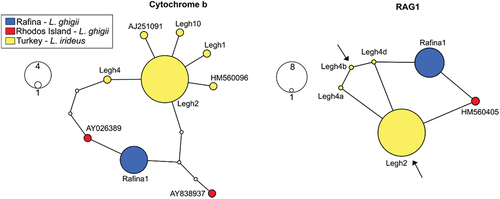
Both analyses of cyt b (1140 bp) and RAG1 (1442 bp) identified specimens from Rafina as Ladigesocypris (). There are no traces of recent hybridisation with Pelasgus native to the investigated region (). All Ladigesocypris specimens bore solely alleles falling into the clade of this genus on both markers used (). The cyt b resolved Ladigesocypris as an internal lineage within Squalius, with Petroleuciscus borysthenicus as the sister lineage to Squalius/Ladigesocypris (), while RAG1 placed P. borysthenicus, Squalius and Ladigesocypris in a polytomy (). All sequences of Ladigesocypris are very similar among themselves (max. 5 mutation steps on cyt b, and 2–3 on RAG1, see ). There is no haplotype sharing between the localities/areas (Rafina, Rhodes and the Turkish mainland) (). In terms of cyt b, the Rafina population is more similar to the Rhodes population, while in terms of RAG1 it is more difficult to identify relationships, as all haplotypes are more similar among themselves ().
Figure 2. The 50% majority rule consensus Bayesian phylogenetic reconstructions for both mitochondrial cytochrome b and nuclear RAG1 sequence data. Numbers on branches represent posterior probabilities; only values ≥ 0.7 are shown. Samples from the introduced population near Rafina are in red.
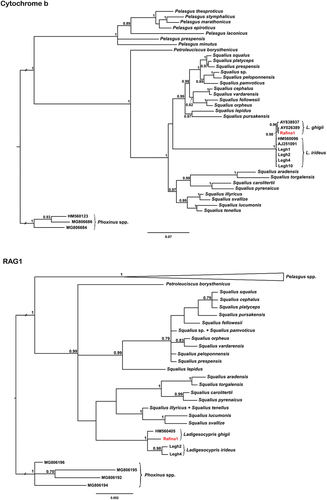
Figure 3. Haplotype networks of Ladigesocypris sequences constructed by a statistical parsimony method for both mitochondrial cytochrome b and nuclear RAG1. For RAG1, all four possible alleles of heterozygotic sample Legh4 are depicted. Arrows in the RAG1 network indicate haplotypes of Legh4 sample with the highest probability as estimated by phasing the dataset by DnaSP (probability 0.65). Intermediate non-occurring haplotypes between our haplotypes are shown as small white circles. A line between two neighbouring haplotypes indicates one mutational step.
Morphology
The collected Ladigesocypris (up to 71.5 mm in standard length (SL)) included individuals that were considerably larger than P. marathonicus (max. reported SL 56 mm, Kottelat & Barbieri Citation2004), but despite this fact, the overall cursory structure of the two minnows is rather similar ().
Ladigesocypris ghigii is a small-bodied freshwater leuciscid (max SL: female 90 mm; male 65 mm) distinguished from other Leuciscidae in south-eastern Europe, and especially from P. marathonicus, by the following combination of characters: incomplete lateral line with 11–17 perforated scales (vs 0–7 in P. marathonicus); 27–33 total scales in midlateral row (vs 36–41 in P. marathonicus); preserved specimens have a broad midlateral stripe from head to caudal base, separating the dark brown back from the white belly (vs missing in P. marathonicus) (Kottelat & Freyhof Citation2007). In living specimens, the shiny golden-silver body is slightly interrupted by this line.
Meristic and morphometric analyses were conducted on 10 individuals of L. ghigii from the sampled sites near Rafina, ranging from 72.7 mm total length (TL) to 91.2 mm TL (57.5–71.5 mm SL). All specimens showed an incomplete lateral line. The following combination of meristic characters confirmed the identification of the species: lateral line pored scales 12–15, midlateral row scales 28–30 (32) + (1) 2 (3), pre-dorsal scales (18) 19–20, transverse scales 1/210–111/2, transverse scales at caudal peduncle 1/2(5) 6 (7)1/2 (vs 1/27–81/2 in P. marathonicus), branched pectoral (P) fin rays (11) 12, branched pelvic (V) fin rays 7 (8), branched dorsal (D) fin rays 7–81/2, branched anal (A) fin rays (7) 81/2 (vs usually 71/2 in P. marathonicus), branched caudal (C) fin rays (18) 19. Data for P. marathonicus are from Kottelat and Barbieri (Citation2004). Detailed morphometric data of L. ghigii found at Rafina are given in . presents the frequency of occurrence of meristic characters.
Table II. Morphometric data of Ladigesocypris ghigii found at two sites near Rafina (N = 10). SD is standard deviation.
Table III. Frequency of occurrence of meristic characters of Ladigesocypris ghigii found at two sites near Rafina (N = 10). Counts of transverse scales and transverse scales at caudal peduncle do not include the scale row on dorsal and ventral midlines noted as “1/2” in the text.
Fish assemblage at the surveyed sites
The river water bodies investigated in spring were rather shallow (mean depth 0.35 cm), with perennial flow and a mean wetted width of 3.8 m during spring flow. Recorded densities of L. ghigii were high, ranging from 0.73 to 1.1 individuals per square metre (a total of 455 specimens were collected at two separate sites). In most areas the fish formed schools in pooling water areas (in pools, in deep runs and under bridges) and a full arc of size classes was found. The numbers of fish were fairly high in a variety of habitats, including swampy edges, rocky areas and undercut banks, and less so in faster flowing riffles. No Pelasgus marathonicus were recorded. The only other fish species present was the European eel (Anguilla anguilla), also with rather high densities, ranging from 0.04 to 0.06 per square metre (27 individuals recorded in total, across both surveyed sites). Underwater camera footage and visual observation from shore confirmed the species’ occurrence at the other two locations and provided a minimum river distribution for the species of 9.7 river kilometres in the spring of 2021. A river barrier (road-crossing) prevents fish from moving upstream at one of the stream tributaries (this was documented using underwater video, above and below the barrier). We intentionally withhold precise geographical positions of all surveyed stream segments here so as to not encourage amateur aquarists and the wider public to find and illegally collect the fish.
