Abstract
Leeches feeding on invertebrates are an important component of freshwater communities, including those dominated by fish, but the effects of fish on their abundance and distribution are not well explored. The relative densities and assemblage structure of two leech families, Erpobdellidae and Glossiphoniidae, were studied from May to August 2014 in ponds stocked with low biomass densities of young common carp Cyprinus carpio, too small to prey on leeches (low-fish ponds), and in ponds containing high biomass densities of large (one- or two-year-old) carp (high-fish ponds), using two types of activity traps (placed either on the bottom or near the water surface). The most common and numerous species were Erpobdella octoculata, Helobdella stagnalis, and Erpobdella nigricollis. The traps near the water surface captured relatively small numbers of leeches, yet the catches showed that in summer some of the leeches moved to the upper layers of the water column, which was free of macrophytes. No differences in leech species richness or diversity were found with respect to fish status of ponds; however, bottom erpobdellids were more abundant in high-fish ponds. Redundancy analysis identified the fish status of the ponds and total nitrogen concentration in water as the only significant variables explaining the distribution of leeches among ponds. Species distribution was nested, but the nestedness pattern was not affected by the fish status of the pond. While fish are generally believed to adversely affect macroinvertebrate diversity and abundance, our study shows that some predatory leech species can persist in the presence of fish and possibly indirectly benefit from it.
Introduction
Freshwater leeches (Annelida: Hirudinea) are highly diversified in terms of feeding adaptations and habitat requirements. Most are predators, liquidosomatophagous parasites or sanguivores; the first two groups mainly exploit benthic and planktonic invertebrates, while sanguivores feed on vertebrates (Sket & Trontelj Citation2008; Lynggaard et al. Citation2022). Leeches known as ectoparasites of fish are associated with fish populations and may inflict serious damage on cultured stocks (Billard Citation1999). Predatory leeches are high-level consumers in many freshwater food webs, and their role in structuring freshwater invertebrate communities may be comparable to that of fish (Dahl & Greenberg Citation1997); for example, some species can act as biocontrol agents of alien molluscs (Paul et al. Citation2021, Citation2022). Coexisting fish and leeches may together shape freshwater assemblages, although the strength of these effects and mutual relationships may vary contingent on the densities and size structure of these two taxonomic groups, the type of living environment, and food preferences (Dahl & Greenberg Citation1997; Batzer et al. Citation2000; Ahlgren & Brönmark Citation2012). Leeches may also play a significant role in food chains as a diet component of fish, amphibians and waterbirds, as well as aquatic predatory arthropods (Young & Spelling Citation1986; Cywinska & Davies Citation1989; Michel & Oberdorff Citation1995; Venturelli & Tonn Citation2005; Govedich & Moser Citation2015), and their assemblages are likely to be structured by predation. Fish exert a strong trophic impact on predatory macroinvertebrates and have been widely documented to feed on leeches (reviewed in Young & Spelling Citation1986). Still, little is known about the population-scale effects of fish on invertebrate-feeding leeches and their distribution in natural conditions (Young & Spelling Citation1986; Zimmer et al. Citation2000; Koperski Citation2006). Apart from trophic interactions, a significant role in the distribution of leeches is played by biotic and abiotic features of the aquatic environment, including submerged vegetation structure, pH, turbidity, aerobic conditions, water temperature, nutrient content, or the nature of the substratum (Sawyer Citation1974; Bendell & McNicol Citation1991; Kubová & Schenková Citation2014; Phillips et al. Citation2020), many of which are also influenced by fish activity (Andersson et al. Citation1978; Zambrano et al. Citation2006; Zhang et al. Citation2017).
We investigated the abundance and assemblage composition of two groups of leeches feeding on invertebrates, Glossiphoniidae and Erpobdellidae, in relation to fish trophic status in large, semi-permanent ponds. Although many species belonging to these families are known to be versatile foragers switching between modes of feeding (Kutschera Citation2003; Elliott & Dobson Citation2015), the two groups generally differ in their manner of feeding. Proboscis-bearing glossiphoniid leeches extract body fluids and are an ecologically diverse group of parasites and predators (often both), while erpobdellids have a sectorial, muscular mouth to ingest their prey whole or in large pieces (Davies & Govedich Citation2001). Erpobdellid leeches in particular can occur at high densities, being important regulators of invertebrate populations (Sket & Trontelj Citation2008). Our study ponds were used for extensive cyprinid culture, dominated by common carp Cyprinus carpio, a benthivorous fish introduced worldwide with manifold effects on freshwater invertebrate communities (Kořinek et al. Citation1987; Badiou et al. Citation2011; Nieoczym & Kloskowski Citation2015). Cyprinid ponds are often stocked with a single age (size)-class of fish (Billard Citation1999), which mediates diametric differences in standing biomass and size structure, and thus in the trophic position of fish among otherwise morphologically similar ponds. We exploited the differential stocking of ponds as an opportunity for a large-scale natural experiment on the effects of fish on leeches under conditions of low vs high trophic influence of fish. We also examined the effects of other biotic and abiotic pond characteristics on leech distribution among ponds. As fish can prey on leeches and compete with them for macroinvertebrate resources, we hypothesised that leech abundance and diversity would be lower in ponds with high fish densities and consequently with ecosystem dominance by fish.
Materials and methods
Study area
The fieldwork was conducted in 2014 on large, lake-like ponds dedicated to extensive cyprinid culture in eastern Poland (51°8–33'N, 22°19–51' E, ). All study ponds were drainable, filled and stocked in spring, and drained and harvested in autumn. We examined leech abundance and assemblage structure in ponds stocked with fish fry, and thus with low biomass density at small individual body size, and consequently with low trophic impact (hereafter: “low-fish ponds”, n = 8) or with older, large-sized fish, stocked at high abundance (“high-fish ponds”, n = 16). Low-fish ponds were stocked with 0+ carp larvae at individual total length of 3–4 mm in mid-May; the stocking biomass of young fish was negligible; however, carp fry reached about 4–5 cm total length by the end of June (standing biomass of about 40–60 kg ha−1). We assumed that over most of the study season (May–August) carp fry did not constitute a serious predatory threat to leeches; in fact, in the first few weeks after stocking, fish larvae could have been preyed on. However, with growth, 0+ carp may develop the ability to feed on small leeches (Young & Spelling Citation1986). High-fish ponds were stocked with one- or two-year-old, and thus relatively large-sized fish shortly after ice-out, from mid-March to mid-April. Fish communities in these ponds were dominated by common carp (80–90% of pond biomass production); smaller, less-numerous mixed-species stock, consisting mainly of grass carp Ctenopharyngodon idella, bighead carp Hypophthalmichthys nobilis, and piscivores, pike-perch Sander lucioperca, pike Esox lucius and wels catfish Silurus glanis, were also introduced to the ponds. Carp and the supplemental fish species were of similar individual body size, with individual total length ranging approximately from 12 to 22 cm at stocking and total stocking biomass of 100–380 kg ha−1. Carp densities were within the ranges found in natural eutrophic systems (Panek Citation1987). Minor populations (less than 5% of the biomass production) of small wild fish, mainly Prussian carp Carassius gibelio and perch Perca fluviatilis, persisted in the ponds.
