Abstract
A new maternity colony of lesser horseshoe bats (Rhinolophus hipposideros) was found and described in an abandoned building in Aljezur, Portugal. Between June 25 and July 4, 2022, we conducted emergence and juvenile counts, took light intensity measurements during emergence times and evaluated microclimatic conditions inside the roost, namely temperature and relative humidity. Moreover, we took ultrasound recordings in the open and performed transects to locate foraging areas and commuting corridors. We found the colony amounted to at least 501 individuals (371 adults and 130 juveniles), currently the largest known in Portugal and one of the biggest in Europe. Sixteen matching echolocation calls were recorded away from the colony near a water reservoir, a probable foraging area together with the Vale da Telha valley connecting the roost to the water body, which may also serve as a commuting corridor. Considering the threatened conservation status of the lesser horseshoe bat, the large colony size and its lack of protection, we proposed several conservation measures aimed at preserving the colony. We hope our recommendations will be included in the management plan currently being prepared for the Natura 2000 site Costa Sudoeste. Following up on this research, a radio-tracking study would definitively confirm the bats’ movements and foraging grounds.
Introduction
The lesser horseshoe bat (Rhinolophus hipposideros Bechstein, 1800) has its core distribution in the Mediterranean region, ranging north-west to Ireland and Wales and north-east to Poland and Ukraine (Schober Citation1998; Dietz et al. Citation2009). Habitats preferred by this species as foraging areas are woodlands and riparian zones (Schofield Citation1996; Bontadina et al. Citation2002; Motte & Libois Citation2002; Russo & Jones Citation2003; Reiter Citation2004; Tournant et al. Citation2013). Foraging areas are usually situated within a 2.5 km range from the colony (Bontadina et al. Citation2002; Holzhaider et al. Citation2002; Motte & Libois Citation2002; Kokurewicz et al. Citation2008; Reiter et al. Citation2013), which can reach up to 4 km depending on the reproductive status of the bats and the size and type of their roosts (Bontadina et al. Citation2002; Reiter et al. Citation2013). During commuting flights, lesser horseshoe bats follow linear landscape elements such as tree lines and hedgerows. A distinctive trait of the species is avoidance of open spaces, which makes them sensitive to the lack of linear elements in the landscape (Bontadina et al. Citation2002; Holzhaider et al. Citation2002; Motte & Libois Citation2002; Kokurewicz et al. Citation2008; Zahn et al. Citation2008). When the linear structures are destroyed or not present, the bats fly low over the ground, making them prone to predation and collisions with cars, especially on motorways (Altringham & Kerth Citation2016).
Lesser horseshoe bat populations have been significantly declining in all European countries since the 1950s, with a decrease exceeding 90% (Stebbings Citation1988; Kokurewicz Citation1990; Bontadina et al. Citation2002; Schofield et al. Citation2022). The causes of decline in the 1950s and 1960s were attributed to intensive use of pesticides like DDT, toxic remedial timber treatment such as Lindane, bat banding, habitat connectivity disruption and habitat and roosts loss caused by human activity (Bontadina et al. Citation2000; Schofield et al. Citation2022). Habitat fragmentation has been recognised as negatively impacting R. hipposideros populations, as the woodlands surrounding the colony appear to be positively correlated to the colony size (Reiter Citation2004; Reiter et al. Citation2013; Tournant et al. Citation2013). Food competition with the common pipistrelle bat (Pipistrellus pipistrellus) was also reported to negatively affect their populations (Arlettaz et al. Citation2000). Due to its population decline and sensitivity to habitat changes, the species is under strict protection in the European Union and in the United Kingdom. It is listed in Annex II and IV of the Habitats Directive and in Annex II of the Bern and Bonn Conventions. According to the International Union for Conservation of Nature, the lesser horseshoe bat falls within the “near threatened” category in Europe, with a decreasing population trend (Hutson et al. Citation2007). In the last revision of the Portuguese Red Book of Vertebrates (Citation2005) it is listed as “vulnerable”. While recently a slow recovery of lesser horseshoe bat populations has been observed in the UK and in continental Europe, it has yet to equalize population losses (Schofield et al. Citation2022). To ensure the effective conservation of this species, it is crucial to locate its roosts, commuting corridors and foraging areas (Bontadina et al. Citation2002; Kokurewicz et al. Citation2008; Reiter et al. Citation2013; Schofield et al. Citation2022). In the light of this, we followed up on reports of a R. hipposideros roost in Aljezur, Portugal and produced its preliminary description, made an attempt to locate the foraging areas and commuting corridors and evaluated the existing and potential threats for the colony, as well as the most urgent conservation and research needs.
