Abstract
Two complete nests of Pterocheilus phaleratus (Panzer, 1797) were described from the north-eastern edge of the Pomeranian Flying Club in Toruń (northern Poland). Both nests were built in sandy terrain on a sloping surface (about 20°). The nests consists of a 5 cm long main burrow with a single cell. Digging and backfilling the nest involves carrying fine gravel in the mandibles. Females transported prey at a frequency of 6 to 12 minutes. The cell contained 25 larvae from the Tortricidae family (Acleris spp.). Metopia cf. argyrocephala (Meigen, 1824) (Diptera: Sarcophagidae) was observed following the female into the nest, but specimens of this species were not excavated from the cell.
Introduction
Pterocheilus Klug, 1805 is essentially a Holarctic genus of solitary wasps with a fairly rich diversity in North America (Bohart Citation1940) and one Afrotropical species, Pterocheilus eurystomus Kohl, 1906. In contrast to other genera, both males and females are characterised by very elongated 3-segmented labial palps, with their segments flattened and covered with long light hair on the sides (Schmid-Egger Citation2002).
Pterocheilus (s. str.) phaleratus has been recorded from Europe, Georgia, Azerbaijan, Turkey, Kazakhstan, Mongolia, and Siberia (Fateryga & Mokrousov Citation2019). The nominative subspecies, P. phaleratus phaleratus (Panzer, 1797), occurs in Poland.
Information on the nesting biology of P. phaleratus is limited to a few articles. It nests in sand and the nest consists of a vertical burrow only one centimetre long (Schultess-Rechberg Citation1887), at the end of which there is a horizontal cell that may contain five (Ferton Citation1909) or seven larvae probably belonging to the family Psychidae (Nielsen Citation1942). Tormos et al. (Citation1997) presented a description of the mature larva of P. phaleratus yeguasicus Blüthgen, 1951.
Digging or sealing the main burrow by moving gravel is similar to the genus Ammophila Kirby, 1798 (Nielsen Citation1942). The egg in the cell is attached to the root fibre with a short thread (Ahrens Citation1924; Nielsen Citation1942). Ahrens (Citation1924) found a single horizontal cell at a depth of 3 cm at the end of the channel. Jansson (Citation1922) and Erlandsson (Citation1968) speculate that the chrysidid Spinolia unicolor (Dahlbom, 1831) may be a nest parasite of P. phaleratus.
Haeseler (Citation1975, Citation1978) reported that the species visited flowers of Pilosella officinarum Vaill., Convolvulus arvensis L., Medicago lupulina L., Trifolium dubium Sibth., Anchusa officinalis L. and Lotus corniculatus L.
Information on the biology of the New World species of Pterocheilus is also scarce: Bohart (Citation1940) presented a revision of the North American species of Pterocheilus and notes on related genera. Evans (Citation1956) published a short note and description of a P. (Megapterocheilus) texanus (Cresson, 1872) nest, followed by Habeck et al. (Citation1974) briefly described instances of prey capture by P. texanus. Grissell (Citation1975) presented ethological data and described the P. texanus larva. Iwata (Citation1976) also reviewed the biology of this genus. Isley (Citation1914) and Evans (Citation1977) described nests and provision of P. (Megapterocheilus) quinquefasciatus (Say, 1824), while Evans (Citation1977) described nests and provision of P. (Onchopterocheilus) laticeps (Cresson, 1872).
The objective of this study is to provide the first comprehensive information on the nesting of P. phaleratus in Poland, including: 1) nest digging and transport of prey to the nest; 2) prey range, including species and number of individuals per nest; 3) nest structure: determination of the number of cells, length and diameter of the main burrow and vertical section of the nest.
Material and methods
Our research was conducted at the airfield of the Pomeranian Flying Club in Toruń (53°01′54′′N; 18°33′21′′E) in northern Poland from mid-July to mid-August 2023, on sunny and warm days (with temperatures ranging from 25°C to 35°C). The airport and its immediate surroundings cover over 200 ha of open grassland habitat, on the periphery of which loose groves of oak and pine have developed.
The nest observation site was an area of 5 m2 of sandy grassland, with species such as Berteroa incana (L.) DC.; Calluna vulgaris (L.) Hull., Corynephorus canescens L.; Dianthus caryophyllus L.; Euphorbia cyparissias L.; Fragaria viridis Weston; Galium sp.; Jasione montana L.; Peucedanum oreoselinum (L.); Scabiosa ochroleuca L.; Solidago virgaurea L.; Thymus serpyllum L. and Verbascum sp., growing around it ().
