Abstract
Many marsh birds, like bitterns or rails, are endangered species inhabiting only natural habitats and dependent exclusively on different types of emergent vegetation. In a changing environment, some of these habitat specialists are becoming more flexible, shifting their preferences by colonizing man-made habitats. We studied habitat selection by Little Bitterns (Ixobrychus minutus minutus) breeding in a fishpond landscape in south-eastern Poland. Applying a large-scale research approach, we examined several habitat features in order to predict the presence of the Little Bittern from a direct comparison of areas with and without breeding birds. Partial least squares (PLS) regression identified two components of several variables that explained 53% of the variation in the presence of the Little Bittern in a fishpond habitat. The occurrence of the Little Bittern was limited to high-quality patches of emergent vegetation, the height, width and area of which were all significantly greater in the area with breeding birds than in unoccupied patches. Patches with a highly variable edge line in the form of fringes or indentations were less favoured. An optimal water level throughout the breeding season was crucial for nest-site selection and ensured access to food. The heterogeneity of the pond dykes and pond canals positively predicted the presence of the Little Bittern by creating potential nesting or foraging sites. Interestingly, the proportion of bulrushes (Typha spp.) in the emergent vegetation patches had a negative effect on the presence of the Little Bittern. The main threat to this habitat‐sensitive species was the cutting of perennial emergent vegetation, in particular the Common Reed (Phragmites australis).
Introduction
The availability of high-quality habitat patches and sufficient amounts of food resources during the breeding season are key factors governing the population sizes of many bird species and their future breeding success (Newton Citation2013). Marsh birds depend largely on the availability of suitable wetland habitats, the most limited and degraded areas worldwide (Leibowitz Citation2003; Davidson Citation2014; Amano et al. Citation2018). Currently, some marsh bird species exhibiting greater plasticity may be living in anthropogenic habitats (Tscharntke Citation1992; Ledwoń et al. Citation2014; Pérez-Garcia et al. Citation2014). Artificial fishponds, post-mining lakes or gravel pits are assumed to be suitable alternative breeding habitats for many waterbirds, including piscivorous species such as herons (Santoul et al. Citation2009; Kloskowski et al. Citation2010; Sebastián-González et al. Citation2010; Trnka Citation2020). Therefore, studying the habitat choice of waterbird species in these altered and man-made habitats may improve their conservation prospects (Amano et al. Citation2018).
The Little Bittern (Ixobrychus minutus minutus) is a long-distance migrating heron species which breeds in Eurasia and winters in Africa (Kushlan & Hancock Citation2005). Despite its widespread distribution, this small heron is a difficult-to-study species because it is mostly uncommon, usually nests solitarily in inaccessible habitat (dense emergent vegetation or shrubs), and is generally difficult to observe because of its camouflaging plumage (Voisin Citation1991; Flis & Betleja Citation2015; Flis & Gwiazda Citation2018). The IUCN Red List classifies the conservation status of the Little Bittern as Least Concern, but the overall population trend is still a decreasing one, mainly as a result of changes to or loss of its natural habitats, e.g. old riverbeds (BirdLife International Citation2022). On the other hand, this secretive marsh bird is highly adaptive, displaying great environmental tolerance, and may occupy different natural and artificial habitats, such as flooded river valleys, eutrophic lakes, fishponds or small overgrown waterbodies close to human settlements (Voisin Citation1991; Kushlan & Hancock Citation2005).
Detailed knowledge about the Little Bittern’s habitat preferences during the breeding season is limited and varies depending on the habitat studied and the spatial scale. Small-scale research has focused only on nest-site location in different habitats (Cempulik Citation1994; Delelis & Boin Citation2006; Pardo-Cervera et al. Citation2010; Samraoui et al. Citation2012; Flis Citation2013, Citation2016; Fazili Citation2014b; Filipiuk Citation2018). Such few large-scale studies as have been conducted have addressed habitat use by Little Bitterns on urban waterbodies (Scheckenhofer Citation2013), and these birds’ spatial behaviour in the breeding period in a shallow lake (Pezzo & Benocci Citation2001). But it was Pezzo and Benocci (Citation2001) who were the first to publish data on the spatial distribution pattern of the Little Bittern’s home range. They found that tracked individuals used the marshland habitat selectively, and that the location and quality of emergent vegetation patches (area, height, density) determined which of them would be used for nesting or feeding.