Figure 1. Location of study sites in eastern Poland (Lublin region). Red ovals outline pond complexes comprising the study ponds. Inset shows location of the study region within Poland.
Apart from individual variation, the two pond types did not differ in water surface area (range 2–13 ha and two ponds of about 24–26 ha) and were similar in mean depth (0.9–1.4 m) and consequently in water temperature. All ponds supported extensive shoreline vegetation. During the spring, submerged macrophytes were generally scarce in the study ponds; as the ponds were shallow, they might extend over most of the bottom in summer. The bottom substrate of the ponds was muddy sand with organic contents. The advantage of our study system was that fish effects on leeches could be largely separated from environmental factors, as the two types of ponds were similar in most morphological or environmental features, whereas contrasts in size and depth are typical between natural fish-free and fish-dominated water bodies (Wellborn et al. Citation1996; Koperski Citation2006). The ponds were nutrient-subsidized, as they were manured prior to stocking, and carp, although relying on natural food, were given supplementary feed (cereal grains).
Leech sampling
Two types of activity traps were used to capture leeches: bottom and near-surface traps. The two types of traps differed in size and the depth of deployment and were based on different catching principles. The bottom traps, placed horizontally on the pond bottom, consisted of 1-L plastic cylinders 220 mm in length, with inverted funnels of 85 mm at the entry and 22 mm at the narrow end (cf. Hyvönen & Nummi Citation2000; Nieoczym & Kloskowski Citation2015), with a capturing area of 57 cm2. The near-surface traps were 4-L plastic cylinders 220 mm high with an inverted funnel of 150 mm at the large end and an 18-mm opening at the narrow end, covering an area of 225 cm2, with a design similar to that described by Brinkman and Duffy (Citation1996). They were vertically positioned near the water surface (about 80–120 cm above the bottom, depending on the depth at the sampling site). The traps were mounted to wooden stakes and placed in haphazardly selected sites, roughly evenly distributed across the pond (in total 10 traps of each type per pond). The traps were deployed for 24 hours at approximately monthly intervals from May through August, 2014. The locations of the traps were the same during each trapping session. As leech activity may be related to movements between micro- and mesohabitats (Govedich & Moser Citation2015), and in order to collect species active within and beyond the shoreline vegetation, the traps were set at the interface between the open water and emergent macrophyte stands along the pond margins (cf. Sychra et al. Citation2010). The traps were not baited, as we assumed that baiting would not be relevant in research on invertebrate-feeding species (see also Verdonschot Citation2010). After being removed, each trap was thoroughly inspected, and all individuals inside them were preserved in a 4% formalin solution. Later, in the laboratory, the leeches were counted and identified to species based on morphological characters (Bielecki Citation2013; Elliott & Dobson Citation2015). The 24-h trapping method, which avoids biases resulting from sampling carried out only during daytime or night-time hours, is obviously more effective in capturing predatory leeches than ectoparasites of vertebrate animals, as predatory taxa are generally more active and more nocturnal (Elliott Citation1973; Angstadt & Moore Citation1997; Hampton & Duggan Citation2003). Consequently, we omitted the sanguivorous Piscicolidae (less than 1% of bottom-trap catches and 5% of near-surface catches) from the study. Hirudinidae and Haemopidae, both families with only one species occurring in Poland, were not represented in the catches. Most Erpobdellidae and Glossiphoniidae species recorded in our study feed principally on invertebrates; we also captured and included in the analyses Hemiclepsis marginata (single individuals were found in two ponds by each trapping method), which is a glossiphoniid ectoparasite of fish and tadpoles, but also molluscs and oligochaetes, and one individual of Theromyzon tessulatum, assumed to be a bird parasite (van Haaren et al. Citation2004; Bielecki et al. Citation2011).
Environmental variables
To determine which pond characteristics influence the assemblage structure of leeches, we measured a range of environmental variables along with the sampling of leeches (see supplementary material). We measured in situ conductivity, pH, and dissolved oxygen concentrations using the CX-401 Elmetron multifunction meter. For other analyses, water samples were kept on ice and analysed immediately after being transported to the laboratory. Chlorophyll a, total phosphorus, and total nitrogen concentrations were measured using a Beckman DU 640 spectrophotometer. The load of total suspended solids (TSS) was assessed only once, in early July, with a Pastel UV Secomam spectrophotometer (the estimates were means from three samples per pond). Overall, the abiotic variables did not differ between ponds with different fish status, except for dissolved oxygen concentrations, which tended to be greater in low-fish ponds than in high-fish ponds (but mainly due to the pronounced difference in June; see supplementary material), and TSS, which were greater in high-fish ponds, median 38.3 mg l−1 (17.5–45.3 lower and upper quartile), than in low-fish ponds, median 10.9 mg l−1 (8.4–18.6), Mann–Whitney test, U8,16 = 13.0, p = 0.002. We assessed the abundance (biomass) of submerged vegetation but did not use it in the analyses due to its strong correlation with fish status; submerged vegetation was significantly more abundant in low-fish ponds (see Nieoczym et al. Citation2020 for more details). Percentage cover of emergent vegetation in ponds was determined from digitized aerial photos.
Data analysis
Total densities of Erpobdellidae and Glossiphoniidae (juvenile and adult leeches were pooled) retrieved from the bottom and near-surface traps were separately compared between pond types using repeated measures (RM) ANOVA with the Greenhouse–Geisser correction (GenStat 15.1, VSN International Ltd). Trap catches were expressed as the number of individuals per 10 traps. Sampling session was entered as a repeated factor. Since the ponds were spatially aggregated in four scattered clusters, the clusters were treated as blocks. Least significant difference (LSD) tests were used for post-hoc comparisons.
