Abstract
Global warming has become an undeniable fact, and the frequent occurrence of extremely hot weather is a serious challenge to the survival of animals. As one of the most active components of the ecosystem, birds are sensitive to the constant changes in the climate and environment. To investigate the physiological responses of Eurasian tree sparrows (Passer montanus) to acute heat stress, various thermogenic parameters of the birds, which included resting metabolic rate (RMR), body mass, body temperature, water intake, and liver cellular metabolic capacity, were measured after exposure to 42°C for different times. The antioxidant response of Eurasian tree sparrows to heat stress was investigated by measuring the lipid oxidation indexes and related antioxidant oxidase activities in the serum and liver. Body temperature and water intake were significantly increased after 2 h of heat stress and lasted until the end of the heat stress (12 h). In contrast, RMR was significantly decreased after 2 h of heat stress. At the cellular level, liver COX activity was significantly increased during heat stress from 2 h to 6 h. Additionally, there was a significant increase in MDA content and superoxide dismutase (SOD) and catalase (CAT) activity levels in the serum. The glutathione peroxidase (GSH-PX) activity in the liver was significantly decreased after 2 h of heat stress. Our results indicated that heat production decreased while heat dissipation increased in Eurasian tree sparrows exposed to acute heat stress. At the same time, the internal antioxidant defense system of the birds was activated to counteract the damage caused by high temperatures. Therefore, it was reasonable to speculate that Eurasian tree sparrows might be resistant to high temperatures to the extent that they can maintain normal life activities through the regulation of thermogenic and antioxidant systems under acute heat stress.
Introduction
In recent years, global warming has taken on a more prominent effect with more frequent weather abnormalities, such as drought and flooding, and the frequency and severity of heat waves are expected to increase in the coming decades (Stillman Citation2019). The main effect arising from global warming is rapid climate change. The unpredictable changes linked to rapid climate change present a great challenge to the survival of animals since their habitats may be adversely affected by these changes (Diffenbaugh & Field Citation2013). In many regions of the world, global climate change has already resulted in increased ambient temperatures. Therefore, exploring how organisms adapt to these climatic changes is important for predicting shifts in population distribution and species diversity associated with global climate change. In order to deal with the changes in the environment brought about by rapid climate change, animals may need to adapt to these changes through modifications to their morphology, physiology, and behavior to ensure their survival. Birds are endotherms that are particularly vulnerable to high temperatures because of their high body temperatures and metabolic rates (Nawab et al. Citation2018; Riddell et al. Citation2021).
Basal metabolic rate (BMR) refers to the rate of energy transformation of an animal in the resting and fasting states within the thermal neutral zones (TNZ). It is the minimum heat production rate essential for the birds to maintain a normal physiological status, and it is one of the basic physiological criteria to evaluate the energy consumption related to thermoregulation (Williams & Tieleman Citation2000; Mckechnie et al. Citation2006; Moller Citation2010). Resting metabolic rate (RMR) is the energy the body needs to perform important bodily functions in the resting state. As it is doubtful that a true BMR can be obtained in the laboratory, RMR is often used to refer to such measurements even when the standard conditions for BMR are met (Swanson Citation2010). There is a close relationship between ambient temperature and energy metabolism. Energy metabolism is often plastically regulated in response to changes in ambient temperature. Within the TNZ, animals do not require excess energy to increase heat production and therefore require less energy to maintain normal life activities, and this is usually accompanied by a decrease in food intake and can result in body mass loss (Yamauchi et al. Citation1981). Studies have shown that increased body mass can contribute to a change in BMR (Zhou et al. Citation2016; Swanson et al. Citation2017). Changes in ambient temperature can lead to corresponding adaptive changes in metabolic activities, which in turn can lead to changes in BMR for the birds.
In addition, BMR is significantly lower in birds kept at high temperatures compared with those kept at low temperatures, and such a phenomenon has been demonstrated for a number of bird species, such as red-billed leiothrixes (Leiothrix lutea) (Cui et al. Citation2019), Chinese bulbuls (Pycnonotus sinensis) (Zheng et al. Citation2013), hwameis (Garrulax canorus) (Zhou et al. Citation2016), house sparrows (Passer domesticus) (Swanson et al. Citation2020), and dark-eyed juncos (Junco hyemalis) (Swanson et al. Citation2014). High ambient temperatures also make it difficult for the body to dissipate heat, thereby hindering the maintenance of body temperature. Increasing the efficiency of heat dissipation through increased evaporative water loss is one of the key features that enable birds to adapt to high ambient temperatures (Smith et al. Citation2015; Soorim & Beissinger Citation2019), but this usually requires an increase in water intake (Xia et al. Citation2013) and energy consumption (Xia et al. Citation2013; Smith et al. Citation2017).
At the cell level, one of the potential mechanisms of RMR alteration in birds is linked to changes in tissue catabolic enzyme activities and/or metabolic substrate transport capacity (Zheng et al. Citation2008, Citation2014; Liknes & Swanson Citation2011). Among the mechanisms of RMR alteration are changes in mitochondrial respiration and cytochrome c oxidase (COX) activity in the tissues (Collin et al. Citation2003). State 4 respiration (S4R) generates heat through incomplete coupling with ADP phosphorylation, reflecting heat production driven by mitochondrial proton leak (Else Citation2004). COX is a key regulatory enzyme involved in aerobic metabolism and is located at the end of the mitochondrial respiratory chain (Wang et al. Citation2006; Zheng et al. Citation2008, Citation2014). Changes in the metabolic activity of cells are usually monitored by measuring the changes in mitochondrial S4R or COX activity (Zheng et al. Citation2008, Citation2014; Swanson et al. Citation2014; Zhou et al. Citation2016; Li et al. Citation2020).
Furthermore, heat stress occurs when the external temperatures are too high for the endotherms to dissipate excess heat, and birds are particularly vulnerable to heat stress because of their unique physiology and high metabolic rates (Nawab et al. Citation2018). Heat stress leads to oxidative stress at the cellular level by affecting mitochondrial function (Farag & Mahmoud Citation2018; Castiglione et al. Citation2020). With increasing ambient temperatures, animal metabolism as well as oxidative phosphorylation processes in the mitochondria are accelerated, leading to an increased production of reactive oxygen species (ROS) over time (Akbarian et al. Citation2016). The main reactive oxygen species produced in the tissues are superoxides, peroxides, and hydroxyl radicals (Kunwar & Priyadarsini Citation2011). When ROS are in excess of the capacity of the antioxidant system, they trigger lipid peroxidation (Halliwell & Aruoma Citation1991; Flanagan et al. Citation1998; Lord-Fontaine & Averill-Bates Citation2002; Mujahid et al. Citation2007), which can be detected by an increased level of malondialdehyde (MDA), the main oxidation product of peroxidized polyunsaturated fatty acids, and is widely recognized as one of the biomarkers of oxidative stress and cellular lipid peroxidation (Olalekan et al. Citation2011; Zhou et al. Citation2017). Excess ROS also cause oxidative damage to proteins and DNA (Halliwell & Aruoma Citation1991; Flanagan et al. Citation1998; Lord-Fontaine & Averill-Bates Citation2002; Mujahid et al. Citation2007).