Materials and methods
Study area
This study was carried out during the periods from June 25 to July 4 and September 30 to October 7, 2022, in Aljezur, Portugal. The building where the colony resides is situated in the Aljezur residential area named Vale da Telha and abandoned in the 1980s. We decided not to discolse the exact location of the roost to avoid possible human disturbance to the colony. The building described is a two-storey structure, with the lower floor partially below ground level. On the south-western side of the building, there is a public road and a terrace with an empty swimming pool measuring about 75 m2. The pool occasionally fills with rainwater and can serve as a drinking site for the bat colony. Adjacent to the pool is an underground pump station with a staircase and a window. There are also two underground toilet blocks, each approximately 3 m2 in size, both accessible by staircases. On the southern part of the building, a footpath leads to the eastern side. Near the path there is a window measuring approximately 1.5 m2, situated 0.5 m above the ground.
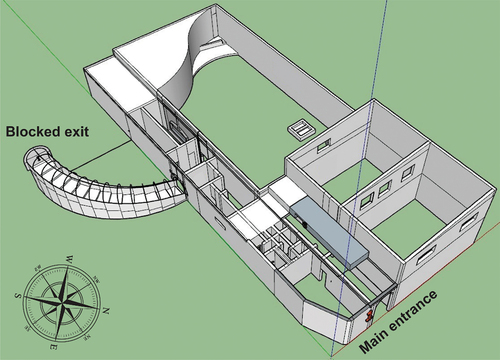
The area in the north-eastern side of the house is covered with thorny vegetation, shrubs and trees, mainly consisting of eucalyptus (Eucalyptus globulus) and acacia species (Acacia spp.). On that side are three windows, each around 2 m2 in size, located 0.5 m above the ground. Additionally, there are two windows on the second floor and the main entrance, which has a height of 2.3 m. The entrance hallway is 4 m long, and there are four rooms on both its left and right sides. To the left of the hall, a staircase leads to an exit on the south-western side of the building, but is blocked by a brick wall. To the left of the exit staircase, there is a passage leading to four small side rooms (). All the windows described above are potential entrances and exits for the bats. The valley to the east of the roost is not urbanized and covered by macchia and garrigue vegetation, creating linear landscape elements that can serve as commuting paths and foraging areas for the bat colony ().
Figure 2. Location of the maternity roost of lesser horseshoe bat (R. hipposideros), walking transect routes with ultrasound bat detectors and locations of their stationary recordings in Vale da Telha, Aljezur taken from June 29 to July 4, 2022.
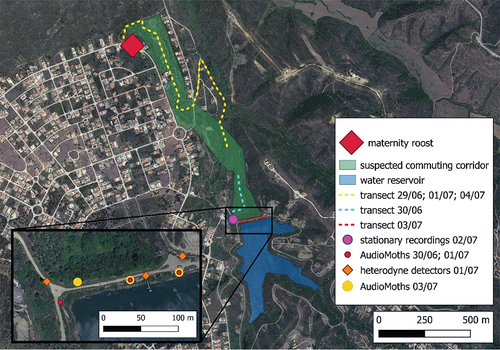
An artificial water reservoir marks the southern end of the Vale da Telha valley at coordinates 37°18′46.6″N 8°50′53.0″W. An unpaved track encircles the reservoir and the dam. The bank side of the reservoir is covered with macchia and garrigue vegetation, interspersed with some scattered trees. The bat colony is located at a straight-line distance of 1.2 km from the lake (). This reservoir is the sole significant water body within a 3 km radius from the maternity roost.
