Abstract
A new genus and species, Jantarineura serafini Gębicki, Walczak and Świerczewski, belonging to the extinct tribe Protodikraneurini of the subfamily Typhlocybinae was described from Baltic amber. The description includes visible external characters based on a single female, especially tegminal venation. Protodikraneurini was classified as a distinctive or at least characteristic element of the fauna inhabiting ecosystems of amber forests.
http://zoobank.org/urn:lsid:zoobank.org:act:AA72A877-BE27-45A0-B960-6511503EE04B
http://zoobank.org/urn:lsid:zoobank.org:act:53C01423-BBE8-4A45-90B5-65FC92D32C89
Introduction
Typhlocybinae are presently one of the two most numerous subfamilies of Cicadellidae (Balme Citation2007), comprising more than 6000 described extant species, in ca. 300 genera and five tribes (Dietrich et al. Citation2023), clearly distinguished morphologically from other representatives of Cicadomorpha (Dietrich Citation2005, Citation2006; Dietrich et al. Citation2022). However, the phylogenetic relations as well as the structure of the subfamily still provoke controversy and are widely discussed. The latest research, including also mitogenomes of the main taxa, reveals the presence of only five tribes with a high degree of affinity, i.e. Alebrini and Empoascini together with Typhlocybini, Dikraneurini and Erythroneurini (Yan et al. Citation2022; Dietrich et al. Citation2022).
Moreover, molecular data show that the main phylogenetic lineages of Typhlocybinae probably separated from the ancestral one in the mid to late Cretaceous (about 76–112 Ma), and thus in the period of the rapid development of Magnoliophyta (Foster et al. Citation2016; Dietrich et al. Citation2017; Yan et al. Citation2022). The age of amber inclusions is still intensively researched and the age of amber deposits, which are often secondary, does not correspond with the age of the amber itself. Eocene amber forests are probably the primary habitat where the species of the subfamily Typhlocybinae evolved and differentiated ecologically.
Protodikraneurini are evolutionarily one of the two oldest groups of Typhlocybinae known from the Eocene. So far, seven species belonging to five genera have been described: Stareono mirabilis Gębicki and Szwedo, Citation2006; Protodikraneura cephalica Gębicki and Szwedo, Citation2006; Protodikraneura ferraria Szwedo and Gębicki, Citation2008; Protodikraneura nasti Gębicki and Szwedo, Citation2006; Microelectrona cladara Szwedo and Gębicki, Citation2010; Retrorsotettix vlaskini Dietrich et al., Citation2023 and Protoparallaxis clavatus Dietrich et al., Citation2023 (Gębicki & Szwedo Citation2006; Szwedo & Gębicki Citation2008; Szwedo et al. Citation2010; Dietrich et al. Citation2023). Recently, also, typical representatives of Dikraneurini tribe have been described, including Eodikraneura obscura Dietrich et al., Citation2023 and Rovnodikra longipes Dietrich et al., Citation2023, and the cited source is the first report about the co-occurrence of these two tribes (Dikraneurini and Protodikraneurini) in the Eocene (Dietrich et al. Citation2023). Four species of Eocene Typhlocybinae described in the 19th century and the beginning of the 20th require redescription and reclassification (Germar & Berendt Citation1856; Bervoets Citation1910; Cockerell Citation1920).
The known representatives of Protodikraneurini can be characterized by the following characters: vertex usually deltoid and convex; frontoclypeus trapezoid, widened in upper part, with gibbosity above sutura epistomalis (in lateral view), genae and lora wide; tegmina linear, without appendix in apical portion, with venation similar to that of Dikraneurini and Empoascini; wings with RP and MA veins separated over the whole length, not fused into common stem, connected by r-m transverse veinlet (sometimes strongly shortened), with well-developed marginal vein, reaching costal margin in 1/3 tegmen length (Gębicki & Szwedo Citation2006; Szwedo & Gębicki Citation2008; Szwedo et al. Citation2010).
It is clear that the taxonomic status of the tribe, its phylogenetic affinities and the relationship with extant fauna require further thorough study. In this article, we describe another representative of Protodikraneurini, giving a detailed description of the species and discussing its relation to other hitherto known species.