In central Europe, many large fishpond complexes continue to represent a semi-natural ecosystem which is a valuable, food-rich habitat that is significant for Little Bittern populations (Wilk et al. Citation2010; Flis & Betleja Citation2015; Filipiuk Citation2018; Trnka Citation2020). It is generally believed that this elusive heron often nests in different types of emergent vegetation along pond dykes and that it forages along the edges of aquatic vegetation and pond canals, but more detailed information about its habitat requirements in such a man-made habitat is not available (Flis Citation2016). In the present paper, based on large-scale research, we investigated which habitat features predict the presence of the Little Bittern in a fishpond landscape by comparing the areas with and without breeding birds.
Material and methods
Study area
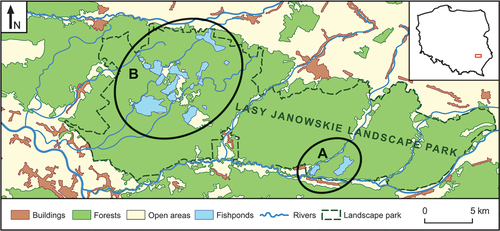
The study was conducted in the breeding season (May–August) of 2010–2012 on fishponds situated in the Lasy Janowskie Landscape Park, south-eastern Poland (50°40′ N 22°20′ E; ). The total area of the eight fishpond complexes was 1380 ha: Stawy Małe 60 ha, Stawy Duże 140 ha, Pieńki 90 ha, Imielty Ług 110 ha, Brzeziny 150 ha, Maliniec 220 ha, Osówek 240 ha, Świdry 370 ha. The areas of the fishponds in the studied complexes varied from 0.5 to 90 ha, and the ponds were 90 − 180 cm deep. The fishponds were partially or wholly covered by emergent vegetation, with dominant Common Reed (Phragmites australis) and bulrush (Typha spp.). The Little Bitterns nested only in the emergent vegetation located along the pond dykes. The water depth in the emergent vegetation varied from 0 to 150 cm. Common Carp (Cyprinus carpio) was the most abundant fish species reared in these ponds (95% of the total fish biomass) (local fishponds managers – pers. comm.). The management of these fishponds involves a semi-intensive production system and a three-year rearing cycle. Three Carp age cohorts were distinguished: Carp fry (0+), Carp after first wintering (1+), and market-size Carp (2+) (Dobrowolski Citation1995). These fishponds are an Important Bird Area (IBA) for many other species, not only the Little Bittern, and a designated Natura 2000 site (Wilk et al. Citation2010).
Monitoring the Little Bittern population
Field procedures were carried out according to the methodology used for monitoring and assessing the population of breeding Little Bitterns in fish farming areas (Morin & Bommé Citation2006; García Citation2009). Poland lies in the northern part of the Little Bittern’s distribution in Europe (EBCC Citation2022), and the first birds arrive at the breeding sites in early May when the new emergent vegetation is starting to appear (Betleja Citation2009). In each breeding season, from May to August, all the pond complexes were monitored regularly, at least once every two weeks. Fieldwork took place mostly in the morning and evening hours or at night. The research was performed using the point-station methodology, where birds were recorded for 30 minutes at selected control points, by walking at 1 km/h along the pond dyke, or by using an inflatable dinghy in areas otherwise hard to reach. The surveys were conducted using a loudspeaker broadcasting the male’s advertising call. All Little Bittern activities were recorded on a 1:5000 map. Potential nesting sites were located by listening for calling males in their territories and observing birds flying over particular reedbed areas. Nests were located by systematically searching all potential breeding sites, wading through patches of emergent vegetation (for detailed information, see Flis Citation2013, Citation2016).
Relationships between habitat features and Little Bittern distribution
The fishpond complexes were divided into two groups: (A) with breeding Little Bitterns, and (B) without breeding Little Bitterns (). Using ArcGIS software, a grid of squares 100 × 100 m was superimposed on the area of each group (ESRI Citation2006). From all the squares located along the pond dykes, selected as the most suitable Little Bittern habitats, 30 study plots were randomly selected for each group (see Supplementary material Figure S1). At the end of August 2012, all the study plots were visited in order to assess their current state and compatibility with high-resolution satellite imagery (cell size 0.25 × 0.25 m) taken in 2012. The satellite images came from the Provincial Center for Geodetic and Cartographic Documentation in Kraków, the Małopolska Region (https://www.malopolska.pl/dla-mieszkanca/rolnictwo-i-geodezja/wojewodzki-osrodek-dokumentacji-geodezyjnej-i-kartograficznej). During these field visits, selected habitat parameters were measured, such as Reed cover or Emergent vegetation width (see Supplementary material Figure S2; ). The other habitat parameters were measured in the ArcGIS environment from satellite imagery, e.g. n patches or Emergent vegetation edge (see Supplementary material Figure S3; ). All the habitat parameters (environmental variables) are described in .