Species identification
We received information about the presence of bats in the abandoned building from groups of students participating in the ERASMUS+ Blended Intensive Programmes held at the CERES field study centre. On June 28, we entered the building to confirm the presence of bats, determine the status of the roost (e.g. maternity colony or colony of males) and identify the species. To minimise disturbance, the inspection was shorter than 5 minutes and undertaken around midday. While it is impossible to distinguish R. hipposideros from R. mehelyi–also occurring in our study area–based solely on recordings of echolocation calls, both species differ by shape of horseshoe-shaped nosepiece (Dietz et al. Citation2009). Therefore, photos of bats and the inside of the building were taken (). Moreover, a faecal sample was collected under the colony and later sent to Ecotype Genetics Ltd (Brighton, UK) for DNA analysis. Total DNA was extracted using a Quick-DNA MiniPrep kit (Zymo Research) by incubating the droppings sample in 800 uL of Genomic Lysis Buffer at room temperature for 30 minutes before proceeding by following the manufacturer’s instructions for solid tissue DNA extraction. Purified DNA was then subject to real-time PCR testing for the presence of R. hipposideros DNA using previously designed species-specific primers “RhipcytbF” and “RhipcytbR” (Harrington et al. Citation2019) which target the mitochondrial cytochrome b gene. PCR reactions were performed in duplicate alongside a negative control (H2O) and consisted of: 5 uL Luna® Universal qPCR Master Mix (New England Biolabs), 1 uL DNA extract and 4 uL of a primer mix both at 0.5 uM. The PCR reaction was performed on an AriaMX qPCR machine (Agilent Technologies) with cycling conditions of: 3 minutes at 95°C, followed by 40 cycles of 15 s at 95°C and 30 s at 62°C. A final dissociation step of 15 s at 95°C, 30 s at 60°C and 15 s at 95°C was used for melt curve analysis to confirm specific amplification. A positive identification was confirmed by the formation of a PCR product in both DNA containing duplicates.
Bat counts inside the roost
At dusk on July 2 and 4, after most of the adults left the roost and none was seen emerging for 15 minutes, we entered the building and the bats inside were counted. The few adults which stayed inside to look after the juveniles were distinguished by their flight abilities from the non-volant juveniles and counted separately (). Inspections inside the roost lasted no more than 10 minutes per time to minimise disturbance to the colony. For the same reason, we did not enter the roost on June 30 to count the bats inside, and the microclimatic measuring devices were placed during these short visits.
Table I. The results of bat counts during emergence and inside the roost.
Emergence counts and light intensity measurements
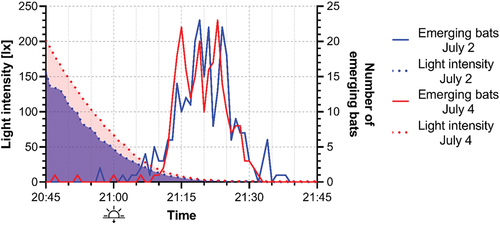
The emergence counts were performed on June 30, July 2 and 4 (). All potential exits were assessed and observers placed accordingly. The observers started counting bats emerging from the roost at 20:45 (i.e.15 minutes before sunset), and finished at 21:45. Following the methodology of Gojznikar et al. (Citation2020), we waited 15 minutes after the last noted emergence from the roost. To minimise disturbance to the bats, a 2 days interval between the emergence counts was implemented. The first trial count was carried out by six observers, the next counts involved seven observers to increase accuracy. During each count, all the identified exits were observed. To estimate the darkness level triggering the bats emergence, the light intensity was measured during the emergence counts using a digital Luxmeter LX1010B with an accuracy of ±1 lx. The measurements were taken with no cloud cover in the open space near the main entrance in minute intervals ().
Microclimatic conditions inside the roost
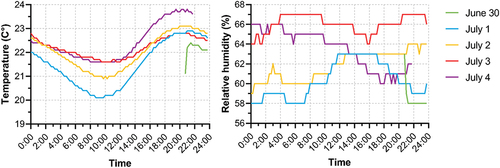
From June 30 to July 4, temperature and relative humidity inside the roost were measured using a Klimalogg Pro (TFA Dostmann; Reicholzheim, Germany) device, placed 1.5 m above the ground to initially assess their suitability for the bats, diurnal variation and eventual need for improvement ().
Ultrasound recordings
Transects were conducted to locate potential commuting corridors and foraging areas. However, due to the dense vegetation and lack of walkable footpaths, the transect route was split into two parts: one along the valley and the other near the water reservoir (). For recording bat activity, an Anabat SD2 ultrasound detector (Titley Electronics, Australia) was used. The recordings started 15 minutes before sunset and continued for 2 hours afterward. AudioMoth bat detectors (Open Acoustic Devices, version 1.2.0; firmware 1.5.0, United Kingdom) were placed near the northern edge of the water reservoir, on the nights of June 30, July 1 and 3 (). The recordings of bat calls captured by the AudioMoth were manually analysed using BatSound 4.4 software. For the recordings taken with the Anabat SD2, the analysis was performed using Analook v4.4a.