Redundancy analysis (RDA), a multivariate ordination technique, was used to evaluate variation in the leech assemblages with regard to environmental variables (ter Braak & Šmilauer Citation2018). To account for phenological variation among species, the maximum numbers of individuals of each species collected in any sampling month were analysed for each pond. For other quantitative factors, median values of monthly measurements were calculated. The two levels of fish status (low-fish vs high-fish ponds) were entered as a nominal term. The number of environmental variables was reduced using the forward selection procedure (CANOCO, version 5.15). Intercorrelations between environmental variables were examined using variation inflation factors (VIFs). In addition to submerged vegetation abundance, pH, TSS, and chlorophyll a were also dropped from the analyses. Following the selection process, all VIFs were ≤4, indicating that collinearity was not a problem. Pond cluster identity was included in the RDA analyses as a covariable. Significance was tested by the Monte Carlo procedure with 999 random permutations (ter Braak & Šmilauer Citation2018).
The Nestedness Ordered by Decreasing Fill (NODF) statistic was applied for the binary species-by-site matrix to examine leech assemblage nestedness. Nestedness reflects the degree to which species-poor assemblages are subsets of richer assemblages. NODF ranges from 0 to 100, with higher numbers indicating greater nestedness (Almeida-Neto et al. Citation2008). To assess whether the observed nestedness differed from a null expectation, the presence-absence matrices of species occurrence were compared with 999 null matrices generated using the proportional column and row totals algorithm. The analyses were run using the free web application NeD (Strona et al. Citation2014). To check the importance of the fish status of ponds for the patterns of nestedness, the Mann–Whitney test on the nestedness ranks of individual pond assemblages in the maximally packed matrix was performed. One low-fish pond in which no leeches were detected was omitted from the nestedness analyses.
Owing to generally low catches in near-surface traps (see below), RDA and nestedness analyses were run only on samples from bottom traps, and when discussing the results of trapping, we refer to bottom catches, unless otherwise stated.
Results
A total of 2839 leeches were retrieved from benthic traps, representing 6 Erpobdellidae species (90.2% of all leeches caught) and 3 Glossiphoniidae species (9.8%) (). Erpobdella octoculata predominated in terms of species occurrence (present in 22 of 24 ponds). The other most common species were Helobdella stagnalis (14 ponds) and Erpobdella nigricollis (11 ponds). These three species numerically comprised 95.4% of all catches. Near-surface traps, in total, captured only 479 leeches representing 5 Erpobdellidae species (76.4% of all leeches caught) and 6 Glossiphoniidae species (23.6%). Again, E. octoculata prevailed in terms of occurrence (present in 20 of 24 ponds), and the other most common species were E. nigricollis (12 ponds) and H. stagnalis (11 ponds). These species together comprised 90.6% of all catches. Although most species were present in both parts of the water column, glossiphoniids Alboglossiphonia heteroclita, Alboglossiphonia hyalina, and Theromyzon tessulatum were only caught by near-surface traps, while Dina apathyi was caught only by bottom traps (). We did not find significant differences in species richness between low-fish ponds and high-fish ponds (Mann–Whitney test): bottom trap catches, U8,16 = 61.5, p = 0.903, medians 3.0 (1.5–3.0 lower and upper quartile) vs 2.0 (2.0–3.5), respectively; near-surface traps, U8,16 = 53.5, p = 0.540, medians 2.0 (1.5–3.0) vs 2.5 (2.0–3.0). Shannon–Wiener diversity also did not differ between low-fish and high-fish ponds: bottom trap catches, U8,16 = 56.0, p = 0.646, median H’ 0.8 (0.2–1.0) vs 0.5 (0.4–0.6); near-surface traps, U8,16 = 54.5, p = 0.582, median H’ 0.7 (0.2–0.9) vs 0.9 (0.5–1.1).
Table I. Mean numerical percentages of leech species in ponds differing in fish status.
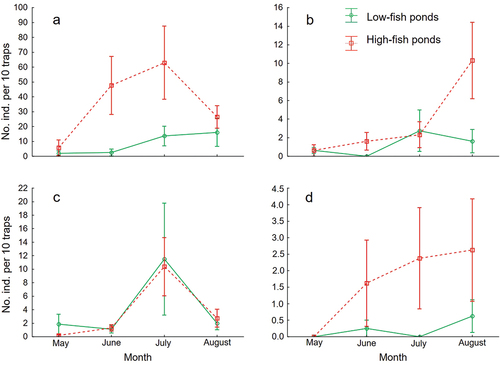
RM ANOVA on the numbers of leeches in bottom traps indicated that erpobdellids were generally more abundant in high-fish ponds (F1,19 = 4.71, p = 0.043), with significant overall variation during the sampling period (F3,66 = 10.61, p < 0.001), resulting mainly from low catches in May compared to later months (LSD tests); the time*fish status interaction was marginally significant (F3,66 = 2.87, p = 0.05). Time was the only significant factor in RM ANOVA on glossiphoniids in bottom traps, with numbers generally increasing throughout the season (F3,66 = 3.61, p = 0.023; fish status, F1,19 = 0.01, p = 0.932); the time*fish status interaction only approached significance (F3,66 = 2.53, p = 0.074) (). RM ANOVA on the catches in near-surface traps showed a significant effect of time, reflecting a peak in erpobdellid abundance in July (F3,66 = 9.34, p < 0.001, LSD tests) and an overall intra-seasonal increase in glossiphoniids (F3,66 = 2.98, p = 0.047). The other effects were non-significant, all p ≥ 0.269 ().
Figure 2. Mean (± standard error) relative leech abundances (individuals per 10 traps) from bottom traps, Erpobdellidae (a), Glossiphoniidae (b), and from near-surface traps, Erpobdellidae (c) and Glossiphoniidae (d), over time in low-fish ponds and high-fish ponds.
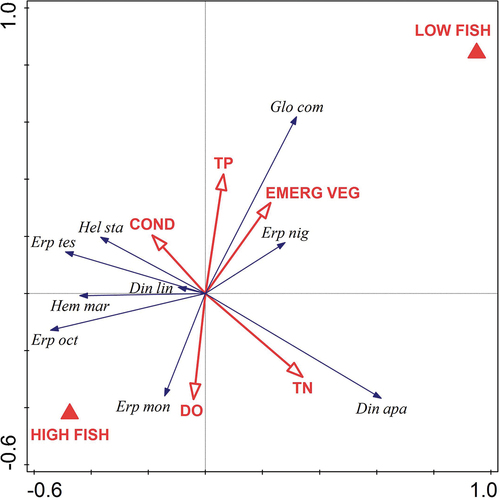
The RDA based on bottom catches showed that fish status, explaining 12.9% of the variability (F = 3.1, p ≤ 0.004), and nitrogen concentration, explaining 11.7% of the variability (F = 2.5, p = 0.042), were significant for leech assemblage structure (). The variables used in the model explained 41.7% of the assemblage variation. The first axis explained 19.7% of the variation, and the second 10.3%. E. octoculata was the only species significantly positively associated with high-fish ponds (Spearman correlation, r = 0.511, p = 0.011), while only E. nigricollis was negatively associated with high-fish ponds (r = −0.453, p = 0.026). The inter-set correlations of the RDA variables with the ordination axes indicated that the first axis captured a gradient of total nitrogen concentration (r = 0.599) and was related to the fish status of the ponds (r = 0.484). The second axis was largely defined by the total phosphorus concentration (r = 0.411) and total nitrogen concentration (r = −0.461). The Monte Carlo permutation test indicated significant associations between species distribution in the ponds and environmental variables (F = 0.6, p = 0.003 for the first canonical axis and F = 1.7, p = 0.013 for the total model; trace value = 0.341).