Material and methods
External structures of the female specimen were examined using an Olympus SZX9 Nikon Eclipse stereoscopic microscope. Photographs were taken with a Canon Eos camera with extension rings. The equipment used is available at the University of Silesia, Faculty of Natural Sciences, Institute of Biology, Biotechnology and Environmental Protection. All photographs and drawings were digitalized and processed using CorelDRAW software. Morphological terminology, especially that referring to tegmina and wings, follows Gębicki and Szwedo (Citation2006), Szwedo and Gębicki (Citation2008) and Szwedo et al. (Citation2010); apical cell numbering follows Dietrich et al. (Citation2023).
Repository. The examined specimen is deposited in the amber collection of the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences (Kraków, Poland), under number MP/1241. The holotype of Jantarineura serafini Gębicki, Walczak and Świerczewski sp. nov. bears two labels. Below, we quote the specimen labels as follows: “[]” is used to enclose the text of a particular label; “/” is used to separate data written in different rows on the label.
Systematics
Order: Hemiptera Linnaeus, Citation1758
Suborder: Cicadomorpha Evans, Citation1946
Superfamily: Membracoidea Rafinesque, Citation1815
Family: Cicadellidae Latreille, Citation1802
Subfamily: Typhlocybinae Kirschbaum, Citation1868
Tribe: Protodikraneurini Gębicki and Szwedo Citation2006 †
Jantarineura gen. nov. Gębicki, Walczak and Świerczewski
urn:lsid:zoobank.org:act:AA72A877-BE27-45A0-B960-6511503EE04B
Type species
Jantarineura serafini sp. nov., by present designation.
Diagnosis
As for the type species.
Etymology
The generic name Jantarineura is an amalgamation of the old Lithuanian word “jantar” meaning amber and the name of the tribe Protodikraneurini, which the new genus belongs to. Gender: feminine.
Jantarineura serafini Gębicki, Walczak and Świerczewski sp. nov.
urn:lsid:zoobank.org:act:53C01423-BBE8-4A45-90B5-65FC92D32C89
Diagnosis
This species differs from other known Protodikraneurini in having the following combination of characters: head with compound eyes wider than pronotum; vertex protruded, almost conical, medially much longer than lateral margins; lateral incision of gena margin beneath eyes very shallow; frontoclypeus lateral sides with two rows of dark stripes; anteclypeus convex, long and narrow, with parallel margins; distal part of CuA1 vein straight, connected with claval margin of tegmen just above the apex; M vein connected directly with RP vein, r-m transverse veinlet reduced; 4th (external) widest, limited by ScRA i RP veins; transverse veinlet r-m of wing distinctly shortened; abdominal sternite VII trapezoidal and wide.
Description
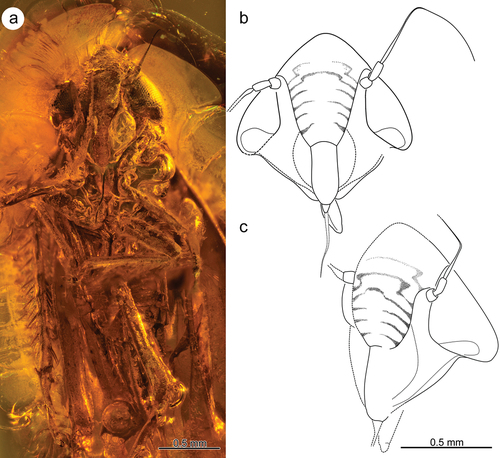
Head. Head with compound eyes wider than pronotum (). Vertex protruded, almost conical, medially much longer than lateral margins (). Disc of vertex convex, margin vertex-frons gently rounded. Sutura coronalis short and weakly visible. Frons medially as wide as long (including compound eyes), widest above antennal fossa. Lateral incisions beneath eyes at the level of genae margin very shallow. Lateral margins of genae slightly convex, arcuate, converging at the top of clypeus. Lora oval and long. Maxillary plates rounded at the top, reaching end of anteclypeus (). Compound eyes large, length equal to anteclypeus width; upper margin longer than the length of upper margin of frontoclypeus (internal side). Ocelli not visible. Antennae a bit shorter than head, in deep fossa, located below upper margin of eyes. Scapus shorter and wider than cylindrical, a bit narrowed basally, pedicellus. Upper half of pedicellus covered with minute tubercles. Base of flagellum widened and ribbed (7 rings, basal ones with lateral spines), upper half narrow and sharpened (). Frontoclypeus convex, with fine-grained texture, forming small gibbosity above upper margin of anteclypeus (sutura epistomalis). Anteclypeus convex, long and narrow, with almost parallel lateral margins, 3 times longer than wide, covered with small, numerous cuticular scales (). Rostrum slightly exceeding mesocoxae ().