On July 1, bats flying up valley towards the water reservoir were counted on their commuting routes that were predicted on the basis of flight behaviour of the lesser horseshoe bat (Schofield Citation1996; Bontadina et al. Citation2002; Kokurewicz et al. Citation2008). The count was done by using heterodyne ultrasound detectors (Batbox III, Stag Electronics and D200 and Pettersson Elektronik) set to 110 kHz in three places near the northern shore of the water body (). These locations were chosen as the narrowest spaces between the vegetation on both sides of the road at the top of the dam, with the assumption that bats would use these spaces while flying up the valley towards the water reservoir. Due to the similar echolocation calls overlapping in frequency, R. hipposideros and Rhinolophus mehelyi cannot be distinguished solely based on recordings of echolocation calls. In fact, both species have a constant frequency pulse (CF) preceded and ended by a short FM part. The CF call has a frequency between 107.3 and 114.0 (usually 111.1) in R. hipposideros, whereas between 104.1 and 113.3 (usually 109.0) in R. mehelyi (Russ Citation2021). Therefore, we treated these species as a pair when analysing recordings taken outside the roost. Recordings of other bat species were omitted.
Results
Species identification
A maternity colony of lesser horseshoe bats (Rhinolophus hipposideros) composed of females with mostly non-volant juveniles was found inside the building. Based on guano accumulation and visual observations, we determined the largest room () to be most frequently inhabited by the colony. Furthermore, sonograms obtained inside the roost were analysed and compared with known R. hipposideros sonograms. The species R. hipposideros was identified by analysing photos taken inside the roost (). Additionally, DNA analysis of a faecal sample confirmed the presence of R. hipposideros. We found no evidence of cohabitation with other bat species.
Bats counts during emergence and inside the roost
The maximum number of emerging bats recorded was 384, and the maximum number of juveniles inside the roost was 130 individuals. Based on three emergence counts followed by two surveys inside the roost, the estimated maximum number of the colony was 501 individuals (371 adults and 130 juveniles; ), and reproductive success was 0.35 juveniles per adults. During the three visits inside the roost, no clustering (huddling) behaviour was observed for either adults or juveniles.
Light intensity measurements during emergence
On July 2, the first two bats emerged from the roost at light intensity of 59 lx, but on July 4 the first emergence occurred at 130 lx. However, the threshold of light intensity triggering the main emergence was estimated to 9 lx on the first night and to 13 lx on the second one, which corresponded to 12 minutes after sunset ().
Microclimatic conditions inside the roost
In the period from June 30 to July 4, 2022 the average temperature inside the roost was 22°C (min: 20.1°C, max: 23.8°C, SD = 0.8°C, n = 387). The lowest temperature values were recorded around 10:00 and the highest around 20:00. The average relative humidity in that period was 62.8% (min: 58%, max: 67%, SD = 2.8%, n = 387). No diurnal pattern of humidity changes was recorded during the observation period (). We noted neither condensation of water on the walls nor any other water sources inside the roost.
Ultrasound recordings outside the roost
Throughout all our observations, 16 contacts with R. hipposideros / R. mehelyi were recorded away from the colony. Eight of them were made on July 1 by heterodyne ultrasound detectors set to 110 kHz, four contacts were recorded on July 2 by use of Anabat SD2 and 4 on July 3 with AudioMoth bat detectors. All recordings were taken on the northern bank of the water reservoir, in narrow spaces between the vegetation (). Out of all the transect routes, only the one carried out along the northern bank of the water reservoir showed a single R. hipposideros/R. mehelyi echolocation call.
Discussion
In the present study, we carried out fieldwork with the aim of characterising the roost type, identifying the species inhabiting the colony, estimating the colony size and locating commuting routes and foraging areas used by the bats living in an abandoned building. The building was inspected during daytime and inside female bats with numerous non-volant pups were observed and photographed (), making this a maternity roost. The species–Rhinolophus hipposideros–was identified by analysing DNA samples, photos and recordings of echolocation calls taken inside the roost.
To estimate the size of the colony, three emergence counts and two counts inside the roost were carried out (). The first emergence count is to be considered a trial count, as the observers were not enough to cover all exits and count efficiently. The second and third count and the following surveys inside the roost showed very small differences on the total numbers, with an estimated maximum size of 501 individuals (371 adults and 130 juveniles) and a reproductive success of 0.35 juveniles per adults. Between the second and third count, we observed 18 more bats emerging from the roost and 21 less juveniles and adults inside it. These oddly similar differences could be explained by those juveniles becoming volant in the span of time between the two counts. Therefore, we may assume that in the last days of June and the beginning of July juveniles become capable of flight and are able to leave the roost.