Figure 3. Ordination biplot from redundancy analysis showing relationships between leech species and environmental variables. Leech abundance estimates were based on bottom trap catches. Species abbreviations are based on the first three letters of genus and species names (see ). Variable abbreviations: low-fish ponds (LOW FISH), high-fish ponds (HIGH FISH), dissolved oxygen (DO), conductivity (COND), total nitrogen concentration (TN), total phosphorus concentration (TP), percentage share of emergent vegetation (EMERG VEG).
Species occurrences based on bottom trapping rendered a significantly nested structure (overall NODF = 56.6, Z = 4.5, p < 0.001). Both NODFcolumns (the occurrence of individual species in ponds; NODF = 44.6, Z = 2.0, p < 0.05), and NODFrows (total numbers of species in each pond; NODF = 58.3, Z = 4.7, p < 0.001) significantly contributed to the overall nestedness. The fish status of the pond did not affect the nestedness of leech assemblages (U7,16 = 49, p = 0.664).
Discussion
The high-fish ponds were found to support greater densities of erpobdellids but not glossiphoniids, while no differences in leech diversity and nestedness with respect to fish status were detected. The total abundances were affected by the prevalence of a few species, especially the ubiquitous and eurytopic E. octoculata, which were able to persist at high densities in the ponds, irrespective of fish status. Also, a few species rarely captured in the study area were recorded only in high-fish ponds. On the other hand, as indicated by RDA, the relatively common E. nigricollis appeared not to tolerate habitats dominated by fish. The leech assemblage structure was significantly influenced by the fish status of the ponds and the total nitrogen concentration in the water.
Various types of traps and hand-operated devices are used to sample leeches, with varied effectiveness depending on habitat conditions and species behavioural attributes (Verdonschot Citation2010; Meyer et al. Citation2011; Kubová & Schenková Citation2014; Lowe et al. Citation2016). We assume that activity traps are superior to manual sampling of mobile invertebrate predators, including free-living leeches (Prus & Kajak Citation1999; Becerra Jurado et al. Citation2008; but see; Bouchard et al. Citation2014; Lowe et al. Citation2016); moreover, they are artificial substrates, which may sample leeches irrespective of the trapping design (De Pauw et al. Citation1986; Adamiak-Brud et al. Citation2018). However, the trapping method could be biased with respect to species activity (Brinkman & Duffy Citation1996). The abundance of glossiphoniids, most of which are relatively poor swimmers and thus less mobile (Govedich & Moser Citation2015), could have been underestimated compared to erpobdellids. Some species use both ambush and active seek-out strategies, depending on the mobility of their prey (Young et al. Citation1993). Pond leeches relying on sit-and-wait hunting are likely to lurk inside vegetation patches (Sawyer Citation1974; Sychra et al. Citation2010), but it is not clear how the abundance of macrophytes affects trap catchability. Finally, invertebrate-feeding leech densities could have been underestimated in high-fish ponds, as they may be less active in the presence of potential predators (Davies & Kasserra Citation1989). Similarly, traps in low-fish ponds captured large numbers of invertebrates and tadpoles (Kloskowski et al. Citation2010; Nieoczym & Kloskowski Citation2015), which potentially could attract predatory and liquidosomatophagous leeches (cf. Verdonschot Citation2010). Nevertheless, we believe that our findings are conservative, as total erpobdellid densities were higher in high-fish ponds than in low-fish ponds.
Trophic effects of fish on leech populations are potentially significant, but not easy to determine; because their bodies are soft and thus rapidly digested, leech remains in fish alimentary tracts are difficult to detect by conventional methods (Young & Spelling Citation1986). Although common carp, the dominant fish in our study system, prefers benthic invertebrates as food (García-Berthou Citation2001; Rahman et al. Citation2009; Kloskowski Citation2011), small quantities of leeches have been recorded in its diet (Prejs Citation1973; Guziur Citation1976; Specziár et al. Citation1997). Fish may also act as competitors for invertebrate prey, which is less abundant in fish-dominated waters (Batzer et al. Citation2000; Leppä et al. Citation2003; Miller & Crowl Citation2006; Nieoczym & Kloskowski Citation2015). In addition, benthivorous species can modify the aquatic environment and indirectly influence the occurrence and abundance of invertebrates (Lougheed et al. Citation1998; Miller & Crowl Citation2006; Badiou et al. Citation2011; Fischer et al. Citation2013). The decline in submerged vegetation in the presence of dense fish populations (cf. Meijer et al. Citation1994) deprives leeches of substrates for attachment and spatial refuges (Tessier et al. Citation2004; Adamiak-Brud et al. Citation2018). Contrary to our expectations (see also Zimmer et al. Citation2000; Koperski Citation2006), the abundance of invertebrate-feeding leeches was not lower in high-fish ponds; in particular, the relatively large densities of some erpobdellids in the presence of dense fish stocks indicate that they evolved effective defensive traits against fish predation. Possible leech adaptations include hiding by burrowing, tenacious sucker attachment, and flattening the body and adhering tightly to the substrate (Sawyer Citation1986). Overall, predatory leeches are known to be more negatively phototactic and nocturnally active than species parasitizing vertebrates, which may be a component of their anti-predator strategies (Elliott Citation1973; Bielecki Citation1999; Davies & Govedich Citation2001). However, many fish species, including common carp, forage during both day and night (Helfman Citation1986; Rahman Citation2015). Leech activity, associated with hunting mode, can be crucial in their exposure to predators. Young and Spelling (Citation1986) state that E. octoculata may be more susceptible to exploitation by fish than glossiphoniid leeches because of its greater activity (but see Koperski Citation2006). The species is known to rest attached to the underside of stones or similar objects during the day, rarely moving, and to become active in search of prey at night (Sawyer Citation1986; Kutschera & Wirtz Citation2001), which may be an effective antipredator strategy. The taxa studied are likely to differ in their responses to predators; good swimming skills compared to glossiphoniid leeches may in fact aid the survival of erpobdellids in the presence of fish (Govedich & Moser Citation2015). Another explanation for the higher numbers of erpobdellids in the presence of dense, large-sized fish stocks than in low-fish ponds could be trade-offs between predation risks from fish and from non-fish predators. Although vertebrate predators are believed to constitute the principal threat to leeches, predatory arthropods can exert a significant trophic effect as well (Young & Spelling Citation1986; Spelling & Young Citation1987; Cywinska & Davies Citation1989; Cobbaert et al. Citation2010). In contrast to high-fish ponds, low-fish ponds abound in amphibians, waterfowl, and predatory insects (Kloskowski et al. Citation2010; Nieoczym & Kloskowski Citation2015). Consequently, leeches, provided that they are able to persist in the presence of fish, may indirectly benefit from the adverse effects of fish on populations of large predatory insects as well as on amphibians and invertebrate-feeding waterbirds.