Table I. Environmental variables (habitat parameters) selected as potential predictors of Little Bittern presence in the study area. The variables were measured in the study plots. A – fishpond complexes with breeding Little Bitterns; B – fishpond complexes without breeding Little Bitterns. The variables are presented as the mean ± standard deviation (SD) and range.
Data analyses
Our data set contained several explanatory environmental variables that were correlated with each other (see Supplementary material Figure S4). Thus, we used partial least squares (PLS) regression to analyse which environmental factors were associated with Little Bittern presence. PLS is a technique used with data that contain correlated predictor variables. The big advantage of this method is that data can be analysed with a large number of predictors (larger than the number of observations). This technique constructs new predictor variables, known as components, as linear combinations of the original predictor variables. PLS constructs these components while maximizing covariance between the predictors and response variables (Esposito Vinzi et al. Citation2010).
To perform PLS we used the “plsdepot” package (Sanchez Citation2012); we chose this because it gives a good visualization of results. We used the optimization procedure of mean squared error examination to choose the optimal number of latent variables (pls components) implemented in the “pls” package (Mevik et al. Citation2016). In order to test which environmental variables had a statistically significant effect on Little Bittern presence, we carried out a regularized variable elimination procedure for parsimonious variable selection in the “plsVarSel” package (Mehmood et al. Citation2011). For each environmental variable we also calculated the variable importance based on PLS coefficients. All the calculations were done in R (R Core Team Citation2017).
Results
In 2010–2012, 8–13 pairs of Little Bittern nested on two of the eight fishpond complexes (). Interestingly, no Little Bittern presence was recorded on the other six fishpond complexes.
We found that two components were selected in the PLS regression () and explained 53% of the variation in the Little Bittern presence data. The selection procedure indicated the environmental variables that were positively related to Little Bittern presence in the fishpond habitat (with the decreasing importance): Emergent vegetation height, Water depth, Pond dyke, Emergent vegetation area, Pond canal, Emergent vegetation width and Pond area (; ). The selection procedure identified six environmental variables that were negatively linked with the presence of Little Bittern (with the decreasing importance): Emergent vegetation edge, Sedge cover, Dry pond, Open water, Bulrush cover and Open area (; ).
Figure 2. Results of partial least squares (PLS) regression. Two latent variables (components) were identified that explained the presence (in red) of Little Bittern in the fishpond habitat. Environmental variables selected as statistically significant are emboldened.
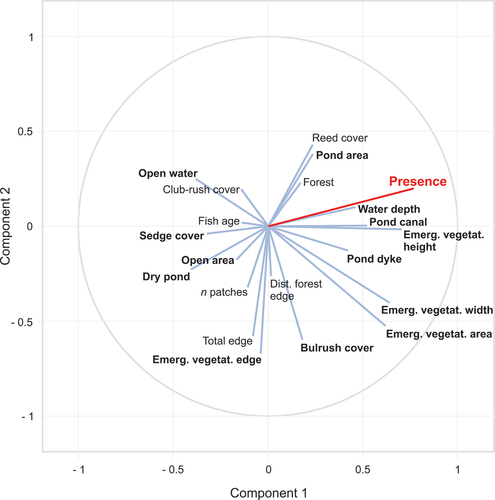
Figure 3. The relative importance of environmental variables in PLS regression. Variables that were selected as statistically significant are shown in red (positive effect of the presence of Little Bittern) or in blue (negative effect of the presence of Little Bittern). Non-significant variables are in grey.