Thorax
Disc of pronotum wide and smooth, medially a bit longer than vertex; anterior margin semicircular, posterior margin almost straight. Scutum (mesonotum) shorter than pronotum.
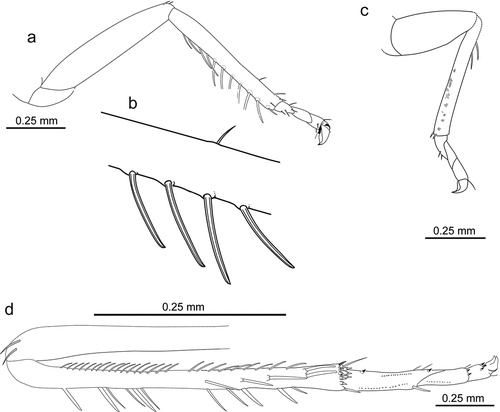
Legs. Protibia interior margin with a row of 6 long and 4 short spines (macrochetae), apex with 2 short conical spines (); macrochetes a bit curved apically, arising from small tubercles (). Protarsus 2.4 times shorter than tibia; segments short, the same length (). Mesotibia 3.0 times narrower than mesocoxa, with sparse and irregular chaetotaxy. Mesotarsus 2.0 times shorter than tibia, with segments almost the same length (). Metatibia with posteroventral margin possessing more than 4 spines and a short row of small chaetae, end with crown of spines, laterally marginated with 2 long chaetae (). Metatarsus about 2.4 times shorter than metatibia: basitarsus more than 2.0 times longer than 2nd segment, with 2 short, conical spines on ventral margin; praetarsus 2.0 times shorter than 2nd segment ().
Figure 3. Morphology of Jantarineura serafini gen. et sp. nov., legs: (a) left fore leg, ventral view; (b) left mid leg, ventral view; (c) right hind leg, ventral view; (d) right hind leg tarsus, ventral view.

Figure 4. Morphology of Jantarineura serafini gen. et sp. nov. legs (reconstruction): (a) left fore leg, ventral view; (b) macrochetes of the fore leg (enlarged); (c) left mid leg, ventral view; (d) right hind leg, ventral view.
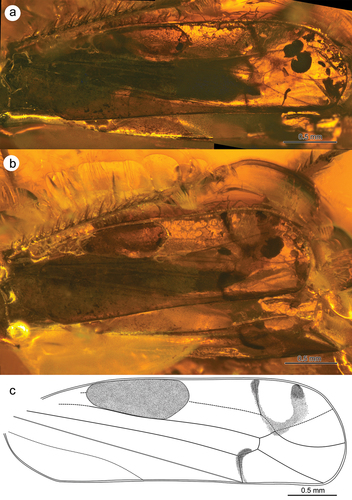
Tegmina elongated with long clavus, much exceeding the top of abdomen (level of CuA2 vein) (). Apical part of tegmen (with apical cells) about 2.5 times shorter than claval part. Internal apical cells (1st and 2nd) almost the same length, 3rd apical cell triangular, limited by RP and M veins, 4th apical cell widest, formed by veins ScRA and RP (). Transverse veinlet r-m reduced, ScRA vein long and slightly curved towards costal margin. CuA1 vein almost straight, connected with claval margin near tegmen apex, CuA2 quite long, only slightly shorter than ScRA. Claval veins invisible. “Wax area” large and strongly elongated, stretching from C vein to M vein. Pterostigma posterior margin with a row of about 7 short spines. Wings poorly visible; RP and M veins distinctly converging in the place of strongly shortened r-m transverse veinlet. MP+CuA vein probably long and forked into separate MP and CuA veins above r-m veinlet.