Risk screening
Based on the calibrated threshold values, the BRA scores for L. ghigii indicated a high-risk category for the Attika region, with a score of 32 (). When considering the potential impact of climate change, the BRA score increased to 38, indicating an even higher risk for the species to become invasive in this RA area under predicted climate change conditions (). Several factors and traits contributed to the increase in the BRA score, such as rapid reproduction, persistence, tolerance and resource exploitation. Conversely, the absence of cultivation of the species in the RA resulted in a decrease in the overall score.
Table IV. Scoring output from the Aquatic Species Invasiveness Screening Kit for Ladigesocypris ghigii in the risk assessment area of Attika region, Greece.
The mean confidence levels (CL) associated with responses to the BRA, CCA, and BRA+CCA questions were as follows: CLBRA = 2.25 ± 0.06, CLCCA = 2.00, and CLTOTAL = 2.22 ± 0.06. These values indicate medium to high confidence in all cases.
Discussion
Phylogenetic and taxonomic implications
The newly established leuciscid fish population in Attika was identified as L. ghigii/irideus. The relationships of the population from Rafina in Attika with those from Rhodes (L. ghigii) and the Turkish mainland (L. irideus) are not completely clear based on the genetic data. However, the results for mitochondrial cyt b, which is considerably more polymorphic than nuclear RAG1, suggest a higher affinity to the Rhodes Island haplotypes, and thus point to Rhodes as the probable origin of the studied introduced population.
The native range of the genus Ladigesocypris includes inland waters of south-western Anatolia and Rhodes (Kottelat & Freyhof Citation2007; Çiçek et al. Citation2020) in the Western Anatolia Ecoregion (Abell et al. Citation2008). However, the taxonomy at the species level as well as at the genus level is not fully resolved (Giannetto et al. Citation2019), and there is great confusion and inconsistency in the nomenclature. Further work is needed to clarify whether Ladigesocypris is a separate genus or whether its species belong to Squalius. Three species are generally recognised, L. mermere from the Gediz River basin (Marmara Lake, Turkey) (Çiçek et al. Citation2020); L. ghigii, suggested to be endemic to the Greek Rhodes Island (Kottelat & Freyhof Citation2007); and L. irideus from the rivers and streams in the south-western Anatolian mainland, from Küçük Menderes to Dalaman (Yılmaz et al. Citation2015). Nevertheless, in both the Eschmeyer’s Catalog of Fishes and FishBase, L. ghigii is listed as Squalius ghigii, while the other two species are listed as belonging to the genus Ladigesocypris. Moreover, Bogutskaya (Citation1996) considered L. ghigii and L. irideus identical based on morphology, with only very minor differences between the Rhodes Island and Anatolian mainland populations, while she recognised L. mermere as a subspecies, with considerable morphological difference, notably a much higher number of lateral line scales. Subsequent genetic investigations provided similar results for L. ghigii and L. irideus, which were found to be genetically almost identical (Perea et al. Citation2010; Geiger et al. Citation2014), although only a small number of specimens were included in both studies. Our results also confirmed very similar haplotypes of L. ghigii and L. irideus, for both mitochondrial and nuclear markers (). Furthermore, Geiger et al. (Citation2014) analysed specimens identified as L. mermere and found they were genetically identical to Petroleuciscus smyrnaeus. Here we provisionally accept a two-species concept for L. ghigii and L. irideus, as a recent speciation event cannot be excluded, and keep the generic name Ladigesocypris while awaiting further comprehensive revision of the Ladigesocypris/Squalius/Petroleuciscus relationships.
Evidence for human-induced translocation
Until now, L. ghigii was known to be confined to its native island of Rhodes which is part of the Western Anatolia Freshwater Ecoregion (Abell et al. Citation2008). Along with other nearshore eastern Greek islands, Rhodes forms part of this Asian ecoregion that has been isolated from the European side of the Aegean at least since the Miocene (Zogaris et al. Citation2009). A population of this genus anywhere west of the Mid-Aegean trench would certainly be presumed translocated by humans.
Recent reviews of the Greek ichthyofauna do not include Ladigesocypris in the area where it was found near Rafina (Economou et al. Citation2007; Barbieri et al. Citation2015). The evidence that stream and nearby areas were surveyed is given in Economou et al. (Citation2007), so the species must have been introduced relatively recently.
An internet search produced anecdotal information of an amateur aquarist’s breeding of Rhodes minnows in Attika after 2009 from stock brought illegally from Rhodes, published on the Greek Aquarists Board internet forum (http://www.aquatek.gr/). The amateur aquarist forum member specifically states that minnows were originally brought to an Athenian home aquarium from Rhodes via a commercial airplane flight in 2009. Although the aquarist had husbandry experience, only a single couple survived the flight and bred in captivity. Although there are several follow-up posts in the forum, in 2014 the hobbyist confessed that he was “quitting the hobby” and would like to part with the fish (he had “50–60 specimens to give for free”). Although we do not have firm evidence of aquarists being the culprits in this invasion, the timing of the alleged transport of these specimens from Rhodes matches the assumed time of their release to the stream near Rafina as no leuciscid species was present in the stream at the time of the survey of Economou et al. (Citation2007). Based on available documentation, it is possible that the species was introduced to Attika between 2014 and 2016, but not before 2010 (HCMR data from wetland surveys in Attika).
Vulnerability to invasion by translocated species is higher in ecosystems near metropolitan megacities such as Athens (Koutsikos et al. Citation2021). Recently, several translocated reptile species have established populations in and near this city (e.g. Christopoulos et al. Citation2022). Finally, aquarium hobbyists have been targeted as one of the main vectors for alien fauna transports in Greece (Papavlasopoulou et al. Citation2014). Since perennially flowing river habitats are scarce around Athens, this system very near Athens, including its international airport and major port facilities, could be vulnerable to human-induced translocations.