Some physicochemical variables which were expected to be determinants of leech distribution, such as dissolved oxygen and conductivity (Davies & Gates Citation1991; Kubová et al. Citation2013), were shown to be non-significant in RDA. One reason could be that their ranges did not differ substantially among ponds, so that food availability and predation risk would then be the main predictors (Sawyer Citation1986; Koperski Citation2006). Also, despite low VIF scores, their importance was likely masked by co-varying environmental conditions, as most of the variables were linked to the trophic status of the ponds. Total nitrogen content significantly contributed to the variation in the leech assemblages in our study, and according to RDA, some species tended to prefer less eutrophic ponds. High concentrations of nutrients and organic matter in water, often associated with benthivorous fish presence (Breukelaar et al. Citation1994; Fischer et al. Citation2013), can positively influence the abundance of leeches, because they are usually associated with the supply of invertebrate prey such as oligochaetes, molluscs, and chironomid larvae (Grantham & Hann Citation1994; van Haaren et al. Citation2004; Govedich & Moser Citation2015). However, densities of chironomid larvae and gastropods, the common prey of invertebrate-feeding leeches (Nieoczym & Kloskowski Citation2015; Nieoczym et al. Citation2020), were lower in high-fish ponds than in low-fish ponds. On the other hand, bioturbation caused by benthivorous fish could favour leeches specializing in benthic prey, as it increases the availability of chironomid larvae for predatory macroinvertebrates (ten Winkel & Davids Citation1985). Within leech assemblages in the highly productive ponds, species adapted to nutrient-rich conditions, such as cosmopolitan E. octoculata and H. stagnalis, are likely to gain a competitive advantage over eutrophication-sensitive species (Sawyer Citation1974; van Haaren et al. Citation2004; Kubová & Schenková Citation2014). Although our data do not provide evidence for interspecific competition, species occurrence of leeches followed a nested pattern, in that rare species occurred in ponds harbouring overall high species richness. These findings suggest that species distribution among ponds was not driven by competitive species replacement (Baselga Citation2012; see also Young & Spelling Citation1989).
The increased density of leeches in ponds in the summer followed a typical phenological pattern of leech abundance (Sawyer Citation1986; Elliott & Dobson Citation2015). The more favourable water temperature conditions and enhanced food resources associated with the production of young by prey species (Young & Ironmonger Citation1982; Nieoczym & Kloskowski Citation2015; Nieoczym et al. Citation2020) yield greater reproductive success of leeches, driven by higher survival of the young (Young & Spelling Citation1989; Martin et al. Citation1994).
Traps deployed near the water surface were used as a tool to check for the vertical use of the water column, including potential vertical migrations, by leeches. The numbers recorded in the near-surface traps were evidently lower than in benthic traps, but, we found that a certain number of leeches, especially E. octoculata and H. stagnalis, the overall most numerous species in the ponds, moved to near-surface water layers. Diel or (intra)seasonal vertical migrations of leeches can be linked to changes in water temperature, oxygen conditions, and feeding behaviour (Blinn & Davies Citation1990; Davies & Gates Citation1991; Govedich & Moser Citation2015). However, owing to the littoral nature of shallow fish ponds, the water mixes quickly and oxygen and thermal stratification is minor (Kořinek et al. Citation1987).
Conclusions
Omnivorous and predatory fish have been documented to reduce the numbers and species richness of macroinvertebrates, especially when introduced to new habitats by humans; hence, they are blamed as factors in biodiversity loss (Batzer et al. Citation2000; Leppä et al. Citation2003; Gozlan et al. Citation2010). However, our findings show that at least some taxonomic groups of leeches can persist at comparatively high densities in ponds dominated by fish. Previous studies on carp-macroinvertebrate interactions have also indicated that certain macroinvertebrate groups, such as chironomids, oligochaetes or water mites, may even benefit from fish presence (Batzer et al. Citation2000; Miller & Crowl Citation2006; Nieoczym et al. Citation2023). Future research should elucidate the mechanisms of successful co-occurrence of individual taxa with fish. Given the natural dominance of fish in large and permanent water bodies and massive fish introductions to fishless wetlands (Eby et al. Citation2006), this would help to develop large-scale management guidelines for maintaining or improving aquatic macroinvertebrate diversity.
Supplemental Material
Download MS Word (21.7 KB)Acknowledgments
This paper is dedicated to Professor Aleksander Bielecki who sadly passed away during the preparation of this manuscript.
We would like to thank the managers of the local fisheries for their cooperation with this study.
This study was conducted under the permit WPN.6401.42.2013.MO provided by the Regional Directorate for Environmental Protection in Lublin.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2289585
References
- Adamiak-Brud Ż, Jabłońska-Barna I, Bielecki A, Kobak J. 2018. Factors shaping leech (Clitellata, Hirudinida) assemblages on artificial and natural substrata in urban water bodies. Limnologica 69:125–134. DOI: 10.1016/j.limno.2018.01.001.
- Ahlgren J, Brönmark C. 2012. Fleeing towards death–leech-induced behavioural defences increase freshwater snail susceptibility to predatory fish. Oikos 121(9):1501–1506. DOI: 10.1111/j.1600-0706.2012.20420.x.
- Almeida-Neto M, Guimaraes P, Guimaraes Jr PR, Loyola RD, Ulrich W. 2008. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 117(8):1227–1239. DOI: 10.1111/j.0030-1299.2008.16644.x.
- Andersson G, Berggren H, Cronberg G, Gelin C. 1978. Effects of planktivorous and benthivorous fish on organisms and water chemistry in eutrophic lakes. Hydrobiologia 59(1):9–15. DOI: 10.1007/BF00017602.
- Angstadt JD, Moore WH. 1997. A circadian rhythm of swimming behavior in a predatory leech of the family Erpobdellidae. The American Midland Naturalist 137(1):165–172. DOI: 10.2307/2426765.
