Abstract
Chinese sleeper is an invasive species from South Asia inhabiting Eastern and Central Europe. So far, no detailed analysis of the reproductive cycle of Chinese sleepers has been conducted. The aim of the study was to describe the reproductive cycle of Chinese sleepers in the Wilanówka River, Vistula tributary (Poland, Central Europe). Fish morphometric data, age, gonadal maturity, and absolute and relative fecundity of females were analysed. A standard paraffin technique and six-stage scales were used to assess the gonad development and maturation in both sexes. The age of the studied fish ranged from 0+ to 6+.The female-to-male ratio was 1:1. Females and males mature at age 1+.The smallest female and male reaching maturity were 3.8 and 3.7 cm long, respectively. An extended spawning season from the beginning of April to mid-September was determined based on gonadal maturity analysis. The mean absolute fecundity was 2,971 (180–19,656) oocytes. The male reproductive system comprises paired testis (spermatogenic region, blackish color) and paired testicular gland (secretory region, whitish color). In both regions, seasonal changes were observed. Male gonads during winter had completed spermatogenesis, and the lobules were filled with spermatozoa. Very fast spermatogenesis and overlapping of reproductive cycles have been observed in Chinese sleeper males. Almost all year round testis contains spermatozoa. In the new habitat examined, this species was characterized by earlier maturation and longer reproduction season, compared to the native habitat and other native species of the examined area. Moreover, high female fecundity, multiple spawning, and lower investment in the development of gonads in males allow maintaining vitality and protecting the nest, contribute to the species competitiveness and successful colonization of new areas.
1. Introduction
Chinese sleeper is a small fish species from the Gobiiformes family. Its natural habitat is south-east Asia, China (Amur area), and North Korea. It is one of the most invasive fish species in Central and Eastern Europe (Copp et al. Citation2005). In the 20th century, the species spread from Asia, through the former Soviet Unions to, e.g., Ukraine (Movchan Citation1989), Lithuania (Repečka Citation2003), Slovakia (Kautman Citation1999) and to the Balkans (Nalbant et al. Citation2004; Horvatić et al. Citation2022), Germany (Reshetnikov & Schliewen Citation2013), Italy (Reshetnikov & Ficetola Citation2011), the Black Sea (Kvach et al. Citation2021). In Poland, Chinese sleeper was first observed in the Vistula River near Dęblin in the 90s of the 20th century (Antychowicz Citation1994) and at subsequent sites in the Vistula drainage (Terlecki & Pałka Citation1999; Rechulicz et al. Citation2015), western Bug River (Terlecki Citation1995), Oder drainage (Andrzejewski et al. Citation2011). It is found in high numbers in small, shallow ponds densely overgrown with plants, the littoral zone of lakes, slowly flowing rivers, wetlands, and old river beds (Witkowski Citation2008). It is characterised by flexible feeding strategy. The fish feeds on insect larvae, small crustaceans, fry of cohabitating species, and amphibian eggs. It may swallow fish representing 2/3 of its own length (Koščo et al. Citation2008; Semenchenko et al. Citation2011; Rau et al. Citation2017).
Chinese sleeper is resistant to unfavorable environmental conditions, such as partial drying of water reservoirs, or freezing, by burying itself in ooze. High fecundity, wide ecological tolerance, predation strategy, opportunistic feeding strategy, and aggressive behavior facilitate the spread of this species, which poses a threat to the European ichthyofauna biodiversity (Reshetnikov & Ficetola Citation2011; Luca & Giorghită Citation2014; Froese & Pauly Citation2023). It shows significant tolerance to high and low temperatures (Bogutskaya & Naseka Citation2002; Witkowski & Grabowska Citation2012) but does not survive in water with a temperature higher than 34–38°C (Golovanova et al. Citation2009). Spawning requires the water temperature to be higher than 15oC (Kirpichnikov Citation1945; Kottelat & Freyhof Citation2007). In Europe, the Chinese sleeper is considered an invasive alien species (European list), very expansive due to ecological flexibility, as well as reproductive potential related to multiple spawning, high fecundity, and egg guarding by males (Bogutskaya & Naseka Citation2002; Witkowski & Grabowska Citation2012). According to FishBase, Chinese sleeper is classified as a potentially harmful species (Froese & Pauly Citation2023), since its predation poses a threat to local fish species, such as: belica (Leucaspnis delineatus), European mudminnow (Umbra krameri), crucian carp (Carassius carassius) and amur bitterling (Rhodeus sericeus).
So far, no detailed analysis of the gonadal structure and reproductive cycle of Chinese sleepers has been conducted. Previous publications include studies on the gonads of other gobioid fishes (Kovacic Citation2007; Hwang & Baek Citation2013; Cole & Parenti Citation2022). The male reproductive tract of Odontobutidae includes the testicular gland as in other gobiids (Fishelson Citation1991; Miller Citation1992; Zhou et al. Citation2022). The testicular gland (also called seminal vesicle, accessory gonadal structure, sperm-duct gland, or secretory lobe) varies in shape, size, and location among species (Nayyar & Sundararaj Citation1970; Schoonen et al. Citation1987; Seiwald & Patzner Citation1989; Lahnsteiner et al. Citation1990; Fishelson Citation1991; Leite et al. Citation2016). The testis structure of Odontobutidae belongs to the unrestricted lobular-type (Grier Citation1981; Parenti & Grier Citation2004; Thacker & Grier Citation2005). Previous histological studies on the histology of Chinese sleeper gonads concerned only on female gonads with regard to pathological lesions of the fish from polluted areas of Prut River and Saratov Reservoir of Volga District, and the occurrence of hermaphroditism in one individual (Mineev Citation2009; Fulga et al. Citation2015; Kirilenko & Shemonaev Citation2015). The aim of the study was to describe the reproductive cycle and to determine the fecundity of female Chinese sleeper in the new habitat in central Poland.
2. Material and methods
Chinese sleeper (Amur sleeper) (Perccottus glenii, Dybowski, 1877 (Teleostei: Gobiiformes: Odontobutidae) was collected from Wilanówka River, Vistula tributary (52°08'18.3''N 21°08'16.5''E) () monthly (every two weeks in the spawning season) in 2022. A total of 575 fish were caught (). The fish collected by electrofishing were transferred to the laboratory and were euthanized in MS-222. The body length (total length TL and standard length SL) of the fish to an accuracy of 0.1 mm was measured and weighed on an electronic scale (to an accuracy of 0.1 g). The age of the fish was determined on the basis of the scale analysis (Steinmetz & Müller Citation1991; Rosecchi et al. Citation1993). From each fish, five scales were removed from the area between the lateral line and the first dorsal fin (Grabowska et al. Citation2011). The scales were cleaned using the alkaline immersion method (Huang et al. Citation2015) and compressed between two glass slides for age determination by counting the number of completely developed annual rings using criteria from Steinmetz and Müller (Citation1991).
Table I. Number of individuals of Chinese sleeper (Perccottus glenii) used in the study in a particular month of the year.
Gonads were dissected and then weighed to the nearest 0.1 mg. The Fulton condition index (CF = gonad mass [g] × length −3 [cm] × 100%) and gonadosomatic index (GSI = gonad mass [g] × fish total body mass −1 [g] × 100%) were calculated. All male and female gonads were fixed in the Bouin’s fluid. The histological sections of 5 µm thickness were cut using the standard paraffin technique. The sections were regressively stained with Heidenhain’s iron hematoxylin (male) or Mayer’s hematoxylin and eosin (female). One microscopic slide contained about 50 histological sections of gonads. The histological sections were analyzed with a light microscope Nikon Eclipse 80i; photographic documentation was performed using the NIS software Elements 3.20 and a digital camera Nikon DS-5Mc-U2, of 5 million pixel resolution.