To our knowledge, this newfound colony of at least 501 individuals is the largest recorded colony of R. hipposideros in Portugal at the present time, the previous one being the maternity colony living in Quinta da Regaleira, Sintra, which was estimated to reach up to 150 bats in the summer (Lino et al. Citation2014, Citation2015). Moreover, after a thorough search in the literature, it can be claimed that the size of the maternity roost in Vale da Telha is one of the largest not only nationally but also one of the biggest in the Europe.
For reference, the United Kingdom is one of the few countries whose lesser horseshoe bat population shows an upward trend in numbers of individuals over the years (The National Bat Monitoring Programme Annual Report Citation2022, 2023; Schofield et al. Citation2022) and in the area near the border between Wales and England, which comprises around 26% of the whole R. hipposideros population of the United Kingdom (Joint Nature Conservation Committee Citation2015), one of the largest and highest quality maternity roost houses around 750 individuals (Knight & Jones Citation2009). On the other hand, roosts of this species can often consist of less than 5 individuals as it was reported in Switzerland (Bontadina et al. Citation2000). Therefore, in our opinion, a roost of at least 501 individuals is to be considered as important both locally and at the European scale.
The first bat emergences from the roost occurred at 130 and 59 lx, which corresponds to nearly full daylight. However, the light intensity during the main emergences was much lower, around 9 and 13 lx, corresponding to 12 minutes after sunset (). Emergence times reported from studies in the UK and Central Europe are typically between 20 and 30 minutes after sunset (Reiter et al. Citation2008; Downs et al. Citation2016; Warchałowski & Pietraszko Citation2019). Early emergence times recorded during our observations are caused by the high energy demands of females at the end of the lactation period: the energy requirements of fully grown juveniles which are not able to forage on their own are the highest of the whole reproductive cycle.
The ambient temperature inside the roost is a primarly important factor determining the growth rate of juveniles and, consequently, their survival during winter (Ransome Citation1990; Schofield et al. Citation2022). Bats tend to select a roost temperature that is within their thermoneutral zone, to minimise the energy costs of maintaining homeothermy (Speakman & Thomas Citation2003). According to Schofield (Citation1996), the thermoneutral zone may be inferred through the temperature found within clustering bats in maternity colonies, which was estimated at 30.9°C, i.e. 14.6°C warmer than the air temperature 0.5 m away of the cluster that gives the roost air temperature of 16.3°C. During our three visits inside the roost we did not observe the clustering (or huddling) behaviour, employed to compensate for low roost temperatures, neither in adults nor in juveniles. Our short-term observations estimating the average roost temperature at 22°C (), confirm the prediction made by Schofield (Citation1996), which assumes that only in roost temperatures falling near 16°C the clustering behaviour occurs, and are contradictory to the findings made in Austria by Reiter (Citation2002), where lesser horseshoe bats are typically found hanging solitary at temperatures above 24°C. Observations of this colony should be continued in the future and cover a longer part of the reproductive season.
The average relative humidity was surprisingly high at 62.8% () compared to its low values in colonies situated in buildings, also considering we did not notice water condensation on the walls nor any other water sources inside the roost. The high values of relative humidity are favourable for the bats as they reduce water loss through evaporation. We assume that due to the hot climate in the study area, the critical factor for reproductive success in the maternity colony under study is the water supply. In our opinion, there is no need for improvement of the microclimatic conditions inside the building.
We managed to record 16 contacts with bats with echolocation calls matching the frequency of R. hipposideros /R. mehelyi, all flying close to the northern bank of the water reservoir, in the narrowest spaces between the vegetation on both sides of the road and on the top of the dike (). Moreover, the times of the recordings are in accordance with their emerging time, which coherently appears to peak right before the times of the detections (). While the number of recordings–especially the single detection during the transects–might not seem exhaustive compared to the size of the colony, it has been previously reported how difficult it is to record lesser horseshoe bats. These bats are small, fly low and fast over the ground and vegetation, and their echolocation is designed for movements in cluttered habitats by use of directional low-intensity calls, detectable only within 5–6 metres from the recording device (Motte & Libois Citation2002). That being the case, the lack of evidence of the bat’s presence along the valley is not enough to discount it as a commuting corridor and foraging area, especially considering the 16 detections in the proximity of the water reservoir. Moreover, the straight-line distance from the maternity roost to the places of recordings was 1.2 km (), and the reservoir is the only large water body within a 3 km radius from the maternity roost under study. In the current state of knowledge, we assume that Vale da Telha, not urbanised, covered by semi-natural vegetation in the advanced stage of re-naturalisation and hosting a large freshwater reservoir, provides foraging areas, drinking place and commuting corridors for the bats in maternity roost under study.