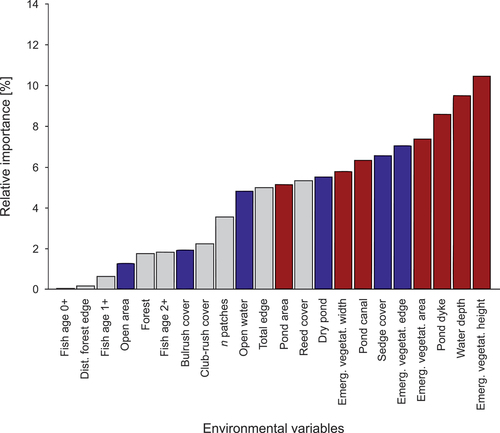
Figure 4. Comparison of values (points) of environmental variables that positively predicted the Little Bittern presence. A/red: fishpond complexes with breeding Little Bitterns; B/blue: fishpond complexes without breeding Little Bitterns. The boxes show the median (bold line), interquartile range (box), min-max values (whiskers) and outliers (points beyond the whiskers).
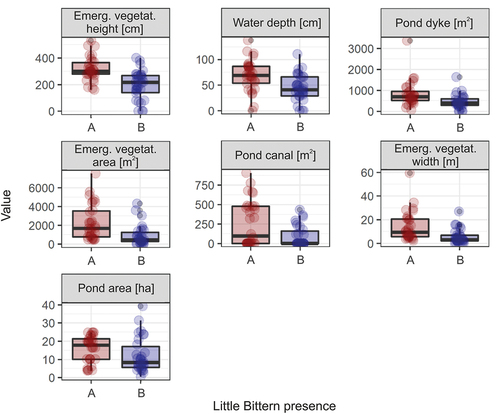
Figure 5. Comparison of values (points) of environmental variables that negatively predicted the Little Bittern presence. A/red: fishpond complexes with breeding Little Bitterns; B/blue: fishpond complexes without breeding Little Bitterns. The boxes show the median (bold line), interquartile range (box), min-max values (whiskers) and outliers (points beyond the whiskers).
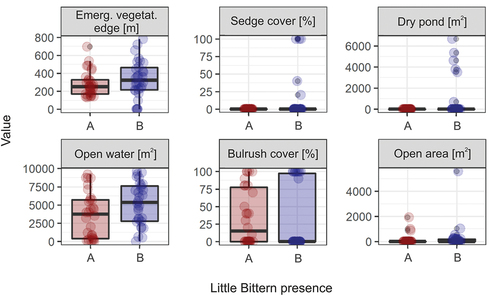
Table II. Partial least squares (PLS) regression coefficients for each explanatory environmental variable. Variables selected as statistically significant are emboldened.
Discussion
From a spatial point of view, comparison of different scales of research under the same environmental conditions offers a mechanistic understanding of habitat selection processes by many bird species (Pickens & King Citation2014; Jedlikowski et al. Citation2016). In our study, the results of large-scale research partially corresponded to those of the small-scale research (nest-site location) previously conducted in the same area (see Flis Citation2016). In both cases, the height of emergent vegetation (Common Reed) was crucial for nest location, because the height and density of aquatic vegetation are the key factors influencing brood survival in many marsh birds, including bitterns (Polak Citation2007; Polak et al. Citation2008; Fazili Citation2014a).
It is known that Little Bitterns often use quite small areas of emergent vegetation and shrubs to nest in, but the size of these patches can vary depending on the habitat occupied, e.g. 0.07 ha on urban waterbodies or 4.6 ha in natural wetlands (Benassi et al. Citation2009; Scheckenhofer Citation2013). We found that the surface area and width of emergent vegetation patches were larger in the study plots with breeding Little Bitterns. These emergent vegetation parameters may therefore be an indication of habitat quality for this species in a fishpond habitat. On the other hand, the length of the emergent vegetation edge was a negative predictor of Little Bittern presence, which is in fact linked to the shape of emergent vegetation patches. On fishpond complexes, Little Bitterns preferred compact patches of a regular shape without any great variation in the edge in the form of indentations or fringes. Such regularly shaped patches can also be created by partially cutting the emerging vegetation, a common practice in fishponds, but in many cases, it leads to a significant reduction of the breeding habitat area and thus the disappearance of Little Bitterns (Szlivka Citation1958; Flis & Betleja Citation2015; Flis Citation2016).