The reproductive cycle of females was described using a Grier (Citation2002) scale. In the period between March and September, right and left ovaries were separately weighed to the nearest 0.1 mg. Then, with the use of Discovery.V12 Zeiss stereo microscope, a fragment of left gonad was collected and weighed, and all oocytes were divided into five size classes (nomenclature of oocytes after Grier Citation2002): group 1 – preovulatory and ovulating oocytes, group 2 – final oocyte maturation, group 3 – secondary growth (vitellogenesis), group 4 – primary growth (finally previtellogenesis), group 5 – primary growth (beginning of previtellogenesis). Subsequently, the number of oocytes from each size group was counted for each ovarian lobe using the gravimetric method (Holčík & Hensel Citation1972). In each class, the oocytes were calculated, and the diameter measurements were performed for 30 oocytes from each class. The diameter of 30 oocytes from each size class was measured by taking the longest and shortest diameter (West Citation1990) with an accuracy of 0.01 μm. Absolute fecundity was calculated as the number of 1–3 group oocytes found in the fish gonad, and relative fecundity was the number of 1–3 group oocytes per gram of fish weight. The fecundity did not include the smallest oocytes from group 4 and 5 in previtellogenesis.
The reproductive cycle of males was described using a classification published by Grier (Citation2002).
Measurement of water parameters
Along with fish sampling, water measurements were taken directly in the field. Temperature and water oxygenation were measured using a multiparameter sensor HQD30 produced by Hach (Düsseldorf, Germany).
Statistical analysis
Prior to the statistical analysis, the normal distribution of variables was tested using the Shapiro–Wilk test and the homogeneity of variance using the Levene test (Sokal & Rohlf Citation1995). The following fish characteristics were compared between samples from individual months using the Kruskal–Wallis test: TL, SL, fish weight, CF, GSI, fecundity, and sizes of oocytes at different stages. The significance of differences in the number of females and males was tested with the chi-square test. All analyses were performed at the significance level of 0.05 using the Statistica v.13 software (StatSoft, Inc.).
3. Results
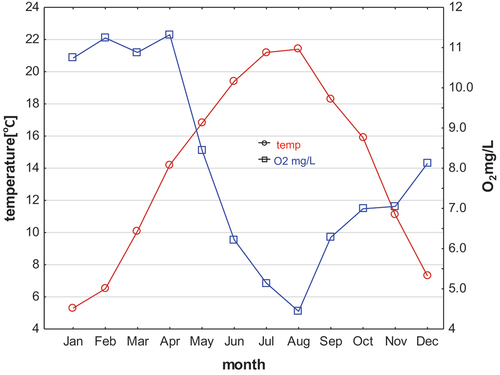
The water temperature in Wilanówka River during the sampling period ranges from 5°C to 21°C, and the oxygen content was between 5 and 22.0 mg L−1 ().
3.1. Length, weight, CF, gonad mass and morphology, GSI of fish
3.1.1. Females
The sex ratio of Chinese sleeper was 1:1 (282:293 individuals) (p ˃ 0.05, chi-square test). The age of females ranged from 0+ to 6+ years. The mean standard length of the collected Chinese sleeper females was 6.38 ± 1.85 [SD] cm. The mean weight of females was 8.27 ± 7.65 g and CF of 2.49 ± 0.55 ().
Table II. Characteristics of specimens of Chinese sleepers.
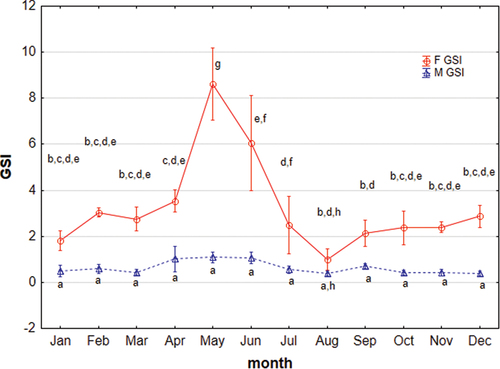
The highest mean GSI in females of 8.62 ± 0.71 was noted in May, while the lowest in August of 0.58 ± 0.5 (). In the period of April–August, the GSI of females with gonads before spawning was 4.61 ± 0.73. The GSI of the mature gonad during the spawning period increased to 13.10 ± 3.29, and after the spawning was of 4.71 ± 1.34. The highest individual GSI was recorded in a female from May − 20.3 (age 6+) (p ˂ 0.05, Kruskal–Wallis test).
Figure 3. Monthly distribution of gonadosomatic index (GSI) of Chinese sleeper (Perccottus glenii) female and male from the study area. Values marked with different letters show differences in significance between females and males and between months (p < 0.05; Kruskal–Wallis test).
Female gonads were paired, with a typical location at the side of the gastrointestinal tract, close to the genital opening. Both gonads revealed a homogeneous structure, dilated from the cephalic side and spindle-shaped in the further part. The lobes of the ovary contain numerous lamellar folds of oocyte tissue, separated by narrow, inter‐lamellar extensions of the lumen of the ovary. Oocytes were distributed on the connective tissue stroma, sometimes containing vacuoles. The weight of the right and left gonads did not significantly differ (p < 0.05, Kruskal–Wallis test). In most cases (70% of individuals), the differences did not exceed 10% of the gonad weight.
3.1.2. Males
The caught specimens of Chinese sleeper males ranged in age from 0+ to 5+ years. Individuals belonging to this sex were smaller than females (p ˂ 0.05, Kruskal–Wallis test); the mean, standard length (SL) of males was 6.10 ± 1.53 cm, weighing of 6.80 ± 5.39 g and CF of 2.44 ± 0.58 (). The gonadosomatic index did not change much throughout the year (p ˃ 0.05, Kruskal–Wallis test). In the breeding season from April to June GSI ranged from 0.73 to 1.11. In this period, male gonads were in late maturation class, and testicular glands were filled with fluid. After spawning GSI is reduced and through the winter until spring the index ranged 0.38–0.65 ().
The male reproductive system is built of two parts, the testis (germinal part) and the testicular gland (seminal vesicle, accessory gonadal structure, sperm duct gland, and secretory lobe). Testis, (T) is located apically, and cephalically. It is constructed of seminiferous lobules running dorsally to the sperm duct. In this part, gamete production takes place. Morphologically, this part of the reproductive system has a blackish color (). The cells in the gonadal capsule contain pigment grains. The pigment is also located in the connective tissue between seminiferous lobules. The testicular gland (TG) is located caudally, closer to the genital opening. It has a white color (). This part is made of secretory lobules lined with a tall columnar secretory epithelium. The amount of fluid accumulated in the lumen, the size of lobules and of this part undergo significant fluctuations in the reproductive cycle.
3.2. Reproductive cycle
3.2.1. Females
From October to February, the gonads of Chinese sleeper were in early vitellogenesis (secondary growth) (). In March, most gonads reached – final oocyte maturation, and about 30% of them had oocytes already before spawning – preovulatory oocyte (individuals from mid-March). In April, most individuals already had ovulation () or after laying the first portion of eggs – post-spawning vesicles are seen in the gonads filled with mature oocytes. These gonads are described as post-spawning, since they have post-spawning vesicles, although they are still filled with preovulatory oocyte (). This is also indicated by low GSI (after laying the first portion) and the presence of numerous oocytes with joined yolk in the gonad (subsequent portion). In May and June, the majority of gonads are spawning or post-spawning, and few are preparing for spawning. In June, there are also gonads with oocytes in primary and secondary growth, with single, degenerating oocytes (). From July to September, there are still individuals with spawning and post-spawning gonads, and the number of individuals in gonads with secondary growth oocytes is increasing (). The gonads of several females aged 2+ and 3+ caught in July, reveal spaces with vacuoles among oocytes in previtellogenesis (). On the other hand, some female gonads collected in September showed single oocytes in the secondary growth phase among oocytes in the primary growth phase and degenerated oocytes ().