In a cell, the oxidative stress caused by increased production of ROS can be eliminated by the activities of the antioxidant systems (such as antioxidant enzymes), and therefore, cell damage may be avoided (Baxter et al. Citation2014; Huang et al. Citation2015). Compared with ectotherms, birds have evolved more effective antioxidant defense mechanisms to defend against damage inflicted by ROS (Costantini Citation2008). Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX) have been recognized as key enzymes in the assessment of the cellular antioxidant defense system in birds (Pamplona & Costantini Citation2011; Jiao et al. Citation2017; Koim-Puchowska et al. Citation2020). SOD, as the first line of defense, reacts with superoxide anions, usually forming relatively unreactive molecules of H2O2 (Skrip & McWilliams Citation2016). CAT and GSH-PX both catalyze the conversion of ROS to H2O2. At the same time, reduced glutathione is converted to an oxidized form. This reaction plays an important role in protecting organisms from oxidative stress (Pamplona & Costantini Citation2011; Chen et al. Citation2015; Habashy et al. Citation2019).
The Eurasian tree sparrow (Passer montanus) is a small, granivorous passerine with strong adaptability that inhabits vast areas of the continents of Europe and Asia (Mackinnon & Phillipps Citation2000). As a typical small bird, tree sparrows have a high BMR and a wide TNZ (Deng & Zhang Citation1990). Tree sparrows respond to changes in ambient temperature mainly through changes in metabolic and respiratory enzyme activities (Dixit et al. Citation2018; Li et al. Citation2020; Swanson et al. Citation2020). The metabolic heat production characteristics of tree sparrows are also affected by other environmental factors. Seasonal variations in metabolic heat production have been explored in tree sparrows, and BMR and physiological and biochemical regulations in tree sparrows are associated with increased heat production in winter (Liu & Li Citation2006; Zheng et al. Citation2008, Citation2014; Li et al. Citation2010). In addition, photoperiod (Li et al. Citation2020), food restriction (Yang et al. Citation2010), and other factors can also affect the metabolic heat production of tree sparrows.
It is not clear what physiological responses occur in tree sparrows when the ambient temperature rises rapidly and sharply above their TNZ or whether they can adapt to such a change. To obtain a greater understanding of how small birds such as tree sparrows might adapt to short-term exposure to a high temperature, we first exposed the birds to 42°C for different durations and then measured the changes in RMR, body mass, body temperature, water intake, liver cellular metabolic capacity, and antioxidant-related enzyme activities to determine how the birds coped with heat stress. We hypothesized that tree sparrows adapt to acute heat stress by altering metabolic heat production and regulating antioxidant capacity. We also predicted that under acute heat stress, RMR and respiratory enzyme activities would decrease, water intake would increase, and antioxidant enzyme activity levels would increase. The findings of this study would no doubt shed further light on the environmental adaptation of small birds.
Materials and methods
Study sites
Animals selected for this study were captured in Wenzhou, Zhejiang Province (27°29=N, 120°51=E). Wenzhou has a subtropical monsoon climate with an average precipitation of 1500 mm in all years. The average summer temperature in Wenzhou ranges from 22 to 28°C but can reach as high as 39°C.
Animals and treatment
Eurasian tree sparrows were captured in the field using mist nets in June 2015. After capture, the body mass of each bird was measured using an electronic balance (Sartorius BT25S, Germany). The birds were kept in individual cages (30 × 30 × 40 cm3) in the laboratory at 25 ± 1°C and under a 12 L:12D photoperiod with lights at 06:00. After adapting to these laboratory conditions for a week, 30 birds were randomly divided into one control group and four heat-stressed groups, with 6 birds per group. The four heat-stressed groups were the 2 H, 4 H, 6 H, and 12 H groups, which were exposed to 42°C for 2 h, 4 h, 6 h, and 12 h, respectively. The body mass and body temperature of the birds were measured before grouping. Each group had a similar mean initial body mass. The birds were given ad libitum access to food and water. In the course of the experiment, two birds were lost, one from the 6 H group and another from the 12 H group, so they were excluded from the data analyses.
Measurements of body mass, body temperature, and water intake
Body mass was measured with an electronic balance (Sartorius BT2S, Germany), accurate to 0.1 g. Body temperature was measured with a lubricated thermocouple inserted in the cloaca, and the output was digitized using an Oakton thermocouple meter (Oakton Stemp-10 K, America) (Zhou et al. Citation2016; Xuanyuan et al. Citation2023).
Before the heat stress treatment, the drinking water was weighed, and after the heat stress treatment, the remaining water was collected and weighed again. The result was corrected for the difference in water evaporated per hour at 42°C and 25°C due to the difference in ambient temperature.
Measurements of metabolic rate
Oxygen consumption was measured by an open-circuit respiratory system (TSE, Germany) according to the method described by Zhou et al. (Citation2016) and Xuanyuan et al. (Citation2023). Briefly, individual birds were allowed to rest in a sealed 1.5-L cylindrical metabolic chamber housed in a temperature-controlled cabinet set at 25 ± 0.5°C, which is near the TNZ of the Eurasian tree sparrows (Deng & Zhang Citation1990; Zheng et al. Citation2008, Citation2014; Li et al. Citation2020). Perches were placed in the metabolic chambers to allow individual birds to rest before their metabolic rates were measured (Wu et al. Citation2015; Bai et al. Citation2016). The air was scrubbed off H2O before being pumped through the metabolic chamber at a rate of 1000 mL·min−1. The air leaving the chamber was also dried and passed through an oxygen analyzer at a flow rate of 300 mL·min−1, as previously described (Wu et al. Citation2014; Zhou et al. Citation2016). The data were recorded and analyzed by an analog-to-digital converter and standard analysis software (Zhou et al. Citation2016; Xuanyuan et al. Citation2023). The lowest rate (over 10 min) was used to quantify the RMR (Zhao et al. Citation2015; Wen et al. Citation2020; Xuanyuan et al. Citation2023). Metabolic rates were expressed as oxygen consumption per hour (mL O2·h−1) and were corrected to standard temperature, pressure, and dry gas conditions. The gas exchange measurements for all groups were obtained during the resting phase of the birds’ circadian cycles (between 24:00 and 02:00) in dark chambers. Oxygen consumption was measured when the birds were observed perching calmly about an hour later.