Evidence for establishment
After a careful inspection and sampling in 2021 and 2022, we confirm that the species is well established and widespread in at least 9.7 km of the small lowland river near Rafina. Estimated population densities were rather high in spring, and are estimated to be even higher when flow and water levels drop in the summer and autumn (Barbieri et al. Citation2003). A variety of size classes are present, and some individuals reached maximum recorded sizes for the species. Stream conditions in Attika are remarkably similar to those of many lowland streams on Rhodes Island. Habitat for reproduction is suitable at both surveyed sites, as well as in other parts of the river. Artificial barriers seem to restrict further upstream dispersal, supporting the assumption that the population is recently established. A delay in identification of the species presumably allowed L. ghigii to establish and spread along the river.
Threats to biodiversity
Ladigesocypris ghigii is a strongly opportunistic generalist but it has never been subject to assessment of invasiveness threat in Greece(Perdikaris et al. Citation2016). Our species-specific risk screening for potential invasiveness in the Attika region through the AS-ISK tool yielded a high risk of being invasive. The species has been called an “opportunistic” breeder, reproducing under various conditions in the wild and easily in captivity as well (Stoumboudi et al. Citation2002; Poncin et al. Citation2005). It is known to undertake mass movements to enter more suitable habitats as summer water levels drop (Kondylatos et al. Citation2014) and is tolerant of varied conditions for feeding and survival. It survives drought periods in remnant river pools under harsh summer droughts with rapid population comebacks (Mamuris et al. Citation2005; SZ personal observation). Moreover, within the species’ native island, successful human-mediated translocations to other streams have been made (Economidis et al. Citation2000), and the species may take up a variety of freshwater habitats including lotic and artificial lentic ecosystems.
During the last three decades, the numbers of translocated fish species in Greece have rapidly increased (Economidis et al. Citation2000; Leonardos et al. Citation2007; Koutsikos et al. Citation2012; Viñuela Rodríguez et al. Citation2021) and the issue is considered a problem in many other European countries as well (Jelić et al. Citation2016; Tarkan et al. Citation2017; Vukić et al. Citation2019; Koutsikos et al. Citation2021; Marić et al. Citation2022). There is also evidence that some translocated freshwater fishes may have a much more invasive ability than other aliens, since they may easily adapt to the roughly similar environmental conditions because they originate from neighbouring areas (Koutsikos et al. Citation2019).
The Rhodes minnow is a fairly attractive small-sized fish, surviving well in small aquarium tanks, and it may be sought out by amateurs who want to develop so-called biotope aquaria with “native fish” in their homes. Although it is not considered an ornamental species, small-bodied fish (usually under 10 cm TL) that survive well in home aquaria are known to be especially problematic invasive threats in other regions (e.g. Florida, see Lawson et al. Citation2015).
Since the said leuciscid is a small fish, similar in appearance to other native schooling minnow-like fishes, the spread may potentially be undetected for some time. The initial misidentification of translocated fish by local researchers is not uncommon; such introductions may occur more frequently than is currently appreciated, since genetic analyses are not widely used to confirm field identifications (Tellier et al. Citation2023). Translocated fish spread could also gravely hamper biodiversity conservation efforts by creating confusion and loss of the identity and distinctive values of endemic-rich inland water bodies. In our study region, very close to the invaded river system near Rafina, protected Natura 2000 river sites host endemic P. marathonicus populations of high conservation value. The conservation value of a protected area may decline if the site becomes infested by non-native fish; this may affect the ecosystem services provided by the protected areas, including various cultural ecosystem services (Vlami et al. Citation2021).
Finally, translocated non-native freshwater species can present direct and indirect pressures and threats to native species and ecosystems in a variety of ways. This includes spreading of diseases and parasites, examples being the well-known cases of chytrid fungus Batrachochytrium dendrobatidis, a pathogen responsible for the global decline of amphibians (Fisher & Garner Citation2007); crayfish plague, Aphanomyces astaci, seriously affecting native crayfish species (Mrugała et al. Citation2017; Svoboda et al. Citation2017); or Anguillicola crassus, a parasitic nematode severely affecting European eel populations (Cesco et al. Citation2001), spread by non-native species. However, only very limited information is available about parasitation of Ladigesocypris (Nejat et al. Citation2023), and the potential risk for spread of parasites and diseases needs further investigation. Another serious potential threat is hybridisation with related fish species, which can lead to the loss of genetic integrity of the native species (Fumagalli et al. Citation2002; Viñuela Rodríguez et al. Citation2021; Marić et al. Citation2022). Hybridisation in leuciscids, even among different genera, is widely documented (e.g. Bianco Citation1988; Freyhof et al. Citation2005; Perea et al. Citation2016; Buj et al. Citation2020; Curto et al. Citation2022). Ladigesocypris was reported to hybridise with Squalius in Turkey (Durand et al. Citation2000). Fortunately, L. ghigii was introduced into a stream with no native leuciscids, although the nearby rivers are inhabited by the endemic P. marathonicus, with which L. ghigii could hypothetically hybridise.
Recommendations
This paper reports on the recent introduction of a fish species from the Western Anatolia to the Western Aegean Freshwater Ecoregion on the European continent, which was assessed as posing a high risk of invasiveness. We may presume that eradication from the specific water bodies and population control by suppression (e.g. through removal programmes) might help prevent its further spread. Such organised conservation and public communication actions might also help inform the public of the severe and rising fish translocation problem (Britton et al. Citation2011). Several carefully orchestrated eradication attempts have been promoted in Europe in recent years (Tiberti et al. Citation2021).
Furthermore, this is a sensitive issue as L. ghigii is a designated IUCN Vulnerable species, for which a published national action plan for its conservation exists (Spala Citation2021), so a careful approach to planning management actions and communicating the problem is required. If an eradication campaign or a containment operation is instigated, animal welfare issues must be taken into careful consideration (Crowley et al. Citation2017). To reduce any potential conflict, a careful study of the issue must be undertaken (Warren Citation2021). We propose a special feasibility study be developed to plan all aspects of the management and potential eradication and/or containment actions including the public communication requirements.