- Badiou P, Goldsborough LG, Wrubleski D. 2011. Impacts of the common carp (Cyprinus carpio) on freshwater ecosystems: A review, 121–146. In: Sanders, J, Peterson S, editors. Carp: Habitat, management and diseases. New York: Nova Science Publishers, Inc. pp. 203.
- Baselga A. 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography 21(12):1223–1232. DOI: 10.1111/j.1466-8238.2011.00756.x.
- Batzer DP, Pusateri CR, Vetter R. 2000. Impacts of fish predation on marsh invertebrates: Direct and indirect effects. Wetlands 20(2):307–312. DOI: 10.1672/0277-5212(2000)020[0307:IOFPOM]2.0.CO;2.
- Becerra Jurado G, Masterson M, Harrington R, Kelly-Quinn M. 2008. Evaluation of sampling methods for macroinvertebrate biodiversity estimation in heavily vegetated ponds. Hydrobiologia 597(1):97–107. DOI: 10.1007/s10750-007-9217-8.
- Bendell BE, McNicol DK. 1991. An assessment of leeches (Hirudinea) as indicators of lake acidification. Canadian Journal of Zoology 69(1):130–133. DOI: 10.1139/z91-019.
- Bielecki A. 1999. The role of eye-like spots of parasitic leeches (Hirudinea, Piscicolidae) in searching for fish-hosts. Annals of Parasitology 45:339–345.
- Bielecki A. 2013. Typ: pierścienice Annelida, 54–66. In: Boroń A, Szlachciak J, editors. Różnorodność i taksonomia zwierząt. Tom II. Olsztyn: Uniwersytet Warmińsko-Mazurski. pp. 228.
- Bielecki A, Cichocka JM, Jeleń I, Świątek P, Adamiak-Brud Z. 2011. A checklist of leech species from Poland. Annals of Parasitology 57:11–20.
- Billard R. 1999. Carp: Biology and culture. Chichester, UK: Springer Praxis Publishing. pp. 342.
- Blinn DW, Davies RW. 1990. Concomitant diel vertical migration of a predatory leech and its amphipod prey. Freshwater Biology 24(2):401–407. DOI: 10.1111/j.1365-2427.1990.tb00719.x.
- Bouchard RW, Genet JA, Chirhart JW. 2014. Does supplementing dipnet samples with activity traps improve the ability to assess the biological integrity of macroinvertebrate communities in depressional wetlands? Wetlands 34(4):699–711. DOI: 10.1007/s13157-014-0535-0.
- Breukelaar AW, Lammens EH, Breteler JGK, Tatrai I. 1994. Effects of benthivorous bream (Abramis brama) and carp (Cyprinus carpio) on sediment resuspension and concentrations of nutrients and chlorophyll a. Freshwater Biology 32(1):113–121. DOI: 10.1111/j.1365-2427.1994.tb00871.x.
- Brinkman MA, Duffy WG. 1996. Evaluation of four wetland aquatic invertebrate samplers and four sample sorting methods. Journal of Freshwater Ecology 11(2):193–200. DOI: 10.1080/02705060.1996.9663478.
- Cobbaert D, Bayley SE, Greter JL. 2010. Effects of a top invertebrate predator (Dytiscus alaskanus; Coleoptera: Dytiscidae) on fishless pond ecosystems. Hydrobiologia 644(1):103–114. DOI: 10.1007/s10750-010-0100-7.
- Cywinska A, Davies RW. 1989. Predation on the erpobdellid leech Nephelopsis obscura in the laboratory. Canadian Journal of Zoology 67(11):2689–2693. DOI: 10.1139/z89-380.
- Dahl J, Greenberg L. 1997. Foraging rates of a vertebrate and an invertebrate predator in stream enclosures. Oikos 78(3):459–466. DOI: 10.2307/3545607.
- Davies RW, Gates TE. 1991. The effects of different oxygen regimes on the feeding and vertical distribution of Nephelopsis obscura (Hirudinoidea). Hydrobiologia 211(1):51–56. DOI: 10.1007/BF00008616.
- Davies RW, Govedich FR. 2001. Annelida: Euhirudinea and Acanthobdellidae, 465–504. In: Thorp J, Covich A, editors. Ecology and classification of North American freshwater invertebrates. 2nd ed. San Diego, California: Academic Press. pp. 1056.
- Davies RW, Kasserra CE. 1989. Foraging activity of two species of predatory leeches exposed to active and sedentary prey. Oecologia 81(3):329–334. DOI: 10.1007/BF00377079.
- De Pauw N, Roels D, Fontoura AP. 1986. Use of artificial substrates for standardized sampling of macroinvertebrates in the assessment of water quality by the Belgian biotic index. Hydrobiologia 133(3):237–258. DOI: 10.1007/BF00005595.
- Eby LA, Roach WJ, Crowder LB, Stanford JA. 2006. Effects of stocking-up freshwater food webs. Trends in Ecology & Evolution 21(10):576–584. DOI: 10.1016/j.tree.2006.06.016.
- Elliott JM. 1973. The diel activity pattern, drifting and food of the leech Erpobdella octoculata (L.) (Hirudinea: Erpobdellidae) in a lake district stream. Journal of Animal Ecology 42(2):449–459. DOI: 10.2307/3297.
- Elliott JM, Dobson M. 2015. Freshwater leeches of Britain and Ireland: Keys to the Hirudinea and a review of their ecology. Ambleside, Cumbria, UK: Freshwater Biological Association. pp. 108. Scientific Publication No. 69.
- Fischer JR, Krogman RM, Quist MC. 2013. Influences of native and non-native benthivorous fishes on aquatic ecosystem degradation. Hydrobiologia 711(1):187–199. DOI: 10.1007/s10750-013-1483-z.
- García-Berthou E. 2001. Size-and depth-dependent variation in habitat and diet of the common carp (Cyprinus carpio). Aquatic Sciences 63(4):466–476. DOI: 10.1007/s00027-001-8045-6.
- Govedich FR, Moser WE. 2015. Clitellata: Hirudinida and Acanthobdellida, 565–588. In: Thorp J, Rogers D, editors. Ecology and general biology. Thorp and Covich’s freshwater invertebrates. Vol. I, 4th. Amsterdam: Academic Press. pp. 1118.
- Gozlan RE, Britton JR, Cowx I, Copp GH. 2010. Current knowledge on non‐native freshwater fish introductions. Journal of Fish Biology 76(4):751–786. DOI: 10.1111/j.1095-8649.2010.02566.x.