At the end of the RMR measurements, the birds were euthanized by cervical dislocation, and blood samples were collected for serum preparation (Zheng et al. Citation2008, Citation2014). Next, the liver was quickly extracted from the dead bird, and the connective tissue and fat attached to the liver were also removed. After that, 50 mg of each liver was taken and added to 9 volumes of ice-cold 0.9% NaCl solution, followed by homogenization using a high-speed homogenizer (IKA, Germany) at 4°C (Xu et al. Citation2022), followed by centrifugation at 3000 × g force for 15 min. After that, the supernatant was collected and used for subsequent analyses. All animal experimental procedures were carried out according to the Animal Care and Use Committee of Wenzhou University.
Measurements of tissue S4R and COX activity
The frozen liver tissue (50 mg) was homogenized in 450 μL of buffer containing 5 mM Tris-HCl (pH = 7.4), 250 mM sucrose, 1 mM MgCl2-6 H2O, and 0.5 mM EDTA using a high-speed homogenizer (IKA, Germany) at 4°C. The liver homogenate was then used for subsequent analyses.
Liver S4R and COX activity were measured at 30°C with a Clark electrode (Hansatech Instruments, UK; DW-1) (Zhou et al. Citation2017; Mao et al. Citation2019). For the S4R measurement, 1.9 mL of respiration medium containing 50 mM Tris·HCl (pH 7.4), 225 mM sucrose, 5 mM MgCl2, 1 mM EDTA, and 5 mM KH2PO4, and 5 mM succinate was first dispensed into the reaction chamber of the electrode, followed by the addition of 100 μL of liver homogenate. In the condition of substrate dependence, succinate was used as a substrate (Williams & Tieleman Citation2000; Zheng et al. Citation2008; Zhou et al. Citation2017). COX activity was measured by dispensing 1.95 mL of respiration medium (3.75 mM ascorbic acid, 0.3 mM TMPD, and 7.5 mM KH2PO4, pH = 7.4) in the reaction chamber, followed by the addition of 50 μL of liver homogenate. The reaction was initiated by the addition of 10 μL of cytochrome C (as a substrate for COX). The progress of the reaction was monitored for one hour. Both S4R and COX activity were expressed as mmol O2 min−1·organ−1.
Protein and oxidative marker assays
Protein concentration in the liver homogenate was determined by the bicinchoninic acid (BCA) method using a commercial kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions. BSA was used as a standard.
MDA levels in the liver homogenate and serum were determined using a commercial Malondialdehyde Kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions and expressed as nmol·mg−1 protein in the case of the liver and nmol·mL−1 protein for the serum. The activities of the antioxidant enzymes SOD, CAT, and GSH-PX were also determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. These particular assay kits were selected because of their high degree of sensitivity, specificity, and inter-assay and intra-assay precision (Chen et al. Citation2014; Xu et al. Citation2022). SOD, CAT, and GSH-PX activities in the liver were expressed as U·mg−1 protein, and in the serum, they were expressed as U·mL−1 protein.
Data analysis
SPSS 20.0 statistical software was used for statistical analysis, and Origin 8.0 was used to plot all graphical data. The Kolmogorov-Smirnov test was used to check the normality of all variables, and the Kruskal-Wallis test was used for non-normally distributed data. Body mass, body temperature, RMR, water intake, liver S4R, and oxidative stress-related indicators were analyzed by one-way ANOVA. Serum SOD activity and liver GSH-PX activity were analyzed by a non-parametric test. Differences between groups after a one-way ANOVA were analyzed by the Least Significant Difference (LSD) test. After the Kruskal-Wallis test, the all pairwise method was used to analyze the differences between groups. All data were expressed as mean ± SE, and statistical significance was considered at the p < 0.05 level.
Results
Body mass, body temperature, water intake, and RMR
The body mass of the Eurasian tree sparrows was not affected by heat stress (F(4,23) = 0.366, p = 0.943, ). However, both body temperature (F(4,23) = 4.945, p = 0.005, ) and water intake (F(4,23) = 14.686, P < 0.001, ) were significantly increased in the heat-stressed groups after 2 h of acclimation to 42°C, and these increases were sustained for the entire duration (12 h) of the experiment. Acute heat stress affected the RMR of tree sparrows to some extent (F(4,23) = 1.595, p = 0.209, ). The treatment groups showed a significant decrease in RMR after 2 h of heat stress compared with the control group (P = 0.026, ). However, with prolonged heat stress, RMR did not differ significantly relative to the control group during 4–12 h (P = 0.121, 4 H group; P = 0.090, 6 H group; P = 0.297, 12 H group, ).
Figure 1. Effect of acute heat stress on body mass (A), body temperature (B), and RMR (C) of Eurasian tree sparrows. The number in brackets within each bar represents the animal number. Data are shown as mean ± SE. Bars with different superscript letters indicate significant differences.
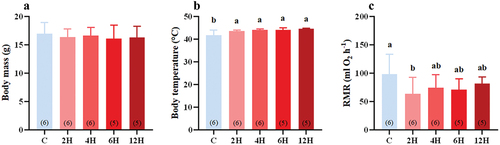
Table I. Effect of acute heat stress on water intake in Eurasian tree sparrows.
Liver COX activity and S4R
There was no significant difference in liver S4R between the control group and the heat-stressed groups (F(4,23) = 0.570, p = 0.687, ), despite the tendency of S4R to increase under heat stress.
Figure 2. Effect of acute heat stress on S4R (A), and liver COX activity (B), of Eurasian tree sparrows. The number in brackets within each bar represents the animal number. Data are shown as mean ± SE. Bars with different superscript letters indicate significant differences.
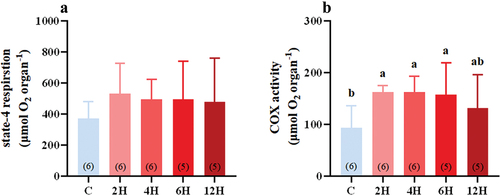
However, acute heat stress had a certain effect on liver COX activity (F(4,23) = 2.616, P = 0.062, ). Compared with the control group (93.203 ± 17.417 μmol O2·organ−1), the heat-stressed group displayed a significant increase in liver COX activity, which reached a peak after 2 h of stress (162.86 ± 4.834 μmol O2·organ−1, P = 0.014, ) and lasted until 6 h (157.71 ± 27.610 μmol O2·organ−1, P = 0.027, ). After 12 hours of stress, there was no significant difference in COX activity between the 12 H group (131.10 ± 29.249 μmol O2·organ−1) and each of the other groups (P = 0.179, the control group; P = 0.257, 2 H group; P = 0.259, 4 H group; P = 0.361, 6 H group, ).