Abdomen. Pygofer elongated, both sides with a row of long and sparsely arranged chaetae. VII sternite trapezoidal and wide, with wide median prolongation (). Ovipositor arcuate, exceeding the top of abdomen at a distance a bit more than its width ().
Figure 6. Morphology of Jantarineura serafini gen. et sp. nov., abdomen: (a) ventral side; (b) terminal sternites with ovipositor (reconstruction).
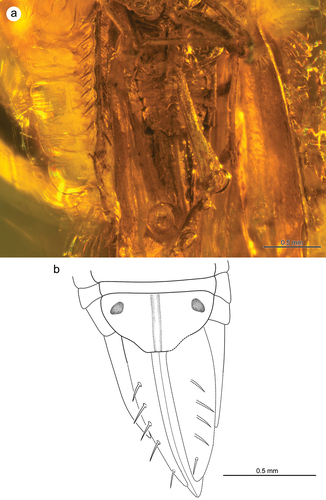
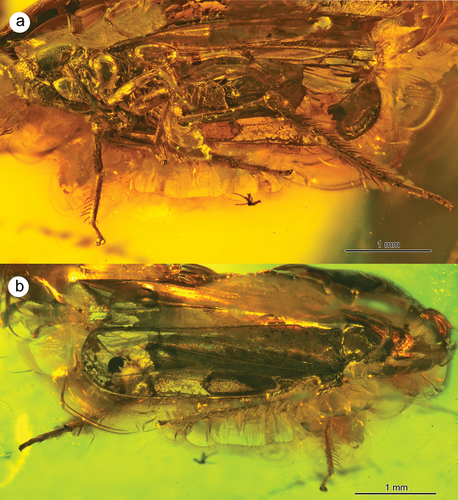
Body (especially dorsal side of thorax) uniformly dark coloured (). Frontoclypeus lateral sides with two rows of dark stripes (). Tegmina transparent; ends of longitudinal veins and claval margin clearly darkened; dark patch in the shape of horseshoe near costal margin. CuA2 and basal part of CuA1 with dark, curved patch (). Two oval, dark spots near VII sternite lateral margin ().
Body measurements: body length including wings − 4.11 mm; body length excluding wings − 2.26 mm; maximum body width − 1.20 mm; median length of vertex − 0.30 mm; width of head including compound eyes − 0.95 mm; basal width of vertex − unknown; length of frons including anteclypeus − 0.96 mm; width of frons including compound eyes − 0.95 mm; width of frons without compound eyes − 0.47 mm; median length of pronotum − 0.52 mm; basal width of pronotum − 0.90 mm; length of scutum − 0.50 mm; length of tegmen − 3.27 mm; width of tegmen − 0.87 mm; tegmen index (length vs width) − 1:0.27; length of wing − 3.05 mm; length of profemur − 0.64 mm; length of protibia − 0.59 mm; length of protarsus − 0.28 mm; length of mesofemur − 0.47 mm; length of mesotibia − 0.53 mm; length of mesotarsus − 0.26 mm; length of metafemur − 0.97 mm; length of metatibia − 1.44 mm; length of metatarsus − 0.68 mm; length of pygofer − unknown; length of ovipositor − 0.93 mm.
Etymology
The specific epithet is dedicated to Mr Jacek Serafin (1940–2022), a Polish amber and amber inclusions collector, one of the co-founders of the International Amber Association (Societas Succinorum Internationalis), and owner of an extensive collection of amber inclusions, who initiated many scientific studies on this subject.
Type material
Holotype, ♀ Eocene Baltic amber inclusion: 1st label (white) – [coll. JACEK SERAFIN – AUC 036JS/Hemiptera: Clypaeorrhyncha/Cicadelloidea: Cicadellidae:/Typhlocybinae], 2nd label (red) – [HOLOTYPE ♀/Jantarineura serafini/gen. et sp. nov./Gębicki, Walczak & Świerczewski].