Taking into account the conservation status of R. hipposideros listed in Annex II of the EU Habitat Directive and the lack of protection of the roost in Vale da Telha, it was necessary to conduct an emergency survey in order to stop the planned demolition of the privately owned building in question. We succeeded in doing so by officially contacting the Local Community Council of Aljezur and notifying them in writing about the presence of protected species inside the abandoned building. The next step is to take action for the conservation of this colony. Previous studies on R. hipposideros showed that the species mainly moves within a 2.5 km radius around their colony; this should be the range within which conservation measures need to be taken, focused on preserving the woodland inside this area which is vital for the colony’s welfare (Bontadina et al. Citation2002; Holzhaider et al. Citation2002; Motte & Libois Citation2002; Kokurewicz et al. Citation2008; Reiter et al. Citation2013).
More specifically, horseshoe bats are known to be strongly light-phobic and to avoid open spaces, flying low and between the gaps in the vegetation (Schofield et al. Citation2022), making them prone to collisions with cars (Altringham & Kerth Citation2016). In the light of this, we suggest leaving the road around the water reservoir as it is–unpaved and unlit–and to keep the speed limit to the minimum. This will reduce the light barrier effect and lower the probability of bats colliding with cars. We also suggest filling the gaps in the vegetation in order to form a constant shrub line along the roadsides. For this purpose we recommend the strawberry tree (Arbutus unedo), a native evergreen shrub known for its ease of cultivation.
The colony currently does not receive any special protection, but we believe that urgent actions are necessary to safeguard it. The most urgent measures we recommend taking are the following:
Restrict human access to the colony by fencing the perimeter of the property and installing information panels indicating that the building is out of bounds to the public.
Maintain the vegetation composed of thorns, shrubs and solitary trees around the building, especially in the north-eastern side of the house. This vegetation serves as the main commuting corridor, allowing bats to leave the roost avoiding open spaces.
Preserve all entrances to the maternity roost as described above.
Implement a monitoring programme to track the number and reproductive success of the colony.
The maternity colony and Vale da Telha are situated in the Natura 2000 site Costa Sudoeste (code: PTZPE0015) established in March 1988 under the Birds Directive (Directive 2009/147/EC) to protect migratory birds. A management plan which includes our study area is currently being prepared. We strongly suggest the roost and valley hereby described to be specifically included in the management plan and prioritised, and our recommendations for the preservation of the roost and its key areas to be taken into account.
Finally, to definitively confirm our hypothesis and more precisely pin down the bats’ foraging areas and migratory routes, we strongly suggest following up with a radio-tracking study of the lactating females from the colony, the planning of which shall be based on the information hereby collected on the reproductive cycle of the colony. Such research has already been successfully carried out on this species multiple times before (Bontadina et al. Citation2002; Holzhaider et al. Citation2002; Motte & Libois Citation2002; Kokurewicz et al. Citation2008; Zahn et al. Citation2008; Knight & Jones Citation2009; Reiter et al. Citation2013). This would greatly help direct the conservation efforts towards key locations that should be put under protection.
Acknowledgments
We would like to thank Dr Samantha Ball (School of Biological, Earth and Environmental Science, University College Cork, Ireland), Dr Jed Kempf (Marine Institute, Galway, Ireland), Wojciech Godlewski and Olga Łuczak (Wrocław University of Environmental and Life Sciences, Poland) for the preliminary bat survey in Vale da Telha in September 2021 and January 2022. We are very grateful to Máté Nagy (Collective Behaviour Research Group, Budapest, Hungary) for making the 3D model of the roost planimetry used in this paper. We also would like to acknowledge all volunteers and supervisors from Leiden University (the Netherlands) and Leeds University (UK) for their help in the fieldwork, namely Prof Peter van Bodegom, Prof Emily Strange, Ashley Sadler, Thomas Pavey, Laurens Kropveld, Anouk Bouma, Thomas Komin and Just Horning. We are grateful to John Haddow (Auritus Wildlife Consultancy, Dunblane, UK), Dr J. Tiago Marques (Mediterranean Institute for Agriculture, Environment and Development University of Évora, Évora, Portugal) and Udo Schwarzer (RWSW Rewilding Sudoeste) for the expert advice on site and critical remarks to the early version of the manuscript. We thank Dr Thomas Etheridge from Ecotype Genetics Ltd for providing us with the qPCR protocol. Finally, we would like to thank Anna Posadowska-Malarz–the Erasmus+ Institutional Coordinator in Wrocław University of Environmental and Life Sciences (Poland)–for the invaluable help in the organisation of the administrative part of the project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- 1303 Lesser horseshoe bat Rhinolophus hipposideros. 2015. Joint Nature Conservation Committee. Available: https://sac.jncc.gov.uk/species/S1303/.