In natural and semi-natural habitats like fishponds, nest predation is a major cause of brood losses among marshland birds so the presence of water below and around the nest is a significance hindrance to predators (Polak Citation2007; Polak et al. Citation2008; Jedlikowski et al. Citation2016). Furthermore, stable hydrological conditions throughout the breeding season ensure access to food (Kloskowski et al. Citation2010). In our study, too, the water level was a significant environmental factor affecting the distribution of Little Bitterns. Although the water depth ranges measured in both survey areas were only approximate, dry patches of pond bed were recorded on the study plots without breeding Little Bitterns, which was not the case on the plots with breeding birds.
The presence of pond dykes and pond canals creates many potential nesting and foraging sites for different bird species associated with aquatic vegetation (Sebastián-González et al. Citation2010; Filipiuk Citation2018). In our fishponds, both dykes and canals were covered by emergent vegetation, and they were also diverse as regards area and the presence of various hydrotechnical structures, such as monks or boat launch slipways. The existence of these habitat parameters had a significant and positive effect on the presence of Little Bitterns, which can use the edge of dykes or canals as a means of obtaining food.
Small-scale research showed that breeding Little Bitterns were closely associated with the Common Reed, because all nests found were located in perennial patches of this emergent plant (Flis Citation2016; Flis & Gwiazda Citation2018). Interestingly, this analysis showed that the actual proportion of Common Reed in emergent vegetation patches was not significant, which suggests that the availability of high-quality Common Reed patches was limited. It has been found that bulrushes are also frequently chosen as nesting sites in different habitats, including Carp fishponds (Pardo-Cervera et al. Citation2010; Samraoui et al. Citation2012; Filipiuk Citation2018), so it is hard to explain why in our research the bulrush cover negatively predicted the presence of Little Bitterns. There were fewer patches of other emergent plants, like club-rushes (Schoenoplectus spp.) and sedges (Carex spp.); as these do not grow very tall, they are a sub-optimal habitat for Little Bitterns.
The Little Bittern has a wide food spectrum (Voisin Citation1991), but like other heron species it is also an opportunist that uses the most readily available food source, which in our case was the Common Carp (Flis & Gwiazda Citation2018). The predominance of fry ponds in particular fishpond complexes could have a positive influence on the Little Bittern presence. In the fishponds surveyed, the proportion of Carp age cohorts was similar in both areas, and fish age had no influence on the presence of the Little Bittern.
Conclusions
Fishpond complexes with extensive or semi-intensive fish farming systems are human-managed habitats known for their high biodiversity. The maintenance of relatively small patches of tall perennial emergent vegetation offers potential breeding sites for Little Bitterns. There was some contrast in the Little Bittern’s habitat preferences compared to other studies conducted in natural and man-made habitats, indicating that this species exhibits environmental plasticity. Its comparatively undemanding breeding requirements enable it to nest in various anthropogenic habitats, an aspect that may be important for arresting its decline right across its breeding range.
Authors’ contributions
Adam Flis conceived the study, designed the methodology, carried out the field work, data analysis and drafted the manuscript. Piotr Skórka performed the data analysis and drafted the manuscript. Wiesław Król took part in designing the methodology, data analysis and drafted the manuscript.
Supplemental Material
Download MS Word (4.5 MB)Acknowledgments
We would like to thank the associate editor and reviewers for very important and helpful comments which improved this manuscript. We would also like to thank Prof. Zbigniew Głowaciński for inspiring this work and Agata Flis for her assistance with the fieldwork. We greatly appreciate the cooperation of the local fish farmers. The fieldwork was carried out with the permission of the General Directorate for Environmental Protection (DOPozgiz-4200/III-162/2212/10/dl). The research methods met all the ethical guidelines for the study of wild birds and were in compliance with the current standards and policies of Polish law.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2023.2291458.
Additional information
Funding
References
- Amano T, Székely T, Sandel B, Nagy S, Mundkur T, Langendoen T, Blanco D, Soykan CU, Sutherland WJ. 2018. Successful conservation of global waterbird populations depends on effective governance. Nature 553:199–202. DOI: 10.1038/nature25139.
- Benassi G, Battisti C, Luiselli L, Boitani L. 2009. Area-sensitivity of three reed bed bird species breeding in Mediterranean marshland fragments. Wetlands Ecology and Management 17:555–564. DOI: 10.1007/s11273-009-9135-9.
- Betleja J. 2009. Little Bittern Ixobrychus minutus. In: Chylarecki P, Sikora A, Cenian Z, editors. Monitoring of breeding birds. Methodological guide. Chief Inspectorate of Environmental Protection. Warsaw, Poland, pp. 109–112. (In Polish).