Figure 5. Histology of the ovary of Chinese sleeper (Perccottus glenii); (a): in early vitellogenesis with primary growth oocytes (P), October to February. Scale bar 100 µm; (b): spawning gonad with mature oocytes (MO) and with post-ovulatory follicles (arrow), April. Scale bar 200 µm; (c): with oocytes in vitellogenesis (V) and with post-ovulatory follicles (arrow), June. Scale bar 100 µm; (d): with oocytes in early vitellogenesis (V) and the oocytes at the beginning of the next generation of previtellogenesis (P) with degenerating oocytes (D), June. Scale bar 100 µm; (e): in spaces with vacuoles (asterisk) among oocytes in previtellogenesis, July. Scale bar 100 µm; (f): single oocytes in vitellogenesis (V) between primary growth oocytes (P), September. Mayer’s hematoxylin and eosin staining. Scale bar 50 µm.
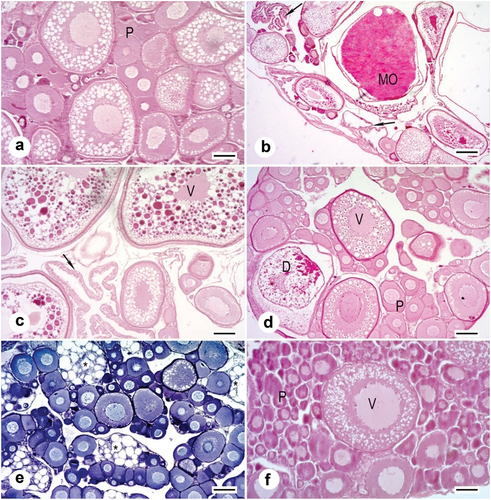
Figure 6. Percent composition of the number of Chinese sleeper (Perccottus glenii) female with ovary in particular classes of maturity in individual months of the calendar year from Wilanówka River.
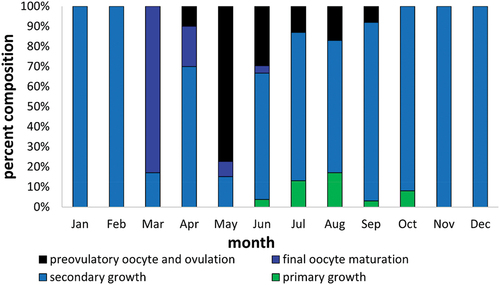
The smallest female Chinese sleeper initiating spawning in the first reproductive season was 3.8 cm long (SL). On the basis of the gonad analysis, we may state that the spawning of Chinese sleepers from the Wilanówka River takes place from the beginning of April until mid-September. Oocyte degeneration was visible in gonads from March, September, and October.
3.2.2. Appearance and size of oocytes
In the gonads of Chinese sleepers in the period March–September, five groups of oocytes were distinguished. The sizes of particular oocyte groups show significant differences (p < 0.05, ANOVA Kruskal–Wallis test, ). The diameter of the largest oocytes (group 1) was 1,126.74 ± 1,4308 µm, and the smallest (group 5) was 114.83 ± 19.90 µm. However, there are no statistically significant differences between oocytes from the same group in subsequent months, except for group 1 (oocytes with completed vitellogenesis), where oocytes increase their size in the spawning season (). In many females during the spawning season, the most developed were oocytes from group 2, which may indicate that another portion of eggs has been laid (oocytes from group 1). The smallest oocytes were especially numerous (group 5), with a mean of 1,847 (±1845) in a female ().
Figure 7. Average sizes of the five groups of Chinese sleeper (Perccottus glenii) oocytes from March-September (group description in the text). Values marked with different letters show the significance of differences in diameter between individual months and groups of oocytes (p < 0.05; Kruskal–Wallis test).
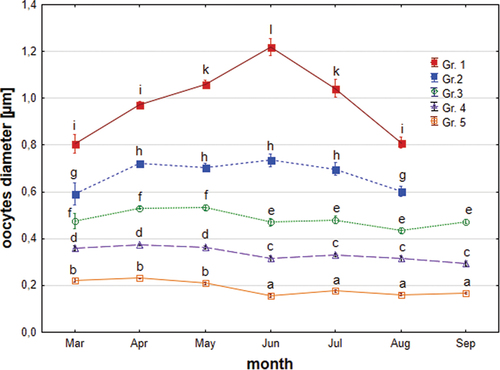
Table III. Sizes of oocytes [µm] at different stages of development in Chinese sleeper (Perccottus glenii).
Table IV. The number of oocytes from 1 to 5 groups in Chinese sleeper (Perccottus glenii).
The absolute fecundity (oocytes from groups 1–3) of Chinese sleepers in the period between March and September was 2,095 (±1,971), and the relative fecundity was 201 (±132) (). Absolute fecundity significantly increased together with the fish age and positively correlated with length of the fish (). Relative fecundity increased up to age 2+ then decreased and slightly negatively correlated with fish length (). The lowest number of oocytes was found in females aged 1+, mean 1,657, and the highest in females aged 6+, mean 26,773. In total, there were 4,721 (±3,900) oocytes of all groups (groups 1–5) per one female, and relatively 476 (±364) oocytes per 1 g of fish. The highest number of all oocytes was found in females before the first spawning, in April, mean of 6,423 (±4,380, range 353–26,867). High fecundity in September observed in our studies was surprising, and it was 7,144 (±3,407, range 1,264–11,361) and 1,496 (±6,011, range 548–3,156) for all oocytes (groups 1–5) and for oocytes from groups 1–3, respectively.
Figure 8. Absolute fecundity in Chinese sleeper (Perccottus glenii) in relation to standard length (SL); (p < 0.05, ANOVA Kruskal–Wallis test).
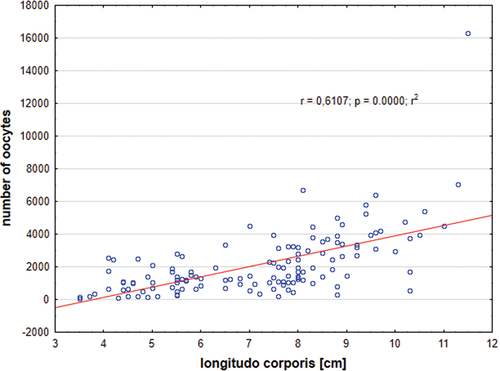
Figure 9. Relative fecundity in Chinese sleeper (Perccottus glenii) in relation to standard length (SL); (p < 0.05, ANOVA Kruskal–Wallis test).
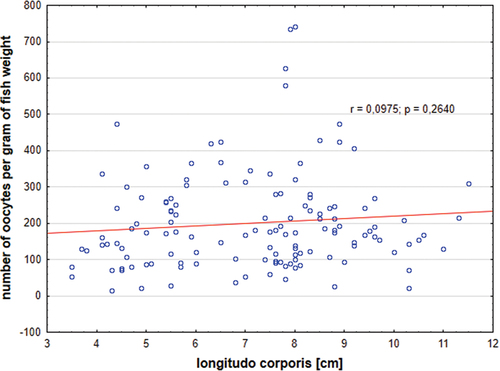
Table V. Absolute and relative fecundity of the Chinese sleeper (Perccottus glenii).
3.3. Males
The testis structure of the Chinese sleeper belongs to the unrestricted lobular-type. Spermatogonia A cells are located over the entire length of seminiferous lobules. In the gonads of the studied species male, spermatogenesis was finalized long before the reproductive season. From mid-October, through winter, until the beginning of the spawning season (which starts in April) gonads were in late maturation class (). Seminiferous lobules contained only spermatozoa and reserve cells of scattered spermatogonia A located at the lobular wall. The wall of seminiferous lobules was tense, and the lumen was filled with a large number of spermatozoa. In some males, part of lobules contained fewer spermatozoa, which were less densely packed in the lobular lumen or they were even empty. The secretory part in autumn and winter typically revealed lobules with collapsed lumen or slight, narrow lumen without fluid. In the middle part, there were some lobules with a moderate amount of fluid. The lumen of some lobules revealed spermatozoa mixed with fluid. In winter, the fluid was darker than in the period of its increased production ().