Discussion
The described species, Jantarineura serafini sp. nov., differs from all other representatives of Protodikraneurini tribe by the following unique combination of characters. Wide genae with small incisions beneath the compound eyes distinguish the species from Stareono mirabilis, characterized by a very deep incision and shortened genal part of the head. Fusion of tegminal R and M veins and complete reduction of transversal r-m veinlet as well as widening of the 4th (external) apical cell clearly distinguish this species from members of the genus Protodikraneura, which possess a well-developed r-m veinlet and in which the 4th apical cell is equal to or a bit narrower than other cells (Gębicki & Szwedo Citation2006). Four well-developed apical cells distinguish the newly described species from Protoparallaxis clavata, which has only three wide apical cells. The conical shape of the head in Jantarineura serafini sp. nov. is different from the head of Retrorsotettix vlaskini, with its semicircular vertex margin (Dietrich et al. Citation2023).
Width of head (including compound eyes) being greater than the width of pronotum is a typical diagnostic character for almost all Protodikraneurini, apart from Microelectrona cladara, which has the head clearly narrower than pronotum (Szwedo et al. Citation2010).
To date, research on Typhlocybinae has revealed a taxonomically separate and morphologically uniform systematic unit, comprising the probably diverse and speciose tribe Protodikraneurini, which is closely related to the extant tribe Dikraneurini, with the oldest representatives known to date from Oligocene Dominican amber (Dietrich & Vega Citation1995). However, recent studies provided information on much older Eocene Dikraneurini occurring in the ecosystems of amber forests, represented by two species, Eodikraneura obscura Dietrich, Simutnik & Perkovsky and Rovnodikra longipes Dietrich, Simutnik and Perkovsky (Dietrich et al. Citation2023). They are characterized by the fusion of R and M veins into one common stem, typical for extant Dikraneurini.
Further studies (Gębicki et al., in prep.) might confirm that Protodikraneurini (as well as fossil Dikraneurini), being fairly common in the pieces of amber resin, are probably a characteristic element of insect communities, which inhabited shrubs and trees (not only resinous) of Eocene amber forests. However, the limited data mean we are still unable to associate particular species of Protodikraneurini with specific biocenoses or to indicate their synecological categories. The shortened piercing-sucking mouthparts, characteristic of most extant Typhlocybinae and also typical for Protodikraneurini, may suggest a similar way of feeding, by the intake of plant sap from mesophyll cells of single and wide leaf blades. There are some examples of interspecific interactions in that group. The first is the case of a typhlocybid male Microelectrona cladara parasitized by the larva of a Pipunculidae fly (Diptera), forming the characteristic thylacium protruding from the body of the leafhopper, known from the middle Eocene (Lutetian − 44 Ma) (Szwedo et al. Citation2010). Finally, the presence in the sample as a syninclusion of a few trichomes from blossoming flower buds suggests that the examined resin formed in early summer, i.e. at the end of the leafhopper mating season (Larsson Citation1978). The same can be observed for other previously described representatives of Protodikraneurini; however, the ecology of the tribe is still poorly documented and requires further study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Balme GR 2007. Phylogeny and systematics of the leafhopper subfamily Typhlocybinae (Insecta: Hemiptera: Typhlocybinae). Ph.D. thesis. Raleigh, NC: North Carolina State University.
- Bervoets R. 1910. Diagnoses de quelques nouvelle espèces de Cicadenes de l`amber de la Baltique. Annales Historico-Naturales Musei Hungarici 8:125–128.
- Cockerell TDA. 1920. Eocene insects from the Rocky Mountains. Proceedings of the United States National Museum 57(2313):233–260. DOI: 10.5479/si.00963801.57-2313.233.
- Dietrich CH. 2005. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). The Florida Entomologist 88(4):502–517. DOI: 10.1653/0015-4040(2005)88[502:KTTFOC]2.0.CO;2.
- Dietrich CH. 2006. Guide to the subfamilies of Cicadellidae. Available: http://www.inhs.uiuc.edu/~dietrich/subfam/guide.html. Accessed Feb 2006 13.
- Dietrich CH, Allen JM, Lemmon AR, Lemmon EM, Takiya DM, Evangelista O, Walden KKO, Grady PGS, Johnson KP. 2017. Anchored hybrid enrichment-based phylogenomics of leafhoppers and treehoppers (Hemiptera: Cicadomorpha: Membracoidea). Insect Systematics and Diversity 1(1):57–72. DOI: 10.1093/isd/ixx003.