MDA and the activity of SOD, CAT, and GSH-PX
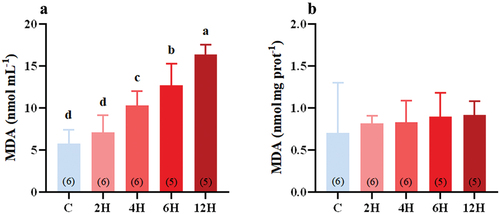
Acute heat stress had a great effect on the serum MDA content of Eurasian tree sparrows (F(4,23) = 27.823, P < 0.001, ). After 2 h of heat stress, the MDA content in the serum increased slightly and continued to increase with the continuation of heat stress (). There was a significant increase in the serum MDA content after 4 h (107 nmol·mL−1) of heat stress compared with that of the control birds (5.761 ± 0.679 nmol·mL−1, P < 0.001). The serum MDA content continued to increase with prolonged heat stress and reached the highest value at 12 h (16.388 ± 0.522 nmol·mL−1, P < 0.001, ). However, there was no significant change in liver MDA content following heat stress (F(4,23) = 0.350, P = 0.841, ).
Figure 3. Effect of acute heat stress on the content of serum (A), and liver (B), MDA levels in Eurasian tree sparrows. The number in brackets within each bar represents the animal number. Data are shown as mean ± SE. Bars with different superscript letters indicate significant differences.
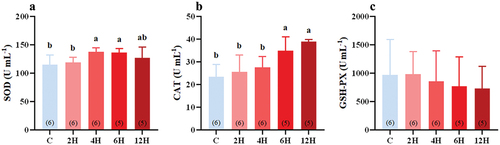
Except for GSH-PX activity (F(4,23) = 0.270, P = 0.894, ), SOD activity (H = 11.700, p = 0.020, ), and CAT (F(4,23) = 7.795, p < 0.001, ), activity in the serum of tree sparrows changed significantly following heat stress. Serum SOD activity first increased and then decreased (), peaking at 137.964 ± 2.844 U·mL−1 after 4 h of stress, and this was significantly different from that of the control group (114.802 ± 7.146 U·mL−1, P = 0.009, ). The increase was maintained up to 6 h of stress, reaching 136.723 ± 2.912 U·mL−1 (). After heat stress for 12 h (127.302 ± 8.524 U·mL−1), SOD activity decreased to a level similar to those of the control group (p = 0.227, ) and the groups subjected to 2–6 h heat treatment (P = 0.275, 2 H group; P = 0.199, 4 H group; P = 0.290, 6 H group, ). After 2 h of heat stress, tree sparrows displayed an increase in serum CAT activity over their control counterparts and reached a maximum level (39.01 ± 0.41 U·mL−1) after 12 h of stress compared with the control birds (P < 0.001, ). In contrast, serum GSH-PX activity was not significantly affected by heat stress since the level of serum GSH-PX activity did not differ significantly between the control and heat-stressed groups, although it tended to decrease in the heat-stressed groups (F(4,23) = 0.270, P = 0.894, ).
Figure 4. Effect of acute heat stress on the activity of SOD (A), CAT (B), and GSH-PX (C), levels in the serum of Eurasian tree sparrows. The number in brackets within each bar represents the animal number. Data are shown as mean ± SE. Bars with different superscript letters indicate significant differences.
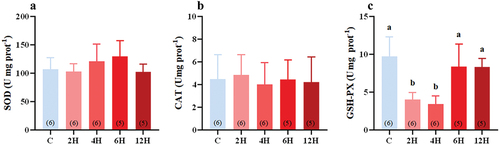
Likewise, heat stress did not cause any significant changes in the liver SOD activity (F(4,23) = 1.643, P = 0.197, ) or CAT activity (F(4,23) = 0.158, P = 0.957, ) of tree sparrows. However, the liver GSH-PX activity of heat-stressed groups was significantly different from that of the control group (H = 19.323, P = 0.001, ), with significantly lower levels after 2 h (4.014 ± 0.387 U·mg·prot−1, P = 0.032), and 4 h (3.419 ± 0.435 U·mg·prot−1, P = 0.005) of heat stress compared with the control group (9.715 ± 1.051 U·mg·prot−1, ). Interestingly, further heat stress appeared to restore the liver GSH-PX activity of the heat-stressed group to a level comparable with that of the control group (8.375 ± 1.340 U·mg·prot−1 at 6 h, P = 1.000; 8.307 ± 0.525 U·mg·prot−1 at 12 h, P = 1.000, ).
Figure 5. Effects of acute heat stress on the activity of SOD (a), CAT (b), and GSH-PX (c) in the liver of Eurasian tree sparrows. The number in brackets within each bar represents the animal number. Data are shown as mean ± SE. Bars with different superscript letters indicate significant differences.
Discussion
When subject to high temperatures, the production of heat in many small birds can be regulated by the adjustment of the indexes associated with metabolic heat production, such as body mass (Zhou et al. Citation2016; Li et al. Citation2020), body temperature (Xia et al. Citation2013), metabolic rate (Zheng et al. Citation2013; Zhou et al. Citation2016; Hu et al. Citation2017), and mitochondrial respiration (Zheng et al. Citation2013; Cui et al. Citation2019). At the same time, the adjustment of heat production is also accompanied by an enhancement of antioxidant enzyme activity to resist heat stress. The data we obtained for Eurasian tree sparrows were consistent with the changes in the indexes associated with metabolic heat production and oxidative responses as a result of the acute heat treatment conducted on the birds.
Effects of acute heat stress on thermoregulation
Ambient temperature is one of the important environmental factors affecting the body mass of birds. A change in body mass in response to a change in the ambient temperature is considered to be an important survival strategy for many small birds (Williams & Tieleman Citation2000; Tieleman et al. Citation2003; Klaassen et al. Citation2004; Vézina et al. Citation2006; Hu et al. Citation2017). When the ambient temperature increases, small birds tend to reduce their body mass to reduce heat production (Gardner et al. Citation2011; Sheridan & Bickford Citation2011; Goodman et al. Citation2012). There was no significant change in body mass in Eurasian tree sparrows after 12 h of acute heat stress (). The reason for this could be that 12 h was too short to cause any adaptive adjustment in the body mass of tree sparrows.
When the ambient temperature approaches or exceeds the bird’s body temperature, the body temperature will increase with the ambient temperature (Talbot et al. Citation2018; Mckechnie et al. Citation2021). This is an acute hyperthermic response, that is, the increase in the bird’s body temperature causes an increase in the gradient between the body and the ambient temperature, thereby increasing heat dissipation to maintain survival through passive heat loss (Tieleman & Williams Citation1999; Smith et al. Citation2017). Tree sparrows have an average body temperature of about 42°C in the TNZ (Deng & Zhang Citation1990). Their body temperature increased when the ambient temperature was increased to 42°C (), probably as a result of increased heat loss via passive heat dissipation. Such a rise in body temperature in response to increased ambient temperature might, therefore, be conducive to their survival under acute heat stress.