Figure 10. Histology of the testes of the Chinese sleeper (Perccottus glenii) male inhabiting the Wilanówka River, Poland. (a): testis in late maturation class, gonad with finalized spermatogenesis, lobules filled with spermatozoa (asterisk), November; (b): testicular gland, lobules were collapsed and contained no or little secretion, November; (c): testis in late maturation class in spawning time, seminiferous lobules filled with spermatozoa, the lobule wall contains multiplying type B spermatogonia (arrow), mid-May; (d): testicular gland during spawning time, lobules filled with a large amount of secretion of light and dark color, mid-May; (e): testis in early maturation class with continuous germinal epithelium, all type of spermatogenic cells are visible in the gonad, first spermatozoa are formed. It is difficult to distinguish the sperm from the current spawning from the newly formed sperm (asterisk), June; (f): testicular gland containing lobules filled with a variable amounts of secretion, June; (g): testis in early maturation class with continuous germinal epithelium, the lobules lumen is filled with spermatozoa, by the lobule wall numerous cysts with maturing cell occurred, September; (h): testicular gland lobules filled with a small amount of secretion, September. Heidenhain’s iron hematoxylin staining. Scale bar 50 μm.
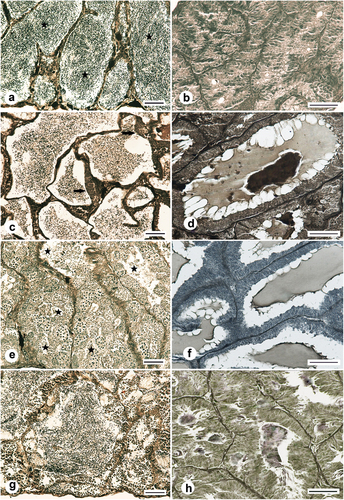
In April and at the beginning of May (the first phase of spawning), male gonads still contained a large number of spermatozoa (late maturation class). The testicular gland lobules significantly increased their size and a large amount of lighter fluid appeared in the lumen. The lobules usually contained areas of light and dark fluid (). From mid-May, in most individuals, spermatogonia multiplication started when lobules were filled with large amount of spermatozoa, so regressed class did not occur (). At the end of May, gonads of all males reach early maturation class with continuous germinal epithelium. The amount of spermatozoa of the current spawning may still be present in the lobules near ducts (cycle overlapping) (). In the glandular part, the lobules of some individuals were still filled with a large amount of fluid with various colour intensities. In some individuals, the amount of fluid in the lobules was smaller () or the lobules were empty. A similar histological picture of both parts of the genital system was visible in June.
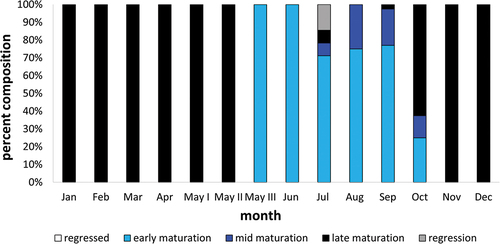
In July, August, and September, the gonads of most individuals were in early maturation class with continuous epithelium and lobules lumen filled with a newly-developed spermatozoa (). Some individuals reached mid and late maturation classes, and germinal epithelium becomes discontinuous. Only in two individuals regression class was noted. In the glandular part, in July, the lobules were filled with a similar amount of fluid as in June. However, in August and September, the amount of fluid was lower ().
In October, most individuals completed spermatogenesis, reaching late maturation class. Some individuals were still in early- and mid-maturation classes. From November, through winter and until the reproductive season, gonads contained only spermatozoa and resting spermatogonia A. In the glandular part, the lobules reduced their diameter, and the amount of fluid was small, if any. Most lobules had collapsed lumen. Detailed percentages of males with gonads at each reproductive class during the calendar year are shown in .
Figure 11. Percent composition of the number of Chinese sleeper (Perccottus glenii) male with testes in particular classes of maturity in individual months of the calendar year from Wilanówka River.
The smallest male attempting maturation was 3.7 cm (SL) in age 1+.
4. Discussion
The Chinese sleeper inhabiting Wilanówka River, Vistula tributary, was aged 0 ± 6+. Age 1+ was the most frequent among the collected individuals (35%). In studies conducted in the Vistula River by Grabowska et al. (Citation2011), the oldest individual was aged 7+, while the dominant age was 5+ (26%). Similarly in the Selenga River, Chinese sleepers reached the age of 7+ (Litvinov & O’Gorman Citation1996). In studies of Reshetnikov (Citation2001) conducted in Lake Glubokoe, older individuals, aged above 10 years, with a length above 25 cm were recorded. The standard length (SL) of the fish we analyzed was within the range 2.4–11.5 cm. In the studies of Terlecki and Pałka (Citation1999) in the Vistula River, the sizes were in the range of 1.5–9.0 cm. In the age group 1+ and 2+, the Chinese sleeper females from the Włocławski Reservoir were larger than males, and at the age of 3 ± 5+, males were larger than females (Grabowska et al. Citation2011). Our females and males aged 1+ and 2+ had a comparable SL, which was 5.11 cm and 5.24 cm, respectively, and in the age group 3+ to 5+ females were larger than males and measured 8.21 and 6.15 cm, respectively. Sex ratio of the analysed fish was 1:1, and was the same as in the studies of Bogutskaya and Naseka (Citation2002) and Grabowska et al. (Citation2011). This was probably due to the fact that there is already a stable population of Chinese sleeper in the Wilanówka River, similarly to the water areas from which individuals of this species were caught and analyzed by Bogutskaya and Naseka (Citation2002) and Grabowska et al. (Citation2011). In invasive species, the first stage of invasion is most often dominated by females (Feiner et al. Citation2012; Brandner et al. Citation2013), whose large share increases the reproductive potential of the population and further expansion. The Chinese sleeper ovary has a typical structure for most teleostean (Grier Citation2000; Grier et al. Citation2007, Citation2009; Cole & Parenti Citation2022). In Chinese sleeper, the GSI of females in spawning months was high (10–18) and low (2–6), due to multiple spawning, depending if the female was before laying eggs (first portion, high GSI) or after laying of the first or subsequent portion, which was also observed by other researchers (Bogutskaya & Naseka Citation2002). The prolonged spawning of the Chinese sleeper is caused not only by laying eggs in batches portions but also by irregular maturation of gonads in the spawning seasons (Bogutskaya & Naseka Citation2002), which was also observed in the analyzed fish from the Wilanówka River. Regarding Chinese sleeper, GSI shows the peak of spawning, before laying the first portion. However, due to multiple spawning, it does not show the whole spawning period. GSI during spawning in the Włocławski Reservoir was 3.69–8.28 for females, and 0.59–1.9 for males, mean 1.1 (Grabowska et al. Citation2011). In the Chinese sleeper population that we studied, the mean value of males during spawning was 1.0 and was similar to that recorded by Grabowska et al. (Citation2011) and in other gobioid fishes (Kovacic Citation2007; Hwang & Baek Citation2013). In the study males of Chinese sleepers, fluctuations of the mean GSI value in the reproductive cycle were not big, i.e. from 0.38 to 1.1. Small GSI changes may result from overlapping of spermatogenesis cycles, no resting period after spawning, presence of spermatozoa in the gonad virtually all calendar year round, and the presence of the glandular part which fills with the fluid synthesized thereby in the period when gametes are ejected from the germinal part. The Chinese sleeper sexes reveal exceptionally unequal investment in reproduction. The GSI of females was more than 8 times higher than in males. Similarly low GSI values in males (0.9) were recorded during spawning in striped goby (Gobius vittatus), while in female was about 5.9 (Kovacic Citation2007). In chameleon goby (Tridentiger trigonocephalus) the difference was greater 1.2 and 16.3 respectively (Hwang & Baek Citation2013). In other fish species, the mean GSI in males during spawning was above 1, and the differences in GSI between sexes were much smaller. In walleye (Sander vitreus), GSI in males and females was 3.2 and 7.6, respectively (Malison et al. Citation1994), and in stone moroko (Pseudorasbora parva) 2–2.4 and 8.6, respectively (Asahina et al. Citation1990; Kirczuk et al. Citation2021). In other cyprinids, GSI in males was higher, reaching a mean of 6–7 in rudd (Scardinius erythrophthalmus) and white bream (Blicca bjoerkna), and in females up to 10–15 (Domagała et al. Citation2015, Citation2020). Low investment in gonads shown by males of gobioid fishes may contribute to long-term reproductive capacity and energy spent on guarding the nest, contributing to the reproductive success of the species.