- BirdLife International. 2022. Species Factsheet: Ixobrychus minutus. Available: http://www.birdlife.org. Accessed May 2022 5.
- Cempulik P. 1994. Population trends, breeding biology and ecology of Little Bittern Ixobrychus minutus at fish ponds and industrial reservoirs in Upper Silesia, Poland. Vogelwelt 115:19–27. (In German).
- Davidson NC. 2014. How much wetland has the world lost? long-term and recent trends in global wetland area. Marine and Freshwater Research 65:934–941. DOI: 10.1071/MF14173.
- Delelis N, Boin S. 2006. Typology of Little Bittern Ixobrychus minutus habitat in the Audomarois marshes (Pas-de-Calais). Alauda 74:65–75. (In French).
- Dobrowolski KA. 1995. Environmental-economic evaluation of fish ponds in Poland. Warsaw: IUCN Poland. (In Polish).
- EBCC. 2022. European breeding bird atlas 2 website. European Bird Census Council. Available: http://ebba2.info. Accessed April 2022 4.
- Esposito Vinzi V, Chin WW, Henseler J, Wang H. 2010. Handbook of partial least squares: Concepts, methods and applications. Heidelberg: Springer.
- ESRI. 2006. ArcGIS, version 9.2. Redlands: Environmental Systems Research Institute.
- Fazili MF. 2014a. Clutch predation in relation to mean vegetation height and reed density at the nest in Little Bittern (Ixobrychus minutus minutus). Journal of Global Biosciences 3:516–519.
- Fazili MF. 2014b. Nesting ecology and breeding success of Little Bittern in Wular Lake Kashmir, India. New York Science Journal 7:109–118.
- Filipiuk MK. 2018. Breeding biology of the Little Bittern Ixobrychus minutus (L., 1766) on fish ponds in the Lublin region. PhD dissertation. Maria Curie-Sklodowska University, Lublin. (In Polish)
- Flis A. 2013. Population status and breeding biology of Little Bittern Ixobrychus minutus on the fishponds in Janowskie Forests Landscape Park (SE Poland). Chrońmy Przyr Ojcz 69:96–105. (In Polish).
- Flis A. 2016. Nest types and nest-site selection of the Little Bittern Ixobrychus minutus breeding in fishpond habitat in south-eastern Poland. Polish Journal of Ecology 64:268–276. DOI: 10.3161/15052249PJE2016.64.2.010.
- Flis A, Betleja J. 2015. Little Bittern Ixobrychus minutus. In: Chylarecki P, Sikora A, Cenian Z, Chodkiewicz T, editors. Monitoring of breeding birds. Methodological guide, edition II. Chief Inspectorate of Environmental Protection. Warsaw, Poland, pp. 349–353. (In Polish).
- Flis A, Gwiazda R. 2018. Diet and feeding of nestling Little Bitterns Ixobrychus minutus at fishponds: Testing a new method for studying a difficult-to-monitor species. Bird Study 65:257–260. DOI: 10.1080/00063657.2018.1446128.
- García P. 2009. Assessment of two methods for estimating Little Bittern Ixobrychus minutus populations in fluvial habitats in central Spain. Revista Catalana d’Ornitologia 25:54–58.
- Jedlikowski J, Chibowski P, Karasek T, Brambilla M. 2016. Multi-scale habitat selection in highly territorial bird species: Exploring the contribution of nest, territory and landscape levels to site choice in breeding rallids (Aves: Rallidae). Acta Oecologica-International Journal of Ecology 73:10–20. DOI: 10.1016/j.actao.2016.02.003.
- Kloskowski J, Nieoczym M, Polak M, Pitucha P. 2010. Habitat selection by breeding waterbirds at ponds with size-structured fish populations. Naturwissenschaften 97:673–682. DOI: 10.1007/s00114-010-0684-9.
- Kushlan JA, Hancock JA. 2005. The herons. Oxford: Oxford University Press.
- Ledwoń M, Betleja J, Stawarczyk T, Neubauer G. 2014. The whiskered tern Chlidonias hybrida expansion in Poland: The role of immigration. Journal of Ornithology 155:459–470. DOI: 10.1007/s10336-013-1027-3.
- Leibowitz SG. 2003. Isolated wetlands and their functions: An ecological perspective. Wetlands 23:517–531. DOI: 10.1672/0277-5212(2003)023[0517:IWATFA]2.0.CO;2.