Acknowledgements
Collecting was conducted under Hellenic Ministry of Environment licence no. 143634/1894/20-7-2016 within water bodies monitored by HCMR. We are grateful to Maximilian Wagner and Sandra Bračun who helped discover the fish with SZ, and to Plato Stephanopoulos who posted a photo of adult individuals on the Facebook group “Freshwater Fishes of Greece and Cyprus” and thus helped to unravel the mystery of the translocated Rhodes minnow. We are also grateful to the Management Unit of Parnitha and Schinias National Parks and Protected Areas of Saronikos Gulf for all their support in this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Contreras-Balderas S, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Sabaj-Perez MH, Petry P. 2008. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 58(5):403–414. DOI: 10.1641/B580507.
- Bampfylde CA, Peters JA, Bobeldyk AM. 2010. A literature analysis of freshwater invasive species research: Are empiricists, theoreticians, and economists working together? Biological Invasions 12:1207–1219. DOI: 10.1007/s10530-009-9540-2.
- Barbieri R, Stoumboudi M, Corsini-Foka M, Kalogianni E, Kondylatos G, Economou AN. 2003. First data for the assessment of the abundance of gizani (Ladigesocypris ghigii). 7th Hellenic Symposium on Oceanography and Fisheries, Heronissos, May, Crete (Greece).
- Barbieri R, Vukić J, Šanda R, Kapakos Y, Zogaris S. 2017. Alburnoides economoui, a new species of spirlin from Central Greece and redescription of Alburnoides thessalicus (Actinopterygii: Cyprinidae). Biologia 72(9):1075–1088. DOI: 10.1515/biolog-2017-0113.
- Barbieri R, Zogaris S, Kalogianni E, Stoumboudi M, Chatzinikolaou Y, Giakoumi S, Kapakos Y, Kommatas D, Koutsikos N, Tachos V, Vardakas L, Economou AN. 2015. Freshwater fishes and lampreys of Greece: An annotated checklist. Monographs on Marine Sciences No. 8. Greece: Hellenic Centre for Marine Research Athens. pp. 1–130.
- Benovics M, Desdevises Y, Šanda R, Vukić J, Šimková A. 2020. Cophylogenetic relationships between Dactylogyrus (Monogenea) ectoparasites and endemic cyprinoids of the north-eastern European peri-Mediterranean region. Journal of Zoological Systematics and Evolutionary Research 58(1):1–21. DOI: 10.1111/jzs.12341.
- Benovics M, Vukić J, Šanda R, Nejat F, Charmpila EA, Buj I, Shumka S, Porcelloti S, Tarkan SA, Aksu S, Emiroğlu O, Šimková A. 2023. Monogeneans and chubs: Ancient host-parasite system under the looking glass. Molecular Phylogenetics and Evolution 179:1–17. DOI: 10.1016/j.ympev.2022.107667.
- Bianco PG. 1988. Leuciscus cephalus (Linnaeus), with records of fingerling adult males, Leuciscus pleurobipunctatus (Stephanidis) and their hybrids from western Greece. Journal of Fish Biology 32:1–16. DOI: 10.1111/j.1095-8649.1988.tb05331.x.
- Bogutskaya NG. 1996. Contribution to the knowledge of leuciscine fishes of Asia Minor. Part 1. Morphology and taxonomic relationships of Leuciscus borysthenicus (Kessler, 1859), L. smyrnaeus Boulenger, 1896 and Ladigesocypris ghigii (Gianferrari, 1927). Publicaciones Especiales, Instituto Español de Oceanografía 21:25–44.
- Bogutskaya NG, Zupančič P, Bogut I, Naseka AM. 2012. Two new freshwater fish species of the genus Telestes (Actinopterygii, Cyprinidae) from karst poljes in Eastern Herzegovina and Dubrovnik littoral (Bosnia and Herzegovina and Croatia). Zookeys 180:53–80. DOI: 10.3897/zookeys.180.2127.
- Boon PJ, Clarke SA, Copp GH. 2020. Alien species and the EU water framework directive: A comparative assessment of European approaches. Biolological Invasions 22:1497–1512. DOI: 10.1007/s10530-020-02201-z.
- Britton JR, Gozlan RE, Copp GH. 2011. Managing non-native fish in the environment. Fish and Fisheries 12(3):256–274. DOI: 10.1111/j.1467-2979.2010.00390.x.
- Buj I, Marčić Z, Čavlović K, Ćaleta M, Tutman P, Zanella D, Duplić A, Raguž L, Ivić L, Horvatic S, Mustafić P. 2020. Multilocus phylogenetic analysis helps to untangle the taxonomic puzzle of chubs (genus Squalius: Cypriniformes: Actinopteri) in the Adriatic basin of Croatia and Bosnia and Herzegovina. Zoological Journal of the Linnean Society 189(3):953–974. DOI: 10.1093/zoolinnean/zlz133.
- Buj I, Šanda R, Zogaris S, Freyhof J, Geiger MF, Vukić J. 2019. Cryptic diversity in Telestes pleurobipunctatus (Actinopterygii; Leuciscidae), as a consequence of historical biogeography in the Ionian freshwater Ecoregion (Greece, Albania). Hydrobiologia 835:147–163. DOI: 10.1007/s10750-019-3935-6.
- Cesco H, Lambert A, Crivelli AJ. 2001. Pseudorasbora parva (Téléostéen, Cyprinidae) espèce invasive, nouvel agent du maintien et de la dissémination de l’anguillicolose en France? Parasite 8:75–76. DOI: 10.1051/parasite/2001081075.
- Christopoulos A, Pantagaki CF, Poulakakis N, Pafilis P, Pafilis P. 2022. First record of Anatololacerta pelasgiana (Mertens, 1959) in mainland Greece: Another new species in Athens. Herpetozoa 35:239–244. DOI: 10.3897/herpetozoa.35.e97649.
- Çiçek E, Sungur S, Fricke R. 2020. Freshwater lampreys and fishes of Turkey; a revised and updated annotated checklist 2020. Zootaxa 4809(2):241–270. DOI: 10.11646/zootaxa.4809.2.2.
- Clement M, Posada D, Crandall KA. 2000. TCS: A computer program to estimate gene genealogies. Molecular Ecology 9(10):1657–1659. DOI: 10.1046/j.1365-294x.2000.01020.x.