Reducing energy expenditure is essential for birds exposed to high temperatures (Williams & Tieleman Citation2000; Cui et al. Citation2019; Xuanyuan et al. Citation2023). It has been reported that Eurasian tree sparrows acclimatized to 30°C have a significantly lower BMR compared with those acclimatized to 10°C (Li et al. Citation2020). Many other small birds also show significantly lower BMR at high temperatures than at lower temperatures, such as red-billed leiothrixes (Cui et al. Citation2019), hwameis (Zhou et al. Citation2016), Chinese bulbuls (Zheng et al. Citation2013; Hu et al. Citation2017), and hoopoe larks (Alaemon alaudipes) (Williams & Tieleman Citation2000). For tree sparrows subjected to heat stress, their RMR decreased rapidly and significantly relative to the control group, but only after 2 h of exposure to 42°C, suggesting that tree sparrows might have a rapid response to acute heat stress. However, the BMR of the heat-stressed birds reversed to that of the control birds after 2 h of acute heat stress (). This was different from the changes in RMR manifested by small birds subjected to warm acclimation, suggesting that small birds might employ different strategies to cope with acute heat stress and warm acclimation. At high temperatures, evaporative water loss also tends to increase to match the increase in heat dissipation (Tieleman et al. Citation2003; Williams et al. Citation2012; Xia et al. Citation2013). When the ambient temperature is too high or even above the body temperature, evaporation is the main way for heat dissipation. However, this can potentially lead to a water imbalance in the body, which requires hydration to maintain water balance (Cooper & Withers Citation2014; Baldo et al. Citation2015). Although evaporative water loss was not directly measured in this study, we found that the water intake of tree sparrows increased when they were exposed to 42°C, a high ambient temperature. This could be due to increased evaporative water loss during acute heat stress, which might consequently slow down the increase in body temperature.
RMR is primarily thought to be functionally derived from metabolically active organs (Swanson et al. Citation2017). The liver is one of the largest and most metabolically active organs in birds (Villarin et al. Citation2003; Zheng et al. Citation2008, Citation2014), contributing up to 25% of total body heat production (Rolfe & Brown Citation1997). S4R is mainly contributed by protein leak, the latter is an ineffective cycle of proton pumping and proton back leaking driven by the respiratory chain, and this is the cytological basis for the differences in metabolic thermogenesis in small birds and mammals (Swanson Citation2010; Zheng et al. Citation2014). COX is located at the end of the mitochondrial respiratory chain. It is an oxidizing enzyme that can transfer electrons from a respiratory substrate directly to molecular oxygen through the cytochrome system in the mitochondrial respiratory chain. A change in COX activity can reflect a change in metabolic heat production in the tissue and a change in aerobic metabolism capacity cells (Wang et al. Citation2006; Zheng et al. Citation2008). Some studies have found that birds kept in a high-temperature environment display changes in S4R and COX activity that are consistent with the changes in their RMR. For example, Chinese bulbuls were found to have reduced RMR accompanied by reduced S4R and COX activity when kept at 30°C compared to 10°C (Zheng et al. Citation2013). Therefore, we predicted that S4R and COX activity in the livers of tree sparrows may decrease under acute heat stress. However, our results revealed no significant change in S4R in tree sparrows exposed to 42°C (), while their RMR was significantly reduced after 2 h of exposure. Contrary to the change in RMR, these birds showed significantly higher liver COX activity, further contradicting our predictions. Our previous studies have found a significant positive correlation between the changes in RMR and liver COX activity in most small birds that underwent temperature adaptation. For example, hwameis, red-billed leiothrixes, and white-shouldered starlings (Sturnus sinensis) were found to have decreased BMR and liver COX activity under warm acclimation compared with cold acclimation (Zhou et al. Citation2016; Cui et al. Citation2019; Li et al. Citation2024). However, for some small birds, changes in BMR are not consistent with changes in liver COX activity. For example, in silky starling (Sturnus sericeus), BMR was found to be significantly higher in winter than in summer, but no significant difference in liver COX activity between the two seasons and no statistically significant correlation between BMR and liver COX activity were found (Li et al. Citation2017). Thus, for birds, a change in liver COX activity is often accompanied by a change in BMR, but such a relationship is not universal for birds under all selective regimes (Swanson et al. Citation2017).
Interestingly, the effect of mitochondrial respiratory function on BMR in birds under different temperatures has also been investigated using a different mitochondrial enzyme. For example, the BMR of black-capped chickadees (Poecile atricapillus) acclimated to −10°C was found to have a significant positive correlation with the activity level of liver mitochondrial oxidative phosphorylation complex enzyme I (OXPHOSCI), although such correlation was not observed when these birds were acclimated to 27°C, which is within their TNZ (Milbergue et al. Citation2022). The finding suggests that the effect of liver mitochondrial function on BMR might be influenced by temperature. Similarly, our data also indicated a lack of effect exerted by mitochondrial respiratory function on BMR and that liver COX activity could only affect the BMR at a low temperature, as in the case of black-capped chickadees. The increase in COX activity observed for the tree sparrows might be related to other biological processes and needs further study.
It should be noted that thermoregulation does not only involve the liver. Although the liver is considered to be the main organ of thermoregulation, the skeletal muscle also plays an important role in thermoregulation. In birds, the skeletal muscle comprises nearly 40% of their body mass, and it provides heat in response to cold primarily through shivering heat production (Hohtola Citation1982; Zhou et al. Citation2016). The skeletal muscle also has a high aerobic capacity, so heat production can be increased by increasing its mass and/or cellular metabolic intensity (Vézina et al. Citation2006; Swanson et al. Citation2014; Li et al. Citation2020). In addition, skeletal muscle is the main site of non-shivering thermogenesis (NST), and seasonal cold can cause it to undergo histological remodeling and mitochondrial adaptation (Pani & Bal Citation2022; Pani et al. Citation2023). We have previously explored the metabolic heat production of bird muscles under low-temperature acclimation or winter adaptation by measuring S4R and COX activity (Zheng et al. Citation2008, Citation2012; Wu et al. Citation2015; Li et al. Citation2017, Citation2020, Citation2024). In general, the muscles of birds exposed to low temperatures show higher S4R and COX activity. We will explore the changes in muscle metabolism in future heat adaptation studies to explore the effect of muscle on the metabolic heat production of birds in a high-temperature environment to adapt to the environmental temperature.