In our studies, the smallest mature female of Chinese sleeper spawning in the first reproductive season had 3.8 cm (SL). In Fishbase, the smallest maturation size of females was 6.0 cm (Kottelat & Freyhof Citation2007). In the population that we studied, maturity was reached by more than 90% of females in the first reproductive season and by all older females. Also, individuals from the Włoclawski Reservoir located in the Vistula River mature at 1+ (mean SL 5.24 cm) (Grabowska et al. Citation2011), earlier than in the natural habitat (2+, 3+ Kirpichnikov Citation1945; Nikolsky Citation1956) and in other areas where they were brought (Spanovskaya et al. Citation1964; Litvinov & O’Gorman Citation1996). In the Selenga River, 12% of Chinese sleepers matured in the first year of life, and the rest in the second year (Litvinov & O’Gorman Citation1996). In our study population, all the males were mature at age 1+, with the smallest measuring 3.7 cm (SL).
Spawning of Chinese sleeper in the analyzed Vistula tributary lasted from April to mid-September and was exceptionally long in comparison to the fish from previously examined sites in Poland, other regions of Europe, and native area. An extended period of reproduction makes this invasive species a potentially greater threat to native ichthyofauna (Cole & Parenti Citation2022). Spawning lasting from April until the end of August was recorded in the Włocławski Reservoir in the Vistula River (Grabowska et al. Citation2011), which was 3 months longer than that in the native area. In Baikal Basin, the spawning seasons were from May to July (Bogutskaya & Naseka Citation2002; Kottelat & Freyhof Citation2007).
In our studies, 5 groups of oocytes were distinguished in the Chinese sleeper gonads, while in the studies of Grabowska et al. (Citation2011), 2 groups of oocytes were distinguished: small (0.1–0.9 mm) and big (1.0–1.8 mm). Our studies are more accurate since the division enables the determination of which oocytes will mature in the current season (groups 1–4), and which represents a reserve for the next season (group 4–5). In our studies, absolute fecundity included only oocytes from groups 1–3, while Grabowska (Citation2011) **included all oocytes. If oocytes from all groups were combined (groups 1–5), the fecundity of the Chinese sleeper analyzed in our studies at the beginning of the reproductive age (in April) would mean 6,423 (1,353–26,866) per female. A comparison of this calculated fecundity of the Chinese sleeper from our analysis with the fecundity in the Włocławski Reservoir 7,766 (1,963–23,479) by Grabowska et al. (Citation2011) the values were similar. A surprising factor observed in our studies was high fecundity in September, amounting to 7,144 (1,264–11,361) (groups 1–5). However, in August and September, 17% and 8% of females, respectively, went for spawning. The prolonged spawning of the Chinese sleeper from Wilanówka River of the Vistula tributary indicates potentially good conditions in this watercourse for the reproduction of this species or the use of more precise research methods, like histological examinations. In Chinese sleeper from the Selenga River, fecundity was mean 19,765 eggs per one female, range from 884 to 37,056, and the highest number of oocytes/g fish was observed in 4- and 5-year-old fish (Litvinov & O’Gorman Citation1996). In the studies of Elovenko (Citation1981), the fish fecundity was in the range of 150–20,000. In the largest Asian individuals, fecundity reaches even 37,000 eggs (iop Citation2022). In our studies, the oocyte count per body weight increases with the fish age, but only in individuals before the first spawning (April). In individuals collected later, no such correlation is observed, probably due to multiple spawning. Chinese sleeper is characterized by multiple spawning, asynchronous development, a wide range of the number of portions laid, which was observed in its natural environment and in newly settled environments (Bogutskaya & Naseka Citation2002; Grabowska et al. Citation2011; iop Citation2022), also in our studies. The fecundity of the Chinese sleeper increases with the age and length of the female, which was observed in the Chinese sleeper from Wilanówka River and at other sites (Litvinov & O’Gorman Citation1996; Grabowska et al. Citation2011). It seems that the lower fecundity found in the Chinese sleeper from the studied waters, compared to other tested waters, is due to the fact that in the Wilanówka River the Chinese sleeper has adapted to the new environment and has already established a stable population. This is not a new phenomenon in invasive species, as it has been reported in other alien species (Copp & Fox Citation2007). It consists in the fact that females when colonizing new areas are characterized by greater fertility than individuals that have already inhabited this area (Bøhn et al. Citation2004). It seems that early maturation of females, multiple spawning and care of the eggs in the Chinese sleeper allow it to achieve reproductive success, even with low fertility.
The structure of the male gonad of the studied Chinese sleeper corresponds to the structure of the male tract of gobioid fishes with two parts, testis and testicular glands (Fishelson Citation1991; Miller Citation1992; Kovacic Citation2007; Cole & Parenti Citation2022). In the studied Chinese sleeper testis and testicular gland showed different colours. Morphologically, a testicular gland in Chinese sleepers is light, and the testis had a darker color. In representative of Odontobutidae family Chinese dark sleeper (Odontobutis sinensis), testis have no pigment (Zhou et al. Citation2022). Testicular glands do not occur in most teleosts (Thacker & Grier Citation2005). Fish males usually have paired testes and without additional reproduction glands (van den Hurk & Resink Citation1992). The gland occurs only in a few groups of fish species belonging to gobiid, blenniid, catfish, and a representative of the Grammatidae family. The shape, size, and location of the gland differ among species (Nayyar & Sundararaj Citation1970; Schoonen et al. Citation1987; Seiwald & Patzner Citation1989; Lahnsteiner et al. Citation1990; Fishelson Citation1991; Miller Citation1992; Leite et al. Citation2016). Testicular gland does not occur in all representatives of Gobidae (Burns & Cole Citation2017). The gland produces mucopolysaccharides, the main constituents of seminal fluid. The content of mucins allows the release of ejaculate in the form of viscous long-branch sperm trails (Mazzoldi et al. Citation2011). The secretory material within the secretory lobules of testicular glands is eosinophilic and PAS+ (Cole & Parenti Citation2022). Shown a positive reaction for acid phosphatase (Seiwald & Patzner Citation1989). Secretion cells contain large amounts of lipids, some secretory cells stain for 3β-hydroxy steroid dehydrogenase (3β-HSD) and glucose-6-phosphate dehydrogenase (G6PD). They synthesise steroids, mainly steroid glucuronides which may also play a role as pheromone (Seiwald & Patzner Citation1989). The size of testicular gland and testis as well as ejaculate performance may differ according to reproductive tactics of males in gobies (Mazzoldi et al. Citation2011). The study of the structure and function of the glandular part requires further, specialist research.