Photographs were taken with Canon EOS M50 and Canon PowerShot S×160IS cameras. Furthermore, a Raynox M-250 macro converter and direct observations were used. The range of prey was determined by nest inspection. The structure of the nest was analysed by wetting the sand and digging gently.
After the female had finished transporting her prey, her nest was unearthed and the prey collected for analysis. An attempt to breed P. phaleratus larvae under laboratory conditions failed. The prey and a specimen of a kleptoparasitic fly flying near the nest have been deposited in the author’s collection.
Results
In order to find a suitable place to dig the main burrow, the female flies short distances, activating her mandibles. Once the site is located, the female starts digging the nest in a characteristic way. All nests located were on a sloping surface (approximately 20°).
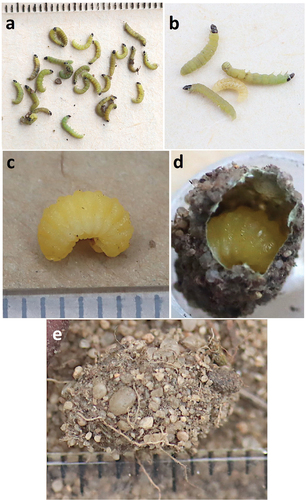
The first nest consists of a 5 cm long burrow. In vertical section, the main burrow descends to a depth of approximately 3.5 cm and then almost horizontally to the side for a length of 3 cm, ending in a single cell (N = 2; ). The diameter of the burrow ranges from 4 to 5 mm. The nest entrance is without a mound, unlike Oxybelus Latreille, 1797; Tachysphex Kohl, 1883; Lindenius Lepeletier & Brullé, 1835, which dig with their legs (). On 16 August 2023, the female started bringing prey to the first nest around 1.45 p.m. to 5.15 p.m. She transported them in flight, supporting them with her mandibles and partly with her legs (). After transporting the prey to the nest, she remained there for about 15 to 20 seconds (N = 15). After depositing the last prey, the female stepped 10 cm outside the entrance and started fetching gravel particles or small pebbles, throwing them into the burrow, and repeated this action. During this time, the structure of the nest was determined. On 17 August 2023, 25 live Tortricidae larvae of the genus Acleris sp. (probably Acleris notana (Donovan, 1806) or Acleris ferrugana (Denis & Schiffermuller, 1775) were deposited in the cell (). Unfortunately, the species affiliation could not be determined. The moth larvae were mature larvae and measured approximately 5 mm in body length. The second nest of the same female of P. phaleratus was built the following day a short distance away (12 cm). However, the female did not bring food to this nest. She was away from the nest for a long time, coming to the nest 3 times in 4 h. The female digs the burrow in a characteristic way using her labial palps (). The material collected during channel digging is removed while hovering in the air for short distances of 12–20 cm. In the last phase of digging, the female empties the nest with a frequency of 8 to 15 seconds. After finishing digging the nest, the female emerged with her back to the burrow. On 18 August, after several hours of searching, the same female was digging another nest within a short distance from the one she had built earlier (15 cm).
Figure 2. Nest of Pterocheilus phaleratus. (a) Lateral view of the nest and brood cells; (b–d) top view of nest entrances; (e–f) nest digging.
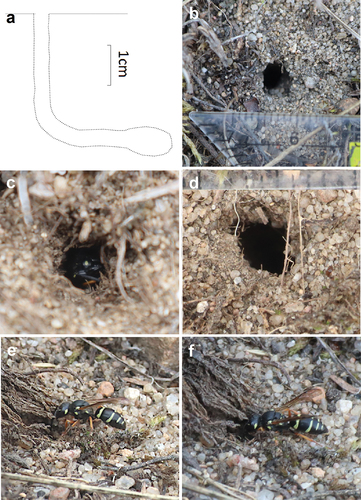
Figure 3. Adult Pterocheilus phaleratus. (a–b) female with prey at the nest entrance; (c) male perching on a withered plant stem; (d) male on Thymus serpyllum; (e) female in flight near the nest.