- Altringham J, Kerth G. 2016. Bats and roads. In: Voigt CC, Kingston T, editors. Bats in the Anthropocene: Conservation of bats in a changing world. New York: Springer Cham. pp. 35–62.
- Arlettaz R, Godat S, Meyer H. 2000. Competition for food by expanding pipistrelle bat populations (Pipistrellus pipistrellus) might contribute to the decline of lesser horseshoe bats (Rhinolophus hipposideros). Biological Conservation 93(1):55–60. DOI: 10.1016/S0006-3207(99)00112-3.
- Bontadina F, Arlettaz R, Fankhauser T, Lutz M, Muhlethaler E, Theiler A, Zingg P. 2000. The lesser horseshoe bat Rhinolophus hipposideros in Switzerland: Present status and research recommendations. Le Rhinolophe 14(May 2014):69–83. Available: http://www.swild.ch/rhinolophus/LeRhino.pdf.
- Bontadina F, Schofield H, Naef-Daenzer B. 2002. Radio-tracking reveals that lesser horseshoe bats (Rhinolophus hipposideros) forage in woodland. Journal of Zoology 258(3):281–290. DOI: 10.1017/S0952836902001401.
- Dietz C, von Helversen O, Nill D. 2009. Bats of Britain, Europe & Northwest Africa. London: A&C Black.
- Downs NC, Cresswell WJ, Reason P, Sutton G, Wells D, Williams L, Wray S. 2016. Activity patterns and use of night roosts by lesser horseshoe bats Rhinolophus hipposideros (Borkhausen, 1797). Acta Chiropterologica 18(1):223–237. DOI: 10.3161/15081109ACC2016.18.1.013.
- Gojznikar J, Zaveršek T, Bolčina A. 2020. Observations made at three church bat (Chiroptera) roosts in central Slovenia. Natura Sloveniae 22(2):85–87. DOI: 10.14720/ns.22.2.85-87.
- Harrington AP, O’Meara DB, Aughney T, McAney K, Schofield H, Collins A, Deenen H, O’Reilly C. 2019. Novel real-time PCR species identification assays for British and Irish bats and their application to a non-invasive survey of bat roosts in Ireland. Mammalian Biology 99:109–118. DOI: 10.1016/j.mambio.2019.10.005.
- Holzhaider J, Kriner E, Rudolph BU, Zahn A. 2002. Radio-tracking a lesser horseshoe bat (Rhinolophus hipposideros) in Bavaria: An experiment to locate roosts and foraging sites. Myotis 40(December):47–54.
- Hutson T, Spitzenberger F, Juste J, Aulagnier S, Fernandes M, Alcaldé JT. 2007. Rhinolophus hipposideros. The IUCN red list of threatened species 2007.
- Knight T, Jones G. 2009. Importance of night roosts for bat conservation: Roosting behaviour of the lesser horseshoe bat Rhinolophus hipposideros. Endangered Species Research 8(1–2):79–86. DOI: 10.3354/esr00194.
- Kokurewicz T. 1990. The decrease in abundance of the lesser horseshoe bat Rhinolophus hipposideros Bechstein, 1800 (Chiroptera: Rhinolophidae) in winter quarters in Poland. Myotis 28:109–118.
- Kokurewicz T, Rusiński M, Haddow J, Furmankiewicz J. 2008. Selekcja siedlisk podkowca małego Rhinolophus hipposideros W Masywie Śnieżnika (Sudety Wschodnie) w okresie zimowania i rozrodu—implikacje dla ochrony gatunku. Przyroda Sudetów Zachodnich 11(supplement 3): 7–26.