- Dietrich CH, Dmitriev DA, Takiya DM, Thomas MJ, Webb MD, Zahniser JN, Zhang Y. 2022. Morphology-based phylogenetic analysis of Membracoidea (Hemiptera: Cicadomorpha) with placement of fossil taxa and description of a new subfamily. Insect Systematics and Diversity 6(5). n. ixac021. DOI: 10.1093/isd/ixac021.
- Dietrich CH, Simutnik SA, Perkovsky E. 2023. Typhlocybinae leafhoppers (Hemiptera, Cicadellidae) from Eocene Rovno amber reveal a transition in wing venation and a defensive adaptation. Journal of Paleontology 97(2):1–14. DOI: 10.1017/jpa.2023.3.
- Dietrich CH, Vega FE. 1995. Leafhoppers (Homoptera: Cicadellidae) from Dominican amber. Annals of the Entomological Society of America 88(3):236–270. DOI: 10.1093/aesa/88.3.263.
- Evans JW. 1946. A natural classification of leaf-hoppers (Jassoidea, Homoptera). Part.1. External morphology and systematic position. Transactions of the Royal Entomological Society of London 96(3):47–60. DOI: 10.1111/j.1365-2311.1946.tb00442.x.
- Foster CSP, Sauquet H, Merwe MVD, McPherson H, Rossetto M, SYW H. 2016. Evaluating the impact of genomic data and priors on Bayesian estimates of the angiosperm evolutionary timescale. Systematic Biology 66:338–351. DOI: 10.1093/sysbio/syw086.
- Gębicki C, Szwedo J. 2006. Protodikraneurini trib. nov. from the Eocene Baltic amber (Hemiptera: Cicadellidae: Typhlocybinae). Annales Zoologici 56(4):763–783.
- Germar EF, Berendt GC. 1856. Die im Bernstein befindlichen Hemipteren und Orthopteren der Vorwelt. Die im Bernstein befindlichen organischen Reste der Vorwelt gesammelt in Verbindung mit Mehreren, bearbeitet und herausgegeben von G.C. Berendt. Nicolaische Buchhandlung, Berlin 2(1):1–40.
- Kirschbaum CL. 1868. Die Cicadinen der gegend von Wiesbaden und Frankfurt a. M. nebst einer anzahl neuer oder Schwer zu unterscheidender Arten aus anderen Gegenden Europa’s Tabellarisch Beschrieben. Jahrbücher des Vereins für Naturkunde im Herzogthum Nassau 21-22:1–202.
- Larsson SG. 1978. Baltic Amber - A Paleontological Study . Klampenborg: Entomograph I. Scandinavian Scientific Press, Vol. 1, pp. 1–192.
- Latreille PA. 1802. Histoire naturelle, générale et particulière des Crustacés et des Insectes: ouvrage faisant suite aux oeuvres de Leclerc de Buffon, et partie du cours complet dˋhistoire naturelle rédigé par C. S. Sonnini. In: membre de plusieurs Sociétés savantes. Vol. 3: ixii. F. Dufart, Paris. p. 467
- Linnaeus C. 1758. Systema Naturae. In: Editio decima, reformata. Laurentius Salvius: Holmiae, Vol. 1, p. 824.
- Rafinesque CS. 1815. Analyse de la nature ou Tableau de l’univers et des corps organisés. Palermo (self-published). p. 224.
- Szwedo J, Gębicki C. 2008. Protodikraneura ferraria sp. nov. from the Eocene Baltic amber (Hemiptera: Cicadellidae: Protodikraneurini). Alavesia 2:177–181.
- Szwedo J, Gębicki C, Kowalewska M. 2010. Microelectrona cladara gen. et sp. nov.: A new Protodikraneurini from the Eocene Baltic amber (Hemiptera: Cicadomorpha: Cicadellidae: Typhlocybinae). Acta Geologica Sinica – English Edition 84(4):696–704. DOI: 10.1111/j.1755-6724.2010.00273.x.
- Yan B, Dietrich CH, Yu X, Jiao M, Dai R, Yang M. 2022. Mitogenomic phylogeny of Typhlocybinae (Hemiptera: Cicadellidae) reveals homoplasy in tribal diagnostic morphological traits. Ecology and Evolution 12(6):1–17. DOI: 10.1002/ece3.8982.