In the annual reproductive cycle of Chinese sleeper males, a trait similar to Perciformes (to which this species was previously classified) was found, i.e., finalization of spermatogenesis of the next reproductive cycle before the winter period (Malison et al. Citation1994; Sulistyo et al. Citation2000; Byczkiewicz et al. Citation2007). Males winter with gonads filled with spermatozoa (late maturation class; ripe stage). In the spring, in the spawning season, these species use gametes produced 5–6 months earlier. In salmonids, spermatogenesis is finalized just before spawning (Dziewulska Citation2001). In other fish species, gonads produce new spermatozoa during spawning (Domagała et al. Citation2014, Citation2016, Citation2020; Kirczuk et al. Citation2021). Gonads of gobioid fish studied by Kovacic (Citation2007) and Hwang and Baek (Citation2013) belong to the latter types finalizing spermatogenesis before or during spawning period while during winter were in growing or recovering spent stages. In numerous species, a new spermatogenesis cycle begins before the gonads are cleared of accumulated sperm (Domagała et al. Citation2014, Citation2016, Citation2020; Kirczuk et al. Citation2021). In others, there is a several-month resting/regressed period after spawning (Dziewulska Citation2001; Byczkiewicz et al. Citation2007). Chinese sleeper reveals an exceptionally early start of spermatogenesis of a new cycle and its rapid course with the formation of gametes as early as from June. This results in the mixing of the spermatozoa produced in winter with the newly produced spermatozoa from the next cycle. The newly developed spermatozoa are likely to be used in the current spawning. After spawning, in some males who finished spermatogenesis, the microscopic picture of the gonad showed, aside from typical lobules filled with spermatozoa, also other lobules with extended walls containing a small number of spermatozoa, or even empty with no spermatozoa. Such a picture of the gonad may be explained by the utilization of spermatozoa during the ongoing spawning, by the movement of the spermatozoa to the canal of the testicular gland, which may serve the purpose of storing gametes or by pressure on the gonads during fish preparation. Interpretation of this case requires further observation.
Conclusion
This study highlights the importance of understanding alien species life‐history traits to assess their invasiveness and vulnerability for developing an appropriate strategy to manage and eliminate the species from the aquatic environment. Especially since the range of the Chinese sleeper in Central Europe has expanded in recent years, and this species was indicated as invasive to European waters in Commission Implementing Regulation (EU) (Citation2016), and its negative impact on native species and their habitats has been documented.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andrzejewski W, Golski J, Mazurkiewicz J, Przybyl A. 2011. Trawianka Perccottus glenii - nowy, inwazyjny gatunek w ichtiofaunie dorzecza Warty [Amur sleeper Perccottus glenii - a new invasive alien species in the Warta River drainage basin] (W Poland). Chrońmy Przyrodę Ojczystą 67:4 [In Polish].
- Antychowicz J. 1994. Percottus glehni w naszych wodach [Percottus glehni in our waters]. Kom Ryb 2:21–22 [in Polish].
- Asahina T, Endoh N, Demura K, Niino T, Masatoshi T. 1990. Temperature dependences of the nonlinearity parameter of liquid-like media. Japanese Journal of Applied Physics 29. DOI: 10.7567/JJAPS.29S1.249.
- Bogutskaya NG, Naseka AM. 2002. Perccottus glenii Dybowski, 1877. Freshwater fishes of Russia. Zool Inst RAS. Available: http://www.zin.ru/Animalia/Pisces/eng/taxbase_e/species_e/perccottus/perccottus_e.htm.
- Bøhn T, Sandlund OT, Amundsen PAA, Primicerio R. 2004. Rapid changing life history during invasion. Oikos 106(1):138–150. DOI: 10.1111/j.0030-1299.2004.13022.x.
- Brandner J, Cerwenka AF, Schliewen UK, Geist J, Tsikliras AC. 2013. Bigger is better: Characteristics of round gobies forming an invasion front in the Danube River. PLoS One 8(9):e73036. DOI: 10.1371/journal.pone.0073036.
- Burns MD, Cole KS. 2017. Reproductive morphology and its application in testing molecular systematic hypotheses in the family Gobiidae (Teleostei, Gobiiformes). Journal of Fish Biology 91(4):1094–1108. DOI: 10.1111/jfb.13403.
- Byczkiewicz J, Domagała J, Dziewulska K. 2007. Development of male perch gonads (Perca fluviatilis L.) in the lower Oder region. The19th Congress of the Pol. Zool. Soc, 12-16 September, Olsztyn. pp. 89.
- Cole KS, Parenti LR. 2022. Gonad morphology of Rhyacichthys aspro (Valenciennes,1837), and the diagnostic reproductive morphology of gobioidfishes. Journal of Morphology 283(3):255–272. DOI: 10.1002/jmor.21440.
- Commission Implementing Regulation (EU). 2016/1141 of 13 July 2016 adopting a list of invasive alien species of union concern pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the council.
- Copp GH, Bianco PG, Bogutskaya NG, Erős T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kováč V, Moreno-Amich R, Naseka AM, Peňáz M, Povž M, Przybylski M, Robillard M, Russell IC, Stakėnas S, Šumer S, Vila-Gispert A, Wiesner C. 2005. To be, or not to be, a non-native freshwater fish? Journal of Applied Ichthyology 21(4):242–262. DOI: 10.1111/j.1439-0426.2005.00690.x.
- Copp GH, Fox MG. 2007. Growth and life-history traits of introduced pumpkinseed (Lepomis gibbosus) in Europe, and the relevance to its potential invasiveness. In: Gherardi F, editor. Biological invaders in inland waters: Profiles, distribution, and threats. Dordrecht: Springer. pp. 289–306.
- Domagała J, Dziewulska K, Kirczuk L, Pilecka-Rapacz M. 2015. Sexual cycle of white bream, Blicca bjoerkna (Actinopterygii, Cypriniformes, Cyprinidae), from three sites of the lower Oder River (NW Poland) differing in temperature regimes. Acta Ichthyologica et Piscatoria 45(3):285–298. DOI: 10.3750/AIP2015.45.3.07.
- Domagała J, Dziewulska K, Kirczuk L, Pilecka-Rapacz M. 2016. Sexual cycle of the blue bream (Ballerus ballerus) from the lower Oder River and Dąbie Lake (NW Poland). Turkish Journal of Fisheries and Aquatic Sciences 16(4):819–829. DOI: 10.4194/1303-2712-v16_4_08.
- Domagała J, Kirczuk L, Dziewulska K, Pilecka-Rapacz M. 2014. Annual development of gonads of pumpkinseed, Lepomis gibbosus (Actinopterygii: Perciformes: Centrarchidae) from a heated-water discharge canal of a power plant in the lower stretch of the Oder River, Poland. Acta Ichthyologica et Piscatoria 44(2):131–143. DOI: 10.3750/AIP2014.44.2.07.
- Domagała J, Kirczuk L, Dziewulska K, Pilecka-Rapacz M. 2020. The annual reproductive cycle of Rudd, Scardinius erythrophthalmus (Cyprinidae) from the lower Oder River and Lake Dąbie, (NW Poland). Folia Biologica 68(1):23–33. DOI: 10.3409/fb_68-1.04.
- Dziewulska K. 2001. Spermatogeneza młodzieży troci wędrownej (Salmo trutta m. trutta L.) Pomorza Zachodniego [Spermatogenesis in the sea trout (Salmo trutta m. trutta L.) parr in Western Pomerania] [ Ph. D. Thesis]. Szczecin University. pp. 102 [in Polish].
- Elovenko VN. 1981. Sistematicheskoe polozhenie igeograficheskoe rasprostranenie ryb semeystva Eleotridae (Gobioidei, Perciformes), introdutsirovannykh v vodoemy Evropeyskoy chasti SSSR, Kazakhstana i Sredney Azii [Taxonomic status and geographical distribution of the fishes (Gobioidei, Perciformes, Eleotridae) introduced in waterbodies of the European part of the USSR, Kazakhstan and Middle Asia]. Zool Zh 60:1517–1522.
- Feiner ZS, Aday D, Rice JA. 2012. Phenotypic shifts in white perch life history strategy across stages of invasion. Biological Invasions 14(11):2315–2329. DOI: 10.1007/s10530-012-0231-z.
- Fishelson L. 1991. Comparative cytology and morphology of seminal vesicles in male gobiid fishes. Japanese Journal of Ichthyology 38(1):17–30. DOI: 10.1007/BF02910104.