- Grantham BA, Hann BJ. 1994. Leeches (Annelida: Hirudinea) in the experimental lakes area, northwestern Ontario, Canada: Patterns of species composition in relation to environment. Canadian Journal of Fisheries and Aquatic Sciences 51(7):1600–1607. DOI: 10.1139/f94-159.
- Guziur J. 1976. The feeding of two year old carp (Cyprinus·carpio L.) in a vendace lake Klawój. Ekologia Polska 24:211–235.
- Hampton SE, Duggan IC. 2003. Diel habitat shifts of macrofauna in a fishless pond. Marine and Freshwater Research 54(7):797–805. DOI: 10.1071/MF02165.
- Helfman GS. 1986. Fish behaviour by day, night and twilight, 366–387. In: Pitcher T, editor. The behaviour of teleost fishes. Boston, MA: Springer. pp. 553. DOI:10.1007/978-1-4684-8261-4_14.
- Hyvönen T, Nummi P. 2000. Activity traps and the corer: Complementary methods for sampling aquatic invertebrates. Hydrobiologia 432(1/3):121–125. DOI: 10.1023/A:1004038707992.
- Kloskowski J. 2011. Differential effects of age-structured common carp (Cyprinus carpio) stocks on pond invertebrate communities: Implications for recreational and wildlife use of farm ponds. Aquaculture International 19(6):1151–1164. DOI: 10.1007/s10499-011-9435-y.
- Kloskowski J, Nieoczym M, Polak M, Pitucha P. 2010. Habitat selection by breeding waterbirds at ponds with size-structured fish populations. Naturwissenschaften 97(7):673–682. DOI: 10.1007/s00114-010-0684-9.
- Koperski P. 2006. Relative importance of factors determining diversity and composition of freshwater leech assemblages (Hirudinea; Clitellata): A metaanalysis. Archiv für Hydrobiologie 166(3):325–341. DOI: 10.1127/0003-9136/2006/0166-0325.
- Kořinek V, Fott J, Fuksa J, Lellák J, Pražáková M. 1987. Carp ponds of central Europe, 29-62. In: Michael R, editor. Managed aquatic ecosystems. Ecosystems of the world 29. Amsterdam: Elsevier Science Publishing Co. pp. 166.
- Kubová N, Schenková J. 2014. Tolerance, optimum ranges and ecological requirements of free-living leech species (Clitellata: Hirudinida). Fundamental and Applied Limnology 185(2):167–180. DOI: 10.1127/fal/2014/0594.
- Kubová N, Schenková J, Horsák M. 2013. Environmental determinants of leech assemblage patterns in lotic and lenitic habitats. Limnologica 43(6):516–524. DOI: 10.1016/j.limno.2013.05.001.
- Kutschera U. 2003. The feeding strategies of the leech Erpobdella octoculata (L.): A laboratory study. International Review of Hydrobiology 88(1):94–101. DOI: 10.1002/iroh.200390008.
- Kutschera U, Wirtz P. 2001. The evolution of parental care in freshwater leeches. Theory in Biosciences 120(2):115–137. DOI: 10.1078/1431-7613-00034.
- Leppä M, Hämäläinen H, Karjalainen J. 2003. The response of benthic macroinvertebrates to whole-lake biomanipulation. Hydrobiologia 498(1/3):97–105. DOI: 10.1023/A:1026224923481.
- Lougheed VL, Crosbie B, Chow-Fraser P. 1998. Predictions on the effect of common carp (Cyprinus carpio) exclusion on water quality, zooplankton, and submergent macrophytes in a Great Lakes Wetland. Canadian Journal of Fisheries and Aquatic Sciences 55(5):1189–1197. DOI: 10.1139/f97-315.
- Lowe TP, Tebbs K, Sparling DW. 2016. A comparison of three macroinvertebrate sampling devices for use in conducting rapid-assessment procedures of Delmarva Peninsula wetlands. Northeastern Naturalist 23(2):321–338. DOI: 10.1656/045.023.0209.
- Lynggaard C, Oceguera‐Figueroa A, Kvist S, Gilbert MTP, Bohmann K. 2022. The potential of aquatic bloodfeeding and nonbloodfeeding leeches as a tool for iDNA characterisation. Molecular Ecology Resources 22(2):539–553. DOI: 10.1111/1755-0998.13486.
- Martin AJ, Seaby RMH, Young JO. 1994. Food limitation in lake-dwelling leeches: Field experiments. Journal of Animal Ecology 63(1):93–100. DOI: 10.2307/5586.
- Meijer M-L, Jeppesen E, van Donk E, Moss B, Scheffer M, Lammens E, van Nes E, van Berkum JA, de Jong GJ, Faafeng BA, Jensen JP. 1994. Long-term responses to fish-stock reduction in small shallow lakes: Interpretation of five-year results of four biomanipulation cases in the Netherlands and Denmark. Hydrobiologia 275(276):457–466. DOI: 10.1007/BF00026734.
- Meyer CK, Peterson SD, Whiles MR. 2011. Quantitative assessment of yield, precision, and cost-effectiveness of three wetland invertebrate sampling techniques. Wetlands 31(1):101–112. DOI: 10.1007/s13157-010-0122-y.
- Michel P, Oberdorff T. 1995. Feeding habits of fourteen European freshwater fish species. Cybium 19:5–46.
- Miller SA, Crowl TA. 2006. Effects of common carp (Cyprinus carpio) on macrophytes and invertebrate communities in a shallow lake. Freshwater Biology 51(1):85–94. DOI: 10.1111/j.1365-2427.2005.01477.x.
- Nieoczym M, Kloskowski J. 2015. Responses of epibenthic and nektonic macroinvertebrate communities to a gradient of fish size in ponds. Journal of Limnology 74(1):50–62. DOI: 10.4081/jlimnol.2014.981.
- Nieoczym M, Mencfel R, Gorzel M, Kloskowski J. 2020. Reduced abundance but increased diversity of chironomid larvae under higher trophic pressure from fish in semi-permanent ponds. Limnologica 82:125778. DOI: 10.1016/j.limno.2020.125778.
- Nieoczym M, Stryjecki R, Buczyński P, Płaska W, Kloskowski J. 2023. Differential abundance, composition and mesohabitat use by aquatic macroinvertebrate taxa in ponds with and without fish. Aquatic Sciences 85(1):25. DOI: 10.1007/s00027-022-00922-y.
- Panek FM. 1987. Biology and ecology of carp, 1–15. In: Cooper E, editor. Carp in North America. Bethesda, MD: American Fisheries Society. pp. 84.