- Mehmood T, Liland KH, Snipen L, Sæbø S. 2011. A review of variable selection methods in partial least squares regression. Chemometrics and Intelligent Laboratory Systems 118:62–69. DOI: 10.1016/j.chemolab.2012.07.010.
- Mevik B-H, Wehrens R, Liland KH. 2016. Pls: Partial least squares and Principal component regression. R package version 2.6-0. Available: https://CRAN.R-project.org/package=pls.
- Morin C, Bommé S. 2006. Methodological contribution to monitoring Little Bittern Ixobrychus minutus in fish farming areas. Alauda 74:143–150. (In French).
- Newton I. 2013. Bird populations. London: HarperCollins Publishers.
- Pardo-Cervera F, Sorensen IH, Jensen C, Ruiz X, Sanchez-Alonso C. 2010. Breeding biology of the Little Bittern Ixobrychus minutus in the Ebro Delta (NE Spain). Ardeola 57:407–416.
- Pérez-Garcia JM, Sebastián-González E, Alexander KL, Sánchez-Zapata JA, Botella F. 2014. Effect of landscape configuration and habitat quality on the community structure of waterbirds using a man-made habitat. European Journal of Wildlife Research 60:875–883. DOI: 10.1007/s10344-014-0854-8.
- Pezzo F, Benocci A. 2001. Spatial behaviour of the Little Bittern Ixobrychus minutus, implications for conservation. Avocetta 25:78.
- Pickens BA, King SL. 2014. Multiscale habitat selection of wetland birds in the Northern Gulf Coast. Estuaries & Coasts 37:1301–1311. DOI: 10.1007/s12237-013-9757-2.
- Polak M. 2007. Nest-site selection and nest predation in the Great Bittern Botaurus stellaris population in eastern Poland. Ardea 95:31–38. DOI: 10.5253/078.095.0104.
- Polak M, Kasprzykowski Z, Kucharczyk M. 2008. Micro-habitat nest preferences of the great bittern, Botaurus stellaris, on fishponds in central-eastern Poland. Annales Zoologici Fennici 45:102–108. DOI: 10.5735/086.045.0202.
- R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: https://www.R-project.org/.
- Samraoui F, Nedjah R, Boucheker A, Alfarhan AH, Samraoui B. 2012. Breeding ecology of the Little Bittern Ixobrychus minutus in northeast Algeria. Bird Study 59:496–503. DOI: 10.1080/00063657.2012.733335.
- Sanchez G. 2012. Plsdepot: Partial least squares (PLS) data analysis methods. R package version 0.1.17. Available: https://CRAN.R-project.org/package=plsdepot.
- Santoul F, Gaujard A, Angélibert S, Mastrorillo S, Céréghino R. 2009. Gravel pits support waterbird diversity in an urban landscape. Hydrobiologia 634:107–114. DOI: 10.1007/s10750-009-9886-6.
- Scheckenhofer C. 2013. Habitat preferences of Little Bitterns Ixobrychus minutus breeding in wetlands embedded in an urban habitat matrix: a case study from Vienna, Austria. MSc dissertation. University of Vienna, Vienna.
- Sebastián-González E, Sánchez-Zapata JA, Botella F. 2010. Agricultural ponds as alternative habitat for waterbirds: Spatial and temporal patterns of abundance and management strategies. European Journal of Wildlife Research 56:11–20. DOI: 10.1007/s10344-009-0288-x.
- Szlivka A. 1958. The little bittern breeding in a colony. Aquila 65:339.
- Trnka A. 2020. Nestling diet and breeding success of Little Bitterns Ixobrychus minutus at two artificial fishpond complexes in south-western Slovakia. Ardea 108:161–169. DOI: 10.5253/arde.v108i2.a2.
- Tscharntke T. 1992. Fragmentation of Phragmites habitats, minimum viable population size, habitat suitability, and local extinction of moths, midges, flies, aphids, and birds. Conservation Biology 6:530–536. DOI: 10.1046/j.1523-1739.1992.06040530.x.
- Voisin C. 1991. The herons of Europe. London: T&AD Poyser.
- Wilk T, Jujka M, Krogulec J, Chylarecki P. 2010. Important bird areas of international importance in Poland. OTOP, Marki, Poland. (In Polish).