Effects of heat stress on oxidative damage and antioxidant enzymes
High temperatures are one of the reasons for the increased ROS production in birds (Magni et al. Citation1994; Mahmoud & Edens Citation2003; Liu et al. Citation2014; Xu et al. Citation2022). Excessive ROS can lead to oxidative stress in organisms, causing various oxidative damage, including lipid peroxidation (Hélène et al. Citation2004; Kwiecien et al. Citation2014). MDA is one of the important lipid peroxidation products that can represent the level of cellular lipid peroxidation (Habashy et al. Citation2019). The MDA content in the serum of Eurasian tree sparrows increased significantly after exposure to 42°C for 4 to 6 h (), suggesting that oxidative stress could be induced even after a brief exposure to heat. Similarly, previous studies found that after 24 h of exposure to 34 ± 1°C, the serum MDA concentration of broilers increased significantly to 3.30 ± 0.18 U·mL, compared with 2.41 ± 0.2 U·mL for broilers exposed to 23 ± 1°C (Ouyang et al. Citation2022). Therefore, acute heat stress in general could lead to oxidative stress in birds. There was no significant change in the content of MDA in the liver for tree sparrows exposed to 42°C for 12 h (). The lack of a significant increase in the liver MDA level might suggest that the liver was less affected by heat stress.
SOD, CAT, and GSH-PX activities in the serum and liver of Eurasian tree sparrows were measured to evaluate the antioxidant capacity of the birds under heat stress. SOD can degrade the superoxide anion (O2·−) into H2O2 and O2 (Fridovich Citation1997; Okoye et al. Citation2019). Tree sparrows exposed to 42°C for 4–6 h displayed a significant increase in serum SOD activity (), consistent with the results reported for other birds. For example, increased serum SOD activity levels were found in Japanese quals and broiler chickens following short-term exposure to heat (Sandhu & Kaur Citation2002; Sahin et al. Citation2003; Altan et al. Citation2003). The pattern of changes in serum CAT activity observed for tree sparrows in response to heat stress was quite similar to that of SOD. Serum CAT activity increased following two hours of heat stress and continued to rise with further exposure, similar to that observed for the MDA level (). In broilers, heat stress at 38°C was also found to induce a significant increase in serum CAT activity necessary to eliminate the increased amount of ROS (Altan et al. Citation2003). We did not observe a significant change in serum GSH-PX activity in tree sparrows under acute heat stress (). This might be due to the fact that H2O2 was produced when ROS was eliminated by SOD, and the clearance of H2O2 consumed GSH-PX.
Different from the results obtained for the serum, the liver SOD and CAT activities of tree sparrows were neither significantly increased nor significantly decreased under acute heat stress ()). The activity of GSH-PX in the liver of tree sparrows decreased significantly after 2 h and 4 h of heat stress. However, liver GSH-PX returned to the control level after 6 h of heat stress. GSH-PX mainly exists in the cytoplasm and mitochondria of liver cells, whereas CAT is mainly present in the microsomes (Krämer Citation2000; Hosokawa et al. Citation2000; Hassan et al. Citation2006). Mitochondria are the power factories of cells and are closely related to the energy metabolism of cells (Walters et al. Citation2016). GSH-PX activity decreased significantly after 2 h of heat exposure, whereas CAT was not significantly changed (). This might be due to the fact that under acute heat exposure, H2O2 production first increased in the mitochondria, the organelles with the most vigorous metabolic activity, so GSH-PX located in mitochondria was the first to clear the H2O2 and, in the process, became inhibited. With prolonged heat stress, GSH-PX activity recovered and reached the control-group level. This might indicate that tree sparrows might have the ability to quickly adjust and regain the activities of antioxidant enzymes in the liver during a short period of time to protect the liver cells and maintain the physiological functions of the liver. This might also be an important reason why MDA in the liver did not increase significantly. This, together with the results of no significant changes in liver SOD and CAT activities, could indicate that heat stress might not have a significant effect on the liver. In summary, MDA content and SOD and CAT activities in the serum increased under acute heat stress, indicating that short-term exposure to a high-temperature environment may cause oxidative stress in tree sparrows. It also showed that tree sparrows could quickly adjust and restore their antioxidant enzyme activity levels in a short time, suggesting that these birds might have good antioxidant ability to resist the adverse effects of high temperatures. However, the antioxidant capacity did not reach the level that could protect fatty acids from oxidative damage.
Conclusion
In conclusion, Eurasian tree sparrows could become resistant to heat stress within a 12-h period of exposure to 42°C. Thus, they were considered sensitive to changes in environmental temperature and could respond to stress when stimulated by high temperatures in the short term. In the face of acute heat stress, tree sparrows showed a decreasing trend in RMR, resulting in lower heat production, a significant increase in serum SOD and CAT activity, and the active elimination of body-produced ROS by liver GSH-PX. Therefore, tree sparrows might have a certain ability to resist high temperatures and could maintain normal life activities through the regulation of heat production and the antioxidant system.
Acknowledgements
This work was supported by the National Natural Science Foundation of China. We would like to express our gratitude to Dr. Alan K. Chang (Wenzhou University) for his helpful discussion and kind effort in revising the language of the manuscript. We also thank the anonymous reviewers for their helpful comments and suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akbarian A, Michiels J, Degroote J, Majdeddin M, Golian A, De Smet S. 2016. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of Animal Science and Biotechnology 7:36. DOI: 10.1186/s40104-016-0097-5.
- Altan O, Pabuçcuoğlu A, Altan A, Konyalioğlu S, Bayraktar H. 2003. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. British Poultry Science 44(4):545–550. DOI: 10.1080/00071660310001618334.
- Bai ML, Wu XJ, Cai KJ, Zheng WH, Liu JS. 2016. Relationships between interspeciifc differences in the mass of internal organs, biochemical markers of metabolic activity, and the thermogenic properties of three small passerines. Avian Research 7(1):32–40. DOI: 10.1186/s40657-016-0046-1.
- Baldo MB, Antenucci CD, Luna F. 2015. Effect of ambient temperature on evaporative water loss in the subterranean rodent Ctenomys talarum. Journal of Thermal Biology 53:113–118. DOI: 10.1016/j.jtherbio.2015.09.002.
- Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signalling. Journal of Experimental Botany 65(5):1229–1240. DOI: 10.1093/jxb/ert375.
- Castiglione GM, Xu Z, Zhou L, Duh EJ. 2020. Adaptation of the master antioxidant response connects metabolism, lifespan and feather development pathways in birds. Nature Communications 11:2476. DOI: 10.1038/s41467-020-16129-4.
- Chen J, Bhandar B, Kavdia M. 2015. Interaction of ROS and RNS with GSH and GSH/GPX Systems. The FASEB Journal 29(S1):636–637. DOI: 10.1096/fasebj.29.1_supplement.636.7.