- Paul P, Das R, Nandy G, Aditya G. 2022. Preferring what others avoid: Differences in the vulnerability of freshwater snails to the exotic and native predators. Hydrobiologia. DOI: 10.1007/s10750-022-05062-w.
- Paul P, Karmakar R, Chatterjee S, Barua A, Banerjee S, Aditya G. 2021. Predation of Glossiphonia weberi (Blanchard, 1897) on the invasive snail Physella acuta (Draparnaud, 1805) in the presence of an alternative prey. Limnological Review 21(4):201–208. DOI: 10.2478/limre-2021-0019.
- Phillips AJ, Govedich FR, Moser WE. 2020. Leeches in the extreme: Morphological, physiological, and behavioral adaptations to inhospitable habitats. International Journal for Parasitology: Parasites and Wildlife 12:318–325. DOI: 10.1016/j.ijppaw.2020.09.003.
- Prejs A. 1973. Experimentally increased fish stock in the pond type Lake Warniak. Feeding of introduced and autochthonous non-predatory fish. Ekologia Polska 21:465–505.
- Prus P, Kajak Z. 1999. Activity of bottom animals-comparison of several trap methods. Acta Hydrobiologica 41:207–217.
- Rahman MM. 2015. Role of common carp (Cyprinus carpio) in aquaculture production systems. Frontiers in Life Science 8(4):399–410. DOI: 10.1080/21553769.2015.1045629.
- Rahman MM, Hossain MY, Jo Q, Kim SK, Ohtomi J, Meyer C. 2009. Ontogenetic shift in dietary preference and low dietary overlap in rohu (Labeo rohita) and common carp (Cyprinus carpio) in semi-intensive polyculture ponds. Ichthyological Research 56(1):28–36. DOI: 10.1007/s10228-008-0062-1.
- Sawyer RT. 1974. Leeches (Annelida: Hirudinea), 81–142. In: Hart Jr. C, Fuller S, editors. Pollution ecology of freshwater invertebrates. New York: Academic Press. pp. 389.
- Sawyer RT. 1986. Leech biology and behaviour. Vols. I–III. Oxford: Clarendon Press. pp. 1065.
- Sket B, Trontelj P. 2008. Global diversity of leeches (Hirudinea) in freshwater. Hydrobiologia 595(1):129–137. DOI: 10.1007/978-1-4020-8259-7_15.
- Specziár A, Tölg L, Bíró P. 1997. Feeding strategy and growth of cyprinids in the littoral zone of Lake Balaton. Journal of Fish Biology 51(6):1109–1124. DOI: 10.1111/j.1095-8649.1997.tb01130.x.
- Spelling SM, Young JO. 1987. Predation on lake-dwelling leeches (Annelida: Hirudinea): An evaluation by field experiment. Journal of Animal Ecology 56(1):131–146. DOI: 10.2307/4804.
- Strona G, Galli P, Seveso D, Montano S, Fattorini S. 2014. Nestedness for dummies (NeD): A user-friendly web interface for exploratory nestedness analysis. Journal of Statistical Software 59(Code Snippet 3):1–9. DOI: 10.18637/jss.v059.c03.
- Sychra J, Adamek Z, Petrivalska K. 2010. Distribution and diversity of littoral macroinvertebrates within extensive reed beds of a lowland pond. Annales de Limnologie-International Journal of Limnology 46(4):281–289. DOI: 10.1051/limn/2010026.
- ten Winkel EH, Davids C. 1985. Bioturbation by cyprinid fish affecting the food availability for predatory water mites. Oecologia 67(2):218–219. DOI: 10.1007/BF00384287.
- ter Braak CJF, Šmilauer P. 2018. Canoco reference manual and user’s guide: Software for ordination (version 5.10). Biometris, Wageningen and České Budějovice. pp. 536.
- Tessier C, Cattaneo A, Pinel-Alloul B, Galanti G, Morabito G. 2004. Biomass, composition and size structure of invertebrate communities associated to different types of aquatic vegetation during summer in Lago di Candia (Italy). Journal of Limnology 63(2):190–198. DOI: 10.4081/jlimnol.2004.190.
- van Haaren T, Hop H, Soes M, Tempelman D. 2004. The freshwater leeches (Hirudinea) of the Netherlands. Lauterbornia 52:113–131.
- Venturelli PA, Tonn WM. 2005. Invertivory by northern pike (Esox lucius) structures communities of littoral macroinvertebrates in small boreal lakes. Journal of the North American Benthological Society 24(4):904–918. DOI: 10.1899/04-128.1.
- Verdonschot RC. 2010. Optimizing the use of activity traps for aquatic biodiversity studies. Journal of the North American Benthological Society 29(4):1228–1240. DOI: 10.1899/09-163.1.
- Wellborn GA, Skelly DK, Werner EE. 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27(1):337–363. DOI: 10.1146/annurev.ecolsys.27.1.337.
- Young JO, Ironmonger JW. 1982. The influence of temperature on the life cycle and occurrence of three species of lake‐dwelling leeches (Annelida: Hirudinea). Journal of Zoology 196(4):519–543. DOI: 10.1111/j.1469-7998.1982.tb03522.x.
- Young JO, Martin AJ, Seaby RMH. 1993. Competitive interactions between the lake-dwelling leeches Glossiphonia complanata and Helobdella stagnalis: An experimental investigation of the significance of a food refuge. Oecologia 93(2):156–161. DOI: 10.1007/BF00317664.
- Young JO, Spelling SM. 1986. The incidence of predation on lake-dwelling leeches. Freshwater Biology 16(4):465–477. DOI: 10.1111/j.1365-2427.1986.tb00989.x.
- Young JO, Spelling SM. 1989. Food utilization and niche overlap in three species of lake‐dwelling leeches (Hirudinea). Journal of Zoology 219(2):231–243. DOI: 10.1111/j.1469-7998.1989.tb02579.x.
- Zambrano L, Perrow MR, Sayer CD, Tomlinson ML, Davidson TA. 2006. Relationships between fish feeding guild and trophic structure in English lowland shallow lakes subject to anthropogenic influence: Implications for lake restoration. Aquatic Ecology 40(3):391–405. DOI: 10.1007/s10452-006-9037-3.
- Zhang X, Mei X, Gulati RD. 2017. Effects of omnivorous tilapia on water turbidity and primary production dynamics in shallow lakes: Implications for ecosystem management. Reviews in Fish Biology and Fisheries 27(1):245–254. DOI: 10.1007/s11160-016-9458-6.
- Zimmer KD, Hanson MA, Butler MG. 2000. Factors influencing invertebrate communities in prairie wetlands: a multivariate approach. Canadian Journal of Fisheries and Aquatic Sciences 57(1):76–85. DOI: 10.1139/f99-180.