- Copp GH, Bianco PG, Bogutskaya NG, Erős T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kováč V, Moreno-Amich R, Naseka AM, Peňáz M, Povž M, Przybylski M, Robillard M, Russell IC, Stakėnas S, Šumer S, Vila-Gispert A, Wiesner C. 2005. To be, or not to be, a non‐native freshwater fish? Journal of Applied Ichthyology 21(4):242–262. DOI: 10.1111/j.1439-0426.2005.00690.x.
- Copp GH, Vilizzi L, Tidbury H, Stebbing PD, Tarkan AS, Moissec L, Goulletquer P. 2016. Development of a generic decision-support tool for identifying potentially invasive aqua tic taxa: AS-ISK. Management of Biological Invasions 7(4):343–350. DOI: 10.3391/mbi.2016.7.4.04.
- Crivelli AJ. 2006. Ladigesocypris ghigii. The IUCN Red List of Threatened Species 2006. Available: https://www.iucnredlist.org/species/11133/3252949. Accessed Oct 2022 9.
- Crowley SL, Hinchliffe S, McDonald RA. 2017. Conflict in invasive species management. Frontiers in Ecology and the Environment 15(3):133–141. DOI: 10.1002/fee.1471.
- Curto M, Morgado-Santos M, Alexandre CM, Alves MJ, Gante HF, Gkenas C, Medeiros JP, Pinheiro PJ, Almeida PR, Magalhães MF, Ribeiro F. 2022. Widespread hybridization between invasive bleak (Alburnus alburnus) and Iberian chub (Squalius spp.): A neglected conservation threat. Fishes 7(5):247. DOI: 10.3390/fishes7050247.
- Dextrase AJ, Mandrak NE. 2006. Impacts of alien invasive species on freshwater fauna at risk in Canada. Biological Invasions 8(1):13–24. DOI: 10.1007/s10530-005-0232-2.
- Durand JD, Tsigenopoulo CS, Ünlü E, Berrebi P. 2002. Phylogeny and biogeography of the family Cyprinidae in the Middle East inferred from cytochrome b DNA—Evolutionary significance of this region. Molecular Phylogenetics and Evolution 22(1):91–100. DOI: 10.1006/mpev.2001.1040.
- Durand JD, Ünlü E, Doadrio I, Pipoyan S, Templeton AR. 2000. Origin, radiation, dispersion and allopatric hybridization in in the chub, Leuciscus cephalus. Proceeding of the Royal Society of London: Series B: Biological Sciences 267:1687–1697. DOI: 10.1098/rspb.2000.1196.
- Economidis PS. 1995. Endangered freshwater fishes of Greece. Biological Conservation 72(2):201–211. DOI: 10.1016/0006-3207(94)00083-3.
- Economidis PS, Dimitriou E, Pagoni R, Michaloudi E, Natsis L. 2000. Introduced and translocated fish species in the inland waters of Greece. Fisheries Management and Ecology 7(3):239–250. DOI: 10.1046/j.1365-2400.2000.00197.x.
- Economou AN, Giakoumi S, Vardakas L, Barbieri R, Stoumboudi MT, Zogaris S. 2007. The freshwater ichthyofauna of Greece-an update based on a hydrographic basin survey. Mediterranean Marine Science 8:91–166. DOI: 10.12681/mms.164.
- Fisher MC, Garner TWJ. 2007. The relationship between the emergence of Batrachochytrium dendrobatidis, the wildlife trade in amphibians and introduced amphibian species. Fungal Biology Reviews 21:2–9. DOI: 10.1016/j.fbr.2007.02.002.
- Freyhof J, Lieckfeldt D, Bogutskaya NG, Pitra C, Ludwig A. 2006. Phylogenetic position of the Dalmatian genus Phoxinellus and description of the newly proposed genus Delminichthys (Teleostei: Cyprinidae). Molecular Phylogenetics and Evolution 38(2):416–425. DOI: 10.1016/j.ympev.2005.07.024.
- Freyhof J, Lieckfeldt D, Pitra C, Ludwig A. 2005. Molecules and morphology: Evidence for introgression of mitochondrial DNA in Dalmatian cyprinids. Molecular Phylogenetics and Evolution 37(2):347–354. DOI: 10.1016/j.ympev.2005.07.018.
- Fumagalli L, Snoj A, Jesenšek D, Balloux F, Jug T, Duron O, Brossier F, Crivelli AJ, Berrebi P. 2002. Extreme genetic differentiation among the remnant populations of marble trout (Salmo marmoratus) in Slovenia. Molecular Ecology 11(12):2711–2716. DOI: 10.1046/j.1365-294X.2002.01648.x.
- Geiger MF, Herder F, Monaghan MT, Almada V, Barbieri R, Bariche M, Berrebi P, Bohlen J, Casal-Lopez M, Delmastro GB, Denys GPJ, Dettai A, Doadrio I, Kalogianni E, Kärst H, Kottelat M, Kovačić M, Laporte M, Lorenzoni M, Marčić Z, Özuluğ M, Perdices A, Perea S, Persat H, Porcelotti S, Puzzi C, Robalo J, Šanda R, Scheneider M, Šlechtová V, Stoumboudi M, Walter S, Freyhof J. 2014. Spatial heterogeneity in the Mediterranean biodiversity hotspot affects barcoding accuracy of its freshwater fishes. Molecular Ecology Resources 14:1210–1221. DOI: 10.1111/1755-0998.12257.
- Giannetto D, Doosti S, Tarkan AS, Yılmaz F. 2019. Ladigesocypris sp. (Ladiges, 1960) complex: Current status of knowledge and implications for conservation of these Cyprinids species endemic to Aegean region. BioEco2019- International Biodiversity & Ecology Sciences Symposium, September, Istanbul (Turkey).
- Gozlan RE. 2008. Introduction of non-native freshwater fish: Is it all bad? Fish and Fisheries 9(1):106–115. DOI: 10.1111/j.1467-2979.2007.00267.x.
- Hall T. 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 4:95–98.
- Helfman GS. 2007. Fish conservation: A guide to understanding and restoring global aquatic biodiversity and fishery resources. (Washington DC): Island Press. pp. 1–688.