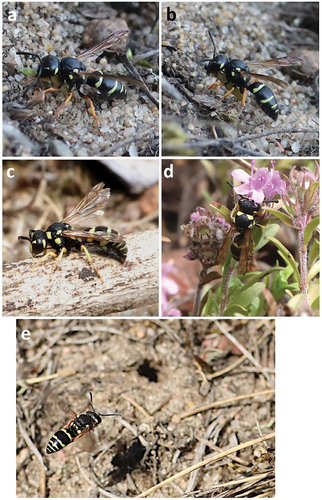
Figure 4. Pterocheilus phaleratus. (a–b) Prey; (b) Prey and feeding larva; (c) Mature larva; (d–e) Cocoon.
On 19 August, the female began provisioning the cell of the third nest. During digging on 27 August, a cocoon 10 mm long and 6 mm in diameter, hard, filled the surface of the nest cell. It consisted of two layers. The outer layer was covered with small pebbles (which formed a concavity on the inner side) and tiny pieces of roots. The inner layer it was smooth pale with a distinct darkening in the lower part. The larva (prepupae) in the cocoon was yellow, measuring 7.5 mm in length ().
No nest kleptoparasites were observed entering the nest. One kleptoparasitic fly Metopia cf. argyrocephala (Meigen, 1824) was flying near the wasp’s nest at a short distance.
In mid-August, females and the male of P. phaleratus () were mainly observed on wild thyme flowers (Thymus serpyllum L.; ) occurring in a short distance from the nest.
Nectar robbing was observed for the male of P. phaleratus on flowers of Thymus serpyllum (). The time spent on the plant was approximately 20–25 seconds.
Discussion
The genus Pterocheilus remains one of the less studied groups of Eumeninae in terms of nesting biology (Evans Citation1977). Our data on P. phaleratus regarding the nest structure, prey for larvae and breeding behaviour of females mostly agree with the observations by Nielsen (Citation1942). Nielsen reported that the species probably preys on larvae of moths from the Psychidae family, and Ferton (Citation1909) and Nielsen (Citation1942) reported that the maximum number of prey in one cell was 5–7 specimens. The egg in the cell is attached to a root with a thread that is several dozen times shorter than the egg itself (it may be ¼ the length of the egg).
In my study, the prey excavated from the cell belonged to the genus Acleris spp. (Lepidoptera: Tortricidae), probably representing one of the two species: Acleris ferrugana (Denis & Schiffermüller, 1775) feeding on oak (Lepiforum 2023) or Acleris notana (Donovan, 1806) feeding mainly on birch (Razowski Citation1984). This assumption seems likely, as both tree species grew at a short distance (3–4 meters) from the nest. Prey of P. texanus Cresson, 1872, a Nearctic species, were late instar larvae of Schinia mitis (Smith, 1891) (Lepidoptera: Noctuidae), collected over a period of 1 to 2 h, with the nest open throughout this time. Habeck et al. (Citation1974) observed the female of P. texanus entering the flower heads and each time she retreated she dragged a mature larva behind her, which she immediately paralysed with her sting. The female wasp transports the larvae (three to nine larvae per cell) into her nest (Grissell Citation1975).
According to Erlandsson (Citation1968), P. phaleratus lives in colonies, but no other nest or other female was observed at the nesting site during our observations.
The cuckoo wasp chrysidid Spinolia unicolor (Dahlbom, 1831) is probable a nest parasite of P. phaleratus (Jansson Citation1922; Erlandsson Citation1968). Unfortunately, this species was not found at the study site. One kleptoparasitic fly Metopia argyrocephala (Meigen, 1824) was observed following the female to the nest, but no individual of this species was dug out from the cell.
In nest of P. phaleratus we can see the similarity in structure and digging and burrowing habit to the genus Ammophila W. Kirby, 1798 (Hymenoptera: Sphecidae).
Nielsen (Citation1942) interprets the nest of Pterocheilus as evolved from the typical nest of Odynerus Latreille, 1802, who nests in loess walls, clay slopes or on old clay-covered buildings. Such a nest contains several nest cells around the main burrow. A characteristic feature is the presence of a chimney at the inlet to the nest, often curving downwards. It is made of lumps of excavated soil glued together with saliva during the construction. Chimneys are usually openwork in structure and very fragile (Iwata Citation1976).