- Chen KX, Wang CM, Wang GY, Zhao ZJ. 2014. Energy budget, oxidative stress and antioxidant in striped hamster acclimated to moderate cold and warm temperatures. Journal of Thermal Biology 44:35–40. DOI: 10.1016/j.jtherbio.2014.06.005.
- Collin A, Buyse J, As PV, Darras VM, Malheiros RD, Moraes VMB, Reyns GE, Taouis M, Decuypere E. 2003. Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. General & Comparative Endocrinology 130:70–77. DOI: 10.1016/S0016-6480(02)00571-3.
- Cooper CE, Withers PC. 2014. Physiological responses of a rodent to heliox reveal constancy of evaporative water loss under perturbing environmental conditions. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 307:R1042–R1048. DOI: 10.1152/ajpregu.00051.2014.
- Costantini D. 2008. Oxidative stress in the ecology and evolution of bird. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 11:1238–1251. DOI: 10.1016/j.cbpa.2008.05.163.
- Cui DQ, Wang N, Ge JR, Xu JY, Liu J. 2019. The role of temperature as a driver of metabolic flexibility in the Red-billed Leiothrix (Leiothrix lutea). Avian Research 10:46. DOI: 10.1186/s40657-019-0184-3.
- Deng HL, Zhang XA. 1990. Standard metabolic rate in several species of passerine birds in alpine meadow. Acta Zoologica Sinica 36:377–381.
- Diffenbaugh NS, Field CB. 2013. Changes in ecologically critical terrestrial climate conditions. Science 341(6145):486–492. DOI: 10.1126/science.1237123.
- Dixit AS, Bamon I, Singh NS. 2018. Temperature modulates photoperiodic seasonal responses in the subtropical tree sparrow, Passer montanus. Journal of Comparative Physiology, A Sensory, Neural, and Behavioral Physiology 204:721–735. DOI: 10.1007/s00359-018-1272-2.
- Else PL. 2004. Respiration rate of hepatocytes varies with body mass in birds. Journal of Experimental Biology 207:2305–2311. DOI: 10.1242/jeb.01017.
- Farag MR, Mahmoud A. 2018. Physiological alterations of poultry to the high environmental temperature. Journal of Thermal Biology 76:101–106. DOI: 10.1016/j.jtherbio.2018.07.012.
- Flanagan SW, Moseley PL, Buettner GR. 1998. Increased flux of free radicals in cells subjected to hyperthermia: Detection by electron paramagnetic resonance spin trapping. FEBS Letters 431:285–296. DOI: 10.1016/S0014-5793(98)00779-0.
- Fridovich I. 1997. Superoxide Anion Radical (O2·−), Superoxide Dismutases, and Related Matters. Journal of Biological Chemistry 272:18515–18517. DOI: 10.1074/jbc.272.30.18515.
- Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. 2011. Declining body size: A third universal response to warming? Trends in Ecology & Evolution 26(6):285–291. DOI: 10.1016/j.tree.2011.03.005.
- Goodman RE, Lebuhn G, Seavy NE, Gardali T, Bluso-Demers JD. 2012. Avian body size changes and climate change: Warming or increasing variability? Global Change Biology 18:63–73. DOI: 10.1111/j.1365-2486.2011.02538.x.
- Habashy WS, Milfort MC, Rekaya R, Aggrey SE. 2019. Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. International Journal of Biometeorology 63:1569–1584. DOI: 10.1007/s00484-019-01769-z.
- Halliwell B, Aruoma OI. 1991. DNA damage by oxygen-derived species. its mechanism and measurement in mammalian systems. FEBS Letters 281:9–19. DOI: 10.1016/0014-5793(91)80347-6.
- Hassan MJ, Zhu Z, Ahmad B, Mahmood Q. 2006. Influence of cadmium toxicity on rice genotypes as affected by zinc, sulfur and nitrogen fertilizers. University of Guilan 4:1–8.
- Hélène M, Tiphaine M, Camille G, François L, Béatrice R. 2004. Seasonal variations in antioxidant defences in blue mussels Mytilus edulis collected from a polluted area: Major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and of glutathione S-transferase. Aquatic Toxicology (Amsterdam, Netherlands) 70:83–93. DOI: 10.1016/j.aquatox.2004.07.003.
- Hohtola E. 1982. Thermal and electromyographic correlates of shivering thermogenesis in the pigeon. Comparative Biochemistry and Physiology Part A: ComparativePhysiology 73(2):159–166. DOI: 10.1016/0300-9629(82)90049-4.
- Hosokawa T, Okabe M, Saito S. 2000. Regional difference of metallothionein induction and DNA damage in rat kidney by cis-diamminedichloroplatinum. Basic & Clinical Pharmacology & Toxicology 86:276–282. DOI: 10.1111/j.0901-9928.2000.860606.x.
- Huang C, Jiao H, Song Z, Zhao J, Wang X, Lin H. 2015. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. Journal of Animal Science 93(5):2144–2153. DOI: 10.2527/jas.2014-8739.
- Hu SN, Zhu YY, Lin L, Zheng WH, Liu JS. 2017. Temperature and photoperiod as environmental cues affect body mass and thermoregulation in Chinese bulbuls Pycnonotus sinensis. Journal of Experimental Biology 220:844–855. DOI: 10.1242/jeb.143842.
- Jiao XY, Yang K, An Y, Teng XY, Teng XH. 2017. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environmental Science & Pollution Research International 24:7555–7564. DOI: 10.1007/s11356-016-8329-y.
- Klaassen M, Oltrogge M, Trost L. 2004. Basal metabolic rate, food intake, and body mass in cold- and warm-acclimated Garden Warblers. Comparative Biochemistry & Physiology Part A Molecular & Integrative Physiology 137(4):639–647. DOI: 10.1016/j.cbpb.2003.12.004.
- Koim-Puchowska B, Drozdz-Afelt JM, Lamparski R, Menka A, Kaminski P. 2020. Antioxidant defence barrier of great tit Parus major nestlings in response to trace elements. Environmental Science and Pollution Research 27(16):20321–20334. DOI: 10.1007/s11356-020-08495-9.
- Krämer R. 2000. The pharmaceutical potential of manganese‐based superoxide dismutase mimics. Angewandte Chemie International Edition 39(24):4469–4470. DOI: 10.1002/1521-3773(20001215)39:24<4469:AID-ANIE4469>3.0.CO;2-9.
- Kunwar A, Priyadarsini KI. 2011. Free radicals, oxidative stress and importance of antioxidants in human health. Journal of Medical & Allied Ences 1:53–60.
- Kwiecien S, Jasnos K, Magierowski M, Sliwowski Z, Pajdo R, Brzozowski B, Mach T, Wojcik D, Brzozowski T. 2014. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress - induced gastric injury. Journal of Physiology & Pharmacology 65:613–622.