- Froese R, Pauly D. 2023. World wide web electronic publication. FishBase. www.fishbase.org, version February. Accessed February 2023.
- Fulga N, Todierasz I, Bulad D, Rajlan N. 2015. Morfologiczeskaja characteristica gonad polowozriwlych samok Perccottus Glenii Dubowski, 1877 reki Lopatna [Morphological and physiological characteristics of the gonads of mature females Perccottus glenii DYBOWSKI, 1877 in Lopatna river]. Studia Universitatis Moldaviae 1:125–131.
- Golovanova IL, Smirnov A, Shlyapkin LV. 2009. The influence of temperature on the activities of digestive carbohydrases in Amur sleeper (Perccottus glenii Dyb.) during the winter. Inland Water Biology 2(2):187–189. DOI: 10.1134/S1995082909020126.
- Grabowska J, Pietraszewski D, Przybylski M, Tarkan AS, Marszał L, Lampart-Kałuzniacka M. 2011. Life-history traits of Amur sleeper, Perccottus glenii, in the invaded Vistula River: Early investment in reproduction but reduced growth rate. Hydrobiologia 661(1):197–210. DOI: 10.1007/s10750-010-0524-0.
- Grier HJ. 1981. Cellular organization of the testis and spermatogenesis in fishes. American Zoologist 21(2):345–357. DOI: 10.1093/icb/21.2.345.
- Grier HJ. 2000. Ovarian germinal epithelium and folliculogenesis in the common snook, Centropomus undecimalis (Teleostei: Centropomidae). Journal of Morphology 243(3):265–281. DOI: 10.1002/(SICI)1097-4687(200003)243:3<265:AID-JMOR4>3.0.CO;2-I.
- Grier HJ. 2002. The germinal epithelium: Its dual role in establishing male reproductive classes and understanding the basis for indeterminate egg production in female fishes. In: Creswell R, editor. Proceedings of the Fifty-Third Annual Gulf and Caribbean Fisheries Institute, November 2000. Fort Pierce, FL: Mississippi/Alabama Sea Grant Consortium. pp 537–552.
- Grier HJ, Uribe MC, Parenti R. 2007. Germinal epithelium, folliculogenesis, and postovulatory follicles in ovaries of rainbow trout, Oncorhynchus mykiss (Walbaum, 1792) (Teleostei, protacanthopterygii, salmoniformes). Journal of Morphology 268(4):293–310. DOI: 10.1002/jmor.10518.
- Grier HJ, Uribe MC, Patiño R. 2009. The ovary, folliculogenesis and oogenesis in teleosts. In: Jamieson B, editor. Reproductive biology and phylogeny of fishes (agnathans and bony fishes). Vol. 8A. Enfield, NH: Science Publishers. pp. 25–84. DOI:10.1201/9781482280609.
- Holčík J, Hensel K. 1972. Handbook of ichthyology. Bratislava, Czechoslovakia: Obzor. p. 220.
- Horvatić S, Zanella D, Marčić Z, Mustafić P, Buj I, Onorato L, Ivić L, Karlović R, Ćaleta M. 2022. First report of the Chinese sleeper Perccottus glenii Dybowski, 1877 in the Drava River, Croatia. BioInvasions Records 11(1):250–266. DOI: 10.3391/bir.2022.11.1.26.
- Huang S, Wang Y, Zheng X, Wang W, Cao X. 2015. Comparative analysis of three methods of making scale specimens for small fish. Environmental Biology of Fishes 98(2):697–703. DOI: 10.1007/s10641-014-0299-7.
- Hwang IJ, Baek HJ. 2013. Reproductive cycle of chameleon goby, Tridentiger trigonocephalus in the Southern Coastal Waters of Korea. Development & Reproduciton 17(4):353–361. PMID: 25949151; PMCID: PMC4382946. DOI: 10.12717/DR.2013.17.4.353.
- iop Kraków. 2022. https://www.iop.krakow.pl/gatunkiobce/default7473.html.
- Kautman J. 1999. Perccottus glenii Dybowski, 1877 vo vodách východného Slovenska [Perccottus glenii Dybowski 1877 from East Slovakian water bodies]. Chránené územia Slovenska 40:20–22 [in Slovak].
- Kirczuk L, Dziewulska K, Czerniejewski P, Brysiewicz A, Rząd I. 2021. Reproductive Potential of Stone Moroko (Pseudorasbora parva, Temminck et Schlegel, 1846) (Teleostei: Cypriniformes: Gobionidae) Inhabiting Central Europe. Animals 11(9):2627. DOI: 10.3390/ani11092627.
- Kirilenko EV, Shemonaev EV. 2015. Intersexual specimen of the Amur sleeper Perccottus gleni (Odontobutidae). Journal of Ichthyology 55(2):285–288. DOI: 10.1134/S0032945215020095.
- Kirpichnikov VS. 1945. Biologia Perccottus glehni Dyb. (Eleotridae). Biull Moskov Obsc Ispytat Prirody s Biol 5-6:14–26.
- Koščo J, Manko P, Miklisová D, Košuthová L. 2008. Feeding ecology of invasive Perccottus glenii (Perciformes, Odontobutidae) in Slovakia. Czech Journal of Animal Science 53(11):479–486. DOI: 10.17221/340-CJAS.
- Kottelat M, Freyhof J. 2007. Handbook of European freshwater fishes. Berlin: Publications Kottelat, Cornol and Freyhof. p. 646.
- Kovacic M. 2007. Reproductive biology of the striped goby, Gobius vittatus (Gobiidae) in the northern Adriatic Sea. Scientia Marina 71(1):145–151. DOI: 10.3989/scimar.2007.71n1145.
- Kvach Y, Karavanskyi Y, Tkachenko P, Zamorov V. 2021. First record of the invasive Chinese sleeper, Perccottus glenii Dybowski, 1877 (Gobiiformes: Odontobutidae) in the Black Sea. BioInvasions Records 10(2):411–418. DOI: 10.3391/bir.2021.10.2.19.
- Lahnsteiner F, Richtarski U, Patzner RA. 1990. Functions of the testicular gland in two blenniid fishes, Salaria (=Blennius) pavo and Lipophrys (= Blennius) dalmatinus (Blenniidae, Teleostei) as revealed by electron microscopy and enzyme histochemistry. Journal of Fish Biology 37(1):85–97. DOI: 10.1111/j.1095-8649.1990.tb05930.x.
- Leite JR, Freitas MO, Sanches EG, Gomes MLM, Hostim-Silva M, Cole KS. 2016. Rediscovering hermaphroditism in Grammatidae with the description of the testicular gland in Brazilian Basslet Gramma brasiliensis. Brazilian Journal of Biology 76(3):743–749. DOI: 10.1590/1519-6984.03115.
- Litvinov AG, O’Gorman R. 1996. Biology of Amur sleeper (Perccottus glehni) in the delta of the Selenga River, Buryatia, Russia. Journal of Great Lakes Research 22(2):370–378. DOI: 10.1016/S0380-1330(96)70962-0.
- Luca M, Giorghită G. 2014. The invasive species Perccottus glenii – a threat for the fresh water ecosystems. Analele Științifice UAIC s. Biologie animală 50:129–138.
- Malison JA, Procarione LS, Barry TP, Kapuscinski AR, Kayes TB. 1994. Endocrine and gonadal changes during the annual reproductive cycle of the freshwater teleost, Stizostedion vitreum. Fish Physiology and Biochemistry 13(6):473–484. DOI: 10.1007/BF00004330.
- Mazzoldi C, Patzner RA, Rossotto MB. 2011. Morphological organization and variability of the reproductive apparatus in gobies. In: Patzner R, Van Tassell JL, Kovacic M, Kapoor BG, editors. The biology of gobies. New York: CRC Press, Taylor & Francis Group. pp. 368–402.