- IPCC, 2005. Guidance notes for lead authors of the IPCC fourth assessment report on addressing uncertainties. Intergovernmental Panel on Climate Change, WMO & UNEP. www.ipcc.ch/site/assets/uploads/2018/02/ar4-uncertaintyguidancenote-1.pdf.
- Jelić D, Špelić I, Žutinić P. 2016. Introduced species community over-dominates endemic ichthyofauna of high Lika plateau (central Croatia) over a 100 year period. Acta Zoologica Academiae Scientiarum Hungaricae 62(2):191–216. DOI: 10.17109/AZH.62.2.191.2016.
- Kondylatos G, Corsini-Foka M, Economidis PS. 2014. First observation of leaping behavior of Ladigesocypris ghigii, a cyprinid fish endemic to Rhodes Island, Greece (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 24:299–300.
- Kottelat M, Barbieri R. 2004. Pseudophoxinus laconicus, a new species of minnow from Peloponnese, Greece, with comments on the west Balkan Pseudophoxinus species (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 15:147–160.
- Kottelat M, Freyhof J. 2007. Handbook of European freshwater fishes. Cornol (Switzerland); Berlin (Germany). pp. 1–646.
- Koutsikos N, Vardakas L, Kalantzi OI, Zogaris S. 2021. Patterns of spatial overlap between non-indigenous and critically endangered freshwater fishes from a Mediterranean biodiversity hotspot. Diversity 13(6):233. DOI: 10.3390/d13060233.
- Koutsikos N, Zogaris S, Vardakas L, Kalantzi OI, Dimitriou E, Economou AN. 2019. Tracking non-indigenous fishes in lotic ecosystems: Invasive patterns at different spatial scales in Greece. Science of the Total Environment 659:384–400. DOI: 10.1016/j.scitotenv.2018.12.324.
- Koutsikos N, Zogaris S, Vardakas L, Tachos V, Kalogianni E, Šanda R, Chatzinikolau Y, Giakoumi S, Economidis PS, Economou AN. 2012. Recent contributions to the distribution of the freshwater ichthyofauna in Greece. Mediterranean Marine Science 13:268–277. DOI: 10.12681/mms.308.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34:772–773. DOI: 10.1093/molbev/msw260.
- Lawson Jr LL, Hill JE, Hardin S, Vilizzi L, Copp GH. 2015. Evaluation of the Fish Invasiveness Screening Kit (FISK v2) for peninsular Florida. Management of Biological Invasions 6:413–422. DOI: 10.3391/mbi.2015.6.4.09.
- Leonardos I, Kagalou I, Triantafyllidis A. 2007. Threatened fishes of the world: Silurus aristotelis (Agassiz 1856) (Siluridae). Environmental Biology of Fishes 78:285–286. DOI: 10.1007/s10641-006-0006-4.
- Mamuris Z, Stoumboudi MT, Stamatis C, Barbieri R, Moutou KA. 2005. Genetic variation in populations of the endangered fish Ladigesocypris ghigii and its implications for conservation. Freshwater Biology 50(9):1441–1453. DOI: 10.1111/j.1365-2427.2005.01410.x.
- Marić S, Stanković D, Sušnik Bajec S, Vukić J, Šanda R, Stefanov T, Nikolić D, Snoj A. 2022. Perils of brown trout (Salmo spp.) mitigation-driven translocations: A case study from the Vlasina Plateau, Southeast Serbia. Biological Invasions 24(4):999–1016. DOI: 10.1007/s10530-021-02688-0.
- Miller RR, Williams JD, Williams JF. 1989. Extinctions of North American fishes during the past century. Fisheries 14:22–38. DOI: 10.1577/1548-8446(1989)014<0022:EONAFD>2.0.CO;2.
- Mrugała A, Šanda R, Petrusek A, Marić D, Vukić J. 2017. Recent acute crayfish mortality reveals Aphanomyces astaci presence in Bosnia and Herzegovina. Journal of Invertebrate Pathology 150:73–75. DOI: 10.1016/j.jip.2017.09.004.
- Muñoz-Mas R, Essl F, van Kleunen M, Seebens H, Dawson W, Casal CMV, García-Berthou E. 2023. Two centuries of spatial and temporal dynamics of freshwater fish introductions. Global Ecology and Biogeography 32:1632–1644. DOI: 10.1111/geb.13714.
- Nejat F, Benovics M, Řehulková E, Vukić J, Šanda R, Kaya C, Tarkan AS, Abdoli A, Aksu S, Šimková A. 2023. Diversity, phylogeny and intraspecific variability of Paradiplozoon species (Monogenea: Diplozoidae) parasitizing endemic cyprinoids in the Middle East. Parasitology 150(8):705–722. DOI: 10.1017/S0031182023000446.
- Papavlasopoulou I, Vardakas L, Perdikaris C, Kommatas D, Paschos I. 2014. Ornamental fish in pet stores in Greece: A threat to biodiversity? Mediterranean Marine Science 15:126–134. DOI: 10.12681/mms.484.
- Perdikaris C, Koutsikos N, Vardakas L, Kommatas D, Simonović P, Paschos I, Detsis V, Vilizzi L, Copp GH. 2016. Risk screening of non-native, translocated and traded aquarium freshwater fish in Greece using fish invasiveness screening Kit. Fisheries Management and Ecology 23:32–43. DOI: 10.1111/fme.12149.
- Perea S, Böhme M, Zupančič P, Freyhof J, Šanda R, Özuluğ M, Abdoli A, Doadrio I. 2010. Phylogenetic relationships and biogeographical patterns in Circum-Mediterranean subfamily Leuciscinae (Teleostei, Cyprinidae) inferred from both mitochondrial and nuclear data. BMC Evolutionary Biology 10(1):1–27. DOI: 10.1186/1471-2148-10-265.
- Perea S, Vukić J, Šanda R, Doadrio I, Bernardi G. 2016. Ancient mitochondrial capture as factor promoting mitonuclear discordance in freshwater fishes: A case study in the genus Squalius (Actinopterygii, Cyprinidae) in Greece. PLOS ONE 11(12):e0166292. DOI: 10.1371/journal.pone.0166292.