Acknowledgments
I am very grateful to Jarosław Buszko (Nicolaus Copernicus University, Poland) for the identification of Acleris spp. (Lepidoptera: Tortricidae) and Alexander Fateryga (T.I. Vyazemsky Karadag Scientific Station – Nature Reserve of RAS, Russia) for critical review of the manuscript and to one anonymous reviewer for their helpful comments.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Ahrens LE. 1924. Sur la biologie et la systematique de Pterochilus chevrieranus Sauss. (Hymenoptera, Eumenidae). Revue Russe d’Entomologie 18:175–180.
- Bohart RM. 1940. A revision of the north American species of Pterocheilus and notes on related genera (Hymenoptera, Vespidae). Annals of the Entomological Society of America 33(1):162–208. DOI: 10.1093/aesa/33.1.162.
- Erlandsson S. 1968. The occurrence of the solitary wasp Pterochilus phaleratus Panz. In the Scandinavian countries (Hym. Eumenidae). Entomologisk Tidskrift 89:173–176.
- Evans HE 1956. Notes on the biology of four species of ground-nesting Vespidae (Hymenoptera). Proceedings of the Entomological Society of Washington 58:265–270.
- Evans HE. 1977. Notes on the nesting behavior and immature stages of two species of Pterocheilus (Hymenoptera: Eumenidae). Journal of the Kansas Entomological Society 50(3):329–334.
- Fateryga AV, Mokrousov MV. 2019. New records of eumenine wasps (Hymenoptera: Vespidae: Eumeninae) from Russia with description of a new species of Leptochilus de Saussure, 1853. Zootaxa 4612(3):412–422. DOI: 10.11646/zootaxa.4612.3.7.
- Ferton C. 1909. Notes détachées sur l’instituct des Hyménoptères mellifères et ravisseurs (5e série) avec la description d’une espèce nouvelle. Annales de la Société entomologique de France 78:401–422.
- Grissell EE. 1975. Ethology and larva of Pterocheilus texanus (Hymenoptera: Eumenidae). Journal of the Kansas Entomological Society 48:244–253.
- Habeck DH, Arbogast RT, Cline LD. 1974. Biology and immature stages of Schinia mitis (Grote) Noctuidae. Journal of the Lepidopterists Society 28(2):152–157.
- Haeseler V. 1975. Pterocheilus phaleratus (Hymenoptera: Vespoidea), ein Nektardieb an den Blüten von Lotus corniculatus (Fabales: Fabaceae). Entomologica Germanica 1(3–4):213–221. DOI: 10.1127/entom.germ/1/1975/213.
- Haeseler V. 1978. Flugzeit, Blütenbesuch, Verbreitung und Häufigkeit der solitären Faltenwespen im Norddeutschen Tiefland (BRD) (Hymenoptera: Vespoidea: Eumenidae). Schriften des Naturwissenschaftlichen Vereins für Schleswig-Holstein 48:63–131.
- Isley D. 1914. The biology of some Kansas Eumenidae. Kansas University Science Bulletin 8:233–309.
- Iwata K. 1976. Evolution of instinct. Comparative ethology of Hymenoptera. New Delhi: Amerind Publishing Co. Pvt. Ltd. Translated from the Japanese edition of 1971; p. 535.
- Jansson A. 1922. Faunistiska och biologiska studier över insektlivet vid Hornsjön på norra Öland. Arkiv för Zoologi 14(23):1–81. DOI: 10.5962/bhl.part.7724.
- Nielsen ET. 1942. Sur les habitudes des Hyménoptères aculéates solitaires; II. Entomologiske Meddelelser 18(2):84–174.
- Razowski J 1984. Tortricini. – Microlepidoptera Palaearctica 6: Textband: I–XV, 1–376, Tafelband, pl. 1–101. Braun (Verlag G. Braun). Available: https://lepiforum.org/wiki/page/Acleris_ferrugana Accessed Oct 2023 1.
- Schmid-Egger C. 2002. Schlüssel für die deutschen Arten der solitären Faltenwespen (Hymenoptera: Vespidae: Eumeninae). Zweite, überarbeitete und ergänzte Ausgabe. Available: http://www.bembix.de/publicationen_pdf/Schmid-Egger%202002%20Eumenidae%20Deutschland.pdf
- Schultess-Rechberg AV. 1887. Fauna insectorum helvetiae, Hymenoptera, Diploptera. Bulletin de la Société entomologique suisse 7.
- Tormos J, Asis JD, Gayubo SF. 1997. Description of the mature larva of Pterocheilus phaleratus yeguasicus. Fragmenta Entomologica 29(2):395–398.