- Lino A, Fonseca C, Goiti U, Pereira MJR. 2014. Prey selection by rhinolophus hipposideros (Chiroptera, Rhinolophidae) in a modified forest in Southwest Europe. Acta Chiropterologica 16(1):75–83. DOI: 10.3161/150811014X683282.
- Lino A, Fonseca C, Mendes G, Pereira MJ. 2015. Roosting behaviour and phenology of the lesser horseshoe bat (Rhinolophus hipposideros) in a breeding colony in Sintra, Portugal. Galemys, Spanish Journal of Mammalogy 27(October):1–12. DOI: 10.7325/galemys.2015.a1.
- Livro vermelho dos vertebrados de Portugal. 2005. Instituto da Conservação da Natureza e das Florestas.
- Motte G, Libois R. 2002. Conservation of the lesser horseshoe bat (Rhinolophus hipposideros Bechstein, 1800) (Mammalia: Chiroptera) in Belgium. A case study of feeding habitat requirements. Belgian Journal of Zoology 132(1):49–54.
- Ransome R. 1990. The natural history of hibernating bats. London: Christopher Helm.
- Reiter G. 2002. Ökologie, Öko-Ethologie und Naturschutzbiologie der Kleinen Hufeisennase (Rhinolophus hipposideros Bechstein 1800) in Österreich. Salzburg, Germany: University of Salzburg.
- Reiter G. 2004. The importance of woodland for Rhinolophus hipposideros (Chiroptera, Rhinolophidae) in Austria. Mammalia 68(4):403–410. DOI: 10.1515/mamm.2004.040.
- Reiter G, Hüttmeir U, Krainer K, Smole-Wiener K, Jerabek M. 2008. Emergence behaviour of lesser horseshoe bats (Rhinolophus hipposideros): Intracolony variation in time and space (Carinthia and Salzburg, Austria). Berichte des Naturwissenschaftlich-medizinischen Vereins in Innsbruck 95:81–93.
- Reiter G, Pölzer E, Mixanig H, Bontadina F, Hüttmeir U. 2013. Impact of landscape fragmentation on a specialised woodland bat, Rhinolophus hipposideros. Mammalian Biology 78(4):283–289. DOI: 10.1016/j.mambio.2012.11.003.
- Russ J. 2021. Bat calls of Britain and Europe. A guide to species identification. (J. Ross (Ed.)). London: Pelagic Publishing.
- Russo D, Jones G. 2003. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: Conservation implications. Ecography 26(2):197–209. DOI: 10.1034/j.1600-0587.2003.03422.x.
- Schober W. 1998. Die Hufeisennasen Europas: Rhinolophidae (Die Neue Brehm-Bücherei). Vol. 647. Magdeburg, Germany: Westarp Wissenschaften.
- Schofield H, Reiter G, Dool SE. 2022. Lesser horseshoe bat rhinolophus hipposideros (André, 1797). In: Hackländer K, Zachos FE, editors. Handbook of the mammals of Europe. New York: Springer Cham. pp. 1–34.
- Schofield HW. 1996. The ecology and conservation biology of Rhinolophus hipposideros, the lesser horseshoe bat. Aberdeen, UK: University of Aberdeen.
- Speakman JR, Thomas DW. 2003. Physiological ecology and energetics of bats. In: Kunz TH, Fenton B, editors. Bat ecology. Chicago: The University of Chicago Press. pp. 430–490.
- Stebbings RE. 1988. Conservation of European bats. London: Christopher Helm.
- The National Bat Monitoring Programme Annual Report 2022. 2023. Available: https://www.bats.org.uk/our-work/national-bat-monitoring-programme/reports/nbmp-annual-report.
- Tournant P, Afonso E, Roué S, Giraudoux P, Foltête JC. 2013. Evaluating the effect of habitat connectivity on the distribution of lesser horseshoe bat maternity roosts using landscape graphs. Biological Conservation 164(April):39–49. DOI: 10.1016/j.biocon.2013.04.013.
- Warchałowski M, Pietraszko M. 2019. The emergence time and flight routes used by lesser horseshoe bats of Radziechowy colony (Poland). Theriologia Ukrainica 17:64–70. DOI: 10.15407/pts2019.17.064.
- Zahn A, Holzhaider J, Kriner E, Maier A, Kayikcioglu A. 2008. Foraging activity of Rhinolophus hipposideros on the Island of Herrenchiemsee, Upper Bavaria. Mammalian Biology 73(3):222–229. DOI: 10.1016/j.mambio.2007.02.005.