- Poncin P, Stoumboudi MT, Gervalle L, Barbieri R, Economou AN, Economidis PS. 2005. The spawning behavior of the endangered freshwater fish Ladigesocypris ghigii (Gianferrari, 1927). Journal of Applied Ichthyology 21:225–228. DOI: 10.1111/j.1439-0426.2005.00604.x.
- Rambaut A. 2018. FigTree v. 1.4.4. Available: https://github.com/rambaut/figtree/releases. Accessed Jan 2019.
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA, Susko E. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67(5):901–904. DOI: 10.1093/sysbio/syy032.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3):539–542. DOI: 10.1093/sysbio/sys029.
- Roy HE, Rabitsch W, Scalera R, Stewart A, Gallardo B, Genovesi P, Essl F, Adriaens T, Bacher S, Booy O, Branquart E, Brunel S, Copp GH, Dean H, D’Hondt B, Josefsson M, Kenis M, Kettunen M, Linnamagi M, Lucy F, Martinou A, Moore N, Nentwig W, Nieto A, Pergl J, Peyton J, Roques A, Schindler S, Schönrogge K, Solarz W, Stebbing PD, Trichkova T, Vanderhoeven S, van Valkenburg J, Zenetos A. 2018. Developing a framework of minimum standards for the risk assessment of alien species. Journal of Applied Ecology 55(2):526–538. DOI: 10.1111/1365-2664.13025.
- Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. 2017. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Molecular Biology and Evolution 34(12):3299–3302. DOI: 10.1093/molbev/msx248.
- Šanda R, Vukić J, Choleva L, Křížek J, Šedivá A, Shumka S, Wilson IF. 2008. Distribution of loach fishes (Cobitidae, Nemacheilidae) in Albania, with genetic analysis of populations of Cobitis ohridana. Folia Zoologica 57:42–50.
- Santos AM, Cabezas MP, Tavares AI, Xavier R, Branco M. 2016. tcsBU: A tool to extend TCS network layout and visualization. Bioinformatics 32(4):627–628. DOI: 10.1093/bioinformatics/btv636.
- Schönhuth S, Vukić J, Šanda R, Yang L, Mayden RL. 2018. Phylogenetic relationships and classification of the Holarctic family leuciscidae (Cypriniformes: Cyprinoidei). Molecular Phylogenetics and Evolution 127:781–799. DOI: 10.1016/j.ympev.2018.06.026.
- Spala Κ. 2021. National Action Plan for the Greek native endemic fish Ladigesocypris ghigii (ghizáni). LIFE-IP 4 NATURA Project: Integrated actions for the conservation and management of Natura 2000 sites, species, habitats and ecosystems in Greece (LIFE16 IPE/GR/000002). Deliverable Action Α.1. Ministry of Environment and Energy. pp. 63. IV Annexes (in Greek).
- Stoumboudi M, Barbieri R, Corsini-Foka M, Economou AN, Economidis PS. 2002. Aspects of the reproduction and early life history of Ladigesocypris ghigii, a freshwater fish species endemic to Rhodes island (Greece): Implementation to conservation. In: Collares-Pereira M, Cowx I, Coelho M, editors. Conservation of freshwater fishes: Options for the future. Fishing news books. Oxford: Blackwell Science. pp. 178–185.
- Svoboda J, Mrugała A, Kozubíková-Balcarová E, Petrusek A. 2017. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. Journal of Fish Diseases 40(1):127–140. DOI: 10.1111/jfd.12472.
- Tarkan AS, Vilizzi L, Top N, Ekmekçi FG, Stebbing PD, Copp GH. 2017. Identification of potentially invasive freshwater fishes, including translocated species, in Turkey using the Aquatic Species Invasiveness Screening Kit (AS-ISK). International Review of Hydrobiology 102(1–2):47–56. DOI: 10.1002/iroh.201601877.
- Tellier JM, Winsmann B, Humphreys M, Minoudi S, Triantafyllidis A, Schultz ET. 2023. What are you doing here? A sculpin endemic to Arkansas and Missouri (Cottus immaculatus) appears in Connecticut. Ichthyology and Herpetology 111(1):1–7. DOI: 10.1643/i2020078.
- Templeton AR, Crandall KA, Sing CF. 1992. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132:619–633. DOI: 10.1093/genetics/132.2.619.
- Tiberti R, Buchaca T, Boiano D, Knapp R, Pou Rovira Q, Tavecchia G, Ventura M, Tenan S, Vamosi S. 2021. Alien fish eradication from high mountain lakes by multiple removal methods: Estimating residual abundance and eradication probability in open populations. Journal of Applied Ecology 58(5):1055–1068. DOI: 10.1111/1365-2664.13857.
- Tsaparis D, Konstantinidis I, Palandačić A, Kalogianni E, Stoumboudi MT, Barbieri R, Vardakas L, Koutsikos N, Tsigenopoulos CS. 2021. DNA barcoding provides new insights on the distribution, systematics and conservation of the freshwater genus Pelasgus (Cypriniformes: Cyprinidae) in Greece. Hydrobiologia 848(5):1163–1176. DOI: 10.1007/s10750-021-04526-9.
- Vardakas L, Perdikaris C, Zogaris S, Kalantzi OI, Koutsikos N. 2022. Stakeholders perceptions of non-indigenous freshwater fish species: A case study from a Mediterranean biodiversity hotspot. Environmental Management 69(6):1091–1101. DOI: 10.1007/s00267-022-01623-w.