- Miller PJ. 1992. The sperm duct gland: A visceral synapomorphy for gobioid fishes. Copeia 1992(1):253–256. DOI: 10.2307/1446565.
- Mineev AK. 2009. Histological anomalies in the gonads of round goby (Perccottus glenii Dibowski, 1877) and Chinese sleeper (Neogobius melanostomus Pallas, 1814) of Saratov Reservoir. Izvestiya Samarskogo Nauchnogo Tsentra RAN 11:185–191.
- Movchan YV. 1989. The first record of Perccottus glehni Dybowski (Pisces, Eleotridae) in the water bodies of the Ukraine. Vest Zool 5:87.
- Nalbant T, Battes K, Pricope F, Ureche D. 2004. First record of Amur sleeper Perccottus glehnii (Pisces: Perciformes: Odontobutidae) in Romania. Travaux du Museum National d’Histoire Naturelle Grigore Antipa 47:279–284.
- Nayyar SK, Sundararaj BI. 1970. Seasonal reproductive activity in the testes and seminal vesicles of the catfish, Heteropneustes fossilis (Bloch). Journal of Morphology 130(2):207–225. DOI: 10.1002/jmor.1051300207.
- Nikolsky GV. 1956. Ryby basseyna Amura. Itogi Amurskoy Ikhthyologicheskoy Ekspedicii 1944–1949. Izdatielstvo Akademii Nauk SSSR. Moskva. pp. 1–551 [in Russian].
- Parenti LR, Grier HJ. 2004. Evolution and phylogeny of gonad morphology in bony fishes. ICB 44(5):333–348. DOI: 10.1093/icb/44.5.333.
- Rau MA, Plavan G, Strungaru SA, Pablo MN, Ureche A, Mihu-Pintilie D, Klimaszyk P. 2017. The impact of amur sleeper (Perccottus glenii Dybowsky, 1877) on the riverine ecosystem: Food selectivity of amur sleeper in a recently colonized river. Oceanological and Hydrobiological Studies 46(1):96–107. DOI: 10.1515/ohs-2017-0010.
- Rechulicz J, Płaska W, Nawrot D. 2015. Occurrence, dispersion and habitat preferences of Amur sleeper (Perccottus glenii) in oxbow lakes of a large river and its tributary. Aquatic Ecology 49(3). DOI:10.1007/s10452-015-9532-5.
- Repečka R. 2003. The species composition of the ichthyofauna in the Lithuanian economic zone of the Baltic Sea and the Curonian Lagoon and its changes in recent years. Acta Zoologica Lituanica 13(2):149–157. DOI: 10.1080/13921657.2003.10512558.
- Reshetnikov AN. 2001. Vliyanie introducyovannoy ryby rotana Perccottus glenii (Odontobutidae, Pisces) na zemnovodnykh v malykh vodoemakh Podmoskovya [Influence of introduced fish Percottus glenii (Odontobutidae, Pisces)]. Zhurnal Obshchei Biologii 62(4):352–361 [in Russian].
- Reshetnikov AN, Ficetola GF. 2011. Potential range of the invasive fish rotan (Perccottus glenii) in the Holarctic. Biological Invasions 13(12):2967–2980. DOI: 10.1007/s10530-011-9982-1.
- Reshetnikov AN, Schliewen UK. 2013. First record of the invasive alien fish rotan Perccottus glenii Dybowski, 1877 (Odontobutidae) in the Upper Danube drainage (Bavaria, Germany). Journal of Applied Ichthyology 29(6):1367–1369. DOI: 10.1111/jai.12256.
- Rosecchi E, Crivelli A, Catsadorakis G. 1993. The establishment and impact of Pseudorasbora parva, an exotic fish species introduced into Lake Mikri Prespa (northwestern Greece). Aquatic Conservation: Marine & Freshwater Ecosystems 3(3):223–231. DOI: 10.1002/aqc.3270030306.
- Schoonen W, Granneman JCM, Lambert JGD, van Oordt PGW. 1987. Steroidogenesis in the testes and seminal vesicles of spawning and non-spawning African catfish, Clarias gariepinus. Aquaculture 63(1–4):1–4, 77–88. DOI: 10.1016/0044-8486(87)90062-7.
- Seiwald M, Patzner RA. 1989. Histological, fine-structural and histochemical differences in the testicular glands of gobiid and blenniid fishes. Journal of Fish Biology 35:631–640. DOI: 10.1111/j.1095-8649.1989.tb03015.x.
- Semenchenko V, Grabowska J, Grabowski M, Rizevsky V, Pluta M. 2011. Non-native fish in Belarusian and Polish areas of the European central invasion corridor. Oceanological and Hydrobiological Studies 40(1):57–67. DOI: 10.2478/s13545-011-0007-6.
- Sokal RR, Rohlf FJ. 1995. Biometry the principles and practice of statistics in biological research. 3rd ed. New York: W.H. Freeman and Co.
- Spanovskaya VD, Savvaitova KA, Potapova TL. 1964. Ob izmenchivosti rotana (Perccottus glenii Dyb. fam. Eleotridae) pri akklimatizatsii. [About the variability of Amur sleeper (Perccottus glenii Dyb. fam. Eleotridae) under acclimatization]. Vopr Ikhtiol 4:632–643 [in Russian].
- Steinmetz B, Müller R. 1991. An atlas of fish scales and other bony structures used for age determination: Non-salmonid species found in European fresh waters. Tresaith, UK: Samara Publishing Ltd. p. 51.
- Sulistyo I, Fontaine J, Gardeur JN, Migaud H, Capdeville B, Kestemont P. 2000. Cycle de reproduction et teneurs en stéroïdes du plasma chez la perche eurasienne mâle Perca fluviatilis. Aquatic Living Resources 13(2):99–106. DOI: 10.1016/S0990-7440(00)00146-7.
- Terlecki J 1995. Percottus glenii Dybowski 1877 (Pisces, Eleotridae) w Polsce. [Percottus glenii Dybowski 1877 (Pisces, Eleotridae) in Poland.]. In: Proceedings of the 11th Congress of the Polish Zoological Society, 14–16 September. Łódź, Poland. p. 163 [In Polish].
- Terlecki J, Pałka R. 1999. Occurrence of Perccottus glenii Dybowski, 1877 (Perciformes Odontobutidae) in middle stretch of the Vistula River, Poland. Archives of Polish Fisheries 7:141–150.
- Thacker C, Grier H. 2005. Unusual gonad structure in the paedomorphic teleost Schindleria praematura (Teleostei: Gobioidei): A comparison with other gobioid fishes. Journal of Fish Biology 66(2):378–391. DOI: 10.1111/j.0022-1112.2005.00603.x.
- van den Hurk R, Resink JW. 1992. Male reproductive system as sex pheromone producer in teleost fish. The Journal of Experimental Zoology 261(2):204–213. DOI: 10.1002/jez.1402610211.
- West G. 1990. Methods of assessing ovarian development in fishes: A review. Marine & Freshwater Research 41(2):199–222. DOI: 10.1071/MF9900199.
- Witkowski A, 2008. Amur sleeper (Perccottus glenii). In: Głowaciński Z, Okarma H, Pawłowski J, Solarz W, editors. Invasive alien species - Polish database. Inst. Ochr. Śród. PAN. http://www.iop.krakow.pl//ias/species.asp?215.
- Witkowski A, Grabowska J. 2012. The non-indigenous freshwater fishes of Poland: Threats to the native Ichthyofauna and consequences for the fishery: A review. Acta Ichthyologica et Piscatoria 42(2):77–87. DOI: 10.3750/AIP2011.42.2.01.
- Zhou L, Yang R, Tian H, Qin X, Cheng Y, Shi X, Xia C, Cai T, Xie Y, Jia Y, Hu G. 2022. Sexual dimorphism in Odontobutis sinensis brain-pituitary-gonad axis and liver highlighted by histological and transcriptomic approach. Gene 819:146264. DOI: 10.1016/j.gene.2022.146264.