- Vilizzi L, Copp GH, Hill JE, Adamovich B, Aislabie L, Akin D, Al-Faisal AJ, Almeida D, Azmai MNA, Bakiu R, Bellati A, Bernier R, Bies JM, Bilge G, Branco P, Bui TD, Canning-Clode J, Cardoso Ramos HA, Castellanos-Galindo GA, Castro N, Chaichana R, Chainho P, Chan J, Cunico AM, Curd A, Dangchana P, Dashinov D, Davison PI, de Camargo MP, Dodd JA, Durland Donahou AL, Edsman L, Ekmekçi FG, Elphinstone-Davis J, Erős T, Evangelista C, Fenwick G, Ferincz Á, Ferreira T, Feunteun E, Filiz H, Forneck SC, Gajduchenko HS, Gama Monteiro J, Gestoso I, Giannetto D, Gilles AS, Gizzi F, Glamuzina B, Glamuzina L, Goldsmit J, Gollasch S, Goulletquer P, Grabowska J, Harmer R, Haubrock PJ, He D, Hean JW, Herczeg G, Howland KL, İ̇lhan A, Interesova E, Jakubčinová K, Jelmert A, Johnsen SI, Kakareko T, Kanongdate K, Killi N, Kim J-E, Kırankaya ŞG, Kňazovická D, Kopecký O, Kostov V, Koutsikos N, Kozic S, Kuljanishvili T, Kumar B, Kumar L, Kurita Y, Kurtul I, Lazzaro L, Lee L, Lehtiniemi M, Leonardi G, Leuven RSEW, Li S, Lipinskaya T, Liu F, Lloyd L, Lorenzoni M, Luna SA, Lyons TJ, Magellan K, Malmstrøm M, Marchini A, Marr SM, Masson G, Masson L, McKenzie CH, Memedemin D, Mendoza R, Minchin D, Miossec L, Moghaddas SD, Moshobane MC, Mumladze L, Naddafi R, Najafi-Majd E, Năstase A, Năvodaru I, Neal JW, Nienhuis S, Nimtim M, Nolan ET, Occhipinti-Ambrogi A, Ojaveer H, Olenin S, Olsson K, Onikura N, O’Shaughnessy K, Paganelli D, Parretti P, Patoka J, Pavia RTB, Pellitteri-Rosa D, Pelletier-Rousseau M, Peralta EM, Perdikaris C, Pietraszewski D, Piria M, Pitois S, Pompei L, Poulet N, Preda C, Puntila-Dodd R, Qashqaei AT, Radočaj T, Rahmani H, Raj S, Reeves D, Ristovska M, Rizevsky V, Robertson DR, Robertson P, Ruykys L, Saba AO, Santos JM, Sarı HM, Segurado P, Semenchenko V, Senanan W, Simard N, Simonović P, Skóra ME, Slovák Švolíková K, Smeti E, Šmídová T, Špelić I, Srėbalienė G, Stasolla G, Stebbing P, Števove B, Suresh VR, Szajbert B, Ta KAT, Tarkan AS, Tempesti J, Therriault TW, Tidbury HJ, Top-Karakuş N, Tricarico E, Troca DFA, Tsiamis K, Tuckett QM, Tutman P, Uyan U, Uzunova E, Vardakas L, Velle G, Verreycken H, Vintsek L, Wei H, Weiperth A, Weyl OLF, Winter ER, Włodarczyk R, Wood LE, Yang R, Yapıcı S, Yeo SSB, Yoğurtçuoğlu B, Yunnie ALE, Zhu Y, Zięba G, Žitňanová K, Clarke S. 2021. A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Science of the Total Environment 788:147868. DOI: 10.1016/j.scitotenv.2021.147868.
- Viñuela Rodríguez N, Šanda R, Zogaris S, Vukić J. 2020. Distribution and genetic diversity of two species of Pelasgus minnows (Leuciscidae) in southern Greece. Knowledge and Management of Aquatic Ecosystems 421(421):27. DOI: 10.1051/kmae/2020019.
- Viñuela Rodríguez N, Šanda R, Zogaris S, Vukić J. 2021. Evolutionary history of the pelasgus minnows (Teleostei: Leuciscidae), an ancient endemic genus from the Balkan Peninsula. Molecular Phylogenetics and Evolution 164:107274. DOI: 10.1016/j.ympev.2021.107274.
- Vitule JRS, Freire CA, Simberloff D. 2009. Introduction of non‐native freshwater fish can certainly be bad. Fish and Fisheries 10(1):98–108. DOI: 10.1111/j.1467-2979.2008.00312.x.
- Vlami V, Kokkoris IP, Zogaris S, Kehayias G, Dimopoulos P. 2021. Cultural ecosystem services in the Natura 2000 network: Introducing proxy indicators and conflict risk in Greece. Land 10:4. DOI: 10.3390/land10010004.
- Vukić J, Eliášová K, Marić D, Šanda R. 2019. Occurrence of alien spirlin (Alburnoides sp.) in the Neretva river basin. Knowledge and Management of Aquatic Ecosystems 420(420):15. DOI: 10.1051/kmae/2019007.
- Warren CR. 2021. Beyond ‘Native V. Alien’: Critiques of the native/alien paradigm in the anthropocene, and their implications. Ethics, Policy & Environment 1–31. DOI: 10.1080/21550085.2021.1961200.
- Witte F, Goldschmidt T, Goudswaard PC, Ligtvoet W, Van Oijen MJP, Wanink J. 1991. Species extinction and concomitant ecological changes in Lake Victoria. Netherlands Journal of Zoology 42:214–232. DOI: 10.1163/156854291X00298.
- Yılmaz F, Yorulmaz B, Giannetto D. 2015. Threatened fishes of the world: Ladigesocypris irideus (Ladiges, 1960) (Cyprinidae). Croatian Journal of Fisheries 73(3):132–133. DOI: 10.14798/73.3.830.
- Zogaris S. 2019. Measures for the restoration of river connectivity of the endemic Marathon Minnow (Pelasgus marathonicus). First Interim Report submitted to the Management Unit of Parnitha and Schinias National Parks and the Protected Areas of the Saronic Gulf, the Natural Environment & Climate Change Agency (NECCA). Hellenic Ministry of Environment and Energy/Hellenic Centre for Marine Research. pp. 44. (in Greek).
- Zogaris S, Apostolou A. 2011. First record of pontian monkey goby, Neogobius fluviatilis (Pallas, 1814) in the Evros river (Greece); is it an alien species? Mediterranean Marine Sciences 12(2):454–461. DOI: 10.12681/mms.47.
- Zogaris S, Economou AN, Dimopoulos P. 2009. Ecoregions in the southern Balkans: Should their boundaries be revised? Environtal Management 43:682–697. DOI: 10.1007/s00267-008-9243-y.