- Li L, Ge JR, Zheng SY, Hong LH, Liu JS. 2020. Thermogenic responses in Eurasian Tree Sparrow (Passer montanus) to seasonal acclimatization and temperature-photoperiod acclimation. Avian Research 11:35. DOI: 10.1186/s40657-020-00222-9.
- Liknes ET, Swanson DL. 2011. Phenotypic flexibility in passerine birds: seasonal variation of aerobic enzyme activities in skeletal muscle. Journal of Thermal Biology 36:430–436. DOI: 10.1016/j.jtherbio.2011.07.011.
- Li M, Sun YQ, Mao HZ, Xu JH, Zheng WH, Liu JS. 2017. Seasonal phenotypic flexibility in body mass, basal thermogenesis, and tissue oxidative capacity in the male Silky Starling (Sturnus sericeus). Avian Research 8:25. DOI: 10.1186/s40657-017-0083-4.
- Liu LL, He JH, Xie HB, Yang YS, Zou Y. 2014. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poultry Science 93:54–62. DOI: 10.3382/ps.2013-03423.
- Liu JS, Li M. 2006. Phenotypic flexibility of metabolic rate and organ masses among tree sparrows Passer montanus in seasonal acclimatization. Acta Zoologica Sinica 533:467–477.
- Li M, Xu MR, Wang J, Yao YQ, Zhang XH, Liu JS. 2024. Phenotypic flexibility in metabolic adjustments and digestive function in white-shouldered starlings: Responses to short-term temperature acclimation. Journal of Experimental Biology 227. DOI: 10.1242/jeb.246214.
- Li M, Yin YJ, Nie CY, Qu LN, Zhang GF, Liang YT, Zhao XJ, Liu JS. 2010. Seasonal variations of serum thyroid hormone and its effect on thermoregulation in the tree sparrow (Passer montanus). Sichuan Journal of Zoology 29:530–534.
- Lord-Fontaine S, Averill-Bates DA. 2002. Heat shock inactivates cellular antioxidant defenses against hydrogen peroxide: Protection by glucose. Free Radical Biology & Medicine 32:752–765. DOI: 10.1016/S0891-5849(02)00769-4.
- Mackinnon J, Phillipps K. 2000. A field guide to the birds of China. Colonial Waterbirds 18:841–843.
- Magni F, Panduri G, Paolocci N. 1994. Hypothermia triggers iron-dependent lipoperoxidative damage in the isolated rat heart. Free Radical Biology and Medicine 16(4):465–476. DOI: 10.1016/0891-5849(94)90124-4.
- Mahmoud KZ, Edens FW. 2003. Influence of selenium sources on age-related and mild heat stress-related changes of blood and liver glutathione redox cycle in broiler chickens (Gallus domesticus). Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology 136:921–934. DOI: 10.1016/S1096-4959(03)00288-4.
- Mao LY, Xu JY, Shi L, Zheng WH, Liu JS. 2019. Food restriction decreases thermoregulation in the silky starling Sturnus sericeus (Aves: Passeriformes). The European Zoological Journal 86:322–332. DOI: 10.1080/24750263.2019.1665114.
- Mckechnie AE, Freckleton RP, Jetz W. 2006. Phenotypic plasticity in the scaling of avian basal metabolic rate. Proceedings of the Royal Society B: Biological Sciences 273:931–937. DOI: 10.1098/rspb.2005.3415.
- Mckechnie AE, Gerson AR, Wolf BO. 2021. Thermoregulation in desert birds: Scaling and phylogenetic variation in heat tolerance and evaporative cooling. Journal of Experimental Biology 224:jeb229211. DOI: 10.1242/jeb.229211.
- Milbergue MS, Vézina F, Desrosiers V, Blier PU. 2022. How does mitochondrial function relate to thermogenic capacity and basal metabolic rate in small birds? The Journal of Experimental Biology 225. DOI: 10.1242/jeb.242612.
- Moller AP. 2010. Basal metabolic rate and risk-taking behaviour in birds. Journal of Evolutionary Biology 22:2420–2429. DOI: 10.1111/j.1420-9101.2009.01850.x.
- Mujahid A, Akiba Y, Toyomizu M. 2007. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poultry Science 86(2):364–371. DOI: 10.1093/ps/86.2.364.
- Nawab A, Ibtisham F, Li G, Kieser B, Wu J, Liu W, Zhao Y, Nawab Y, Li K, Xiao M. 2018. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. Journal of Thermal Biology 78:131–139. DOI: 10.1016/j.jtherbio.2018.08.010.
- Okoye CN, MacDonald-Jay N, Kamunde C. 2019. Effects of bioenergetics, temperature and cadmium on liver mitochondria reactive oxygen species production and consumption. Aquatic Toxicology 214. DOI: 10.1016/j.aquatox.2019.105264.
- Olalekan LA, Folusho LA, Ologundudu A, Yakubu AO, Omonkhua A, ObI F. 2011. Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. Journal of Toxicological Sciences 36:549–557. DOI: 10.2131/jts.36.549.
- Ouyang JX, Zhou H, Li QF, Zheng J, Chen C, Guo SP, You JM, Li GH. 2022. Tryptophan alleviates acute heat stress-induced impairment of antioxidant status and mitochondrial function in broilers. Frontiers in Veterinary Science 9:863156. DOI: 10.3389/fvets.2022.863156.
- Pamplona R, Costantini D. 2011. Molecular and structural antioxidant defenses against oxidative stress in animals. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 301:843–863. DOI: 10.1152/ajpregu.00034.2011.
- Pani P, Bal NC. 2022. Avian adjustments to cold and non-shivering thermogenesis: Whats, wheres and hows. Biological Reviews of the Cambridge Philosophical Society 97:2106–2126. DOI: 10.1111/brv.12885.
- Pani P, Swalsingh G, Pani S, Senapati U, Sahu B, Pati B, Rout S, Bal NC. 2023. Seasonal cold induces divergent structural/biochemical adaptations in different skeletal muscles of Columba livia: Evidence for nonshivering thermogenesis in adult birds. Biochemical Journal 480:1397–1409. DOI: 10.1042/BCJ20230245.
- Riddell EA, Iknayan KJ, Hargrove L, Tremor S, Patton JL, Ramirez R, Wolf BO, Beissinger SR. 2021. Exposure to climate change drives stability or collapse of desert mammal and bird communities. Science 371:633–636. DOI: 10.1126/science.abd4605.
- Rolfe DF, Brown GC. 1997. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological Reviews 77:731–758. DOI: 10.1152/physrev.1997.77.3.731.
- Sahin K, Onderci M, Sahin N, Gursu MF, Kucuk O. 2003. Dietary vitamin C and folic acid supplementation ameliorates the detrimental effects of heat stress in Japanese quail. The Journal of Nutrition 133(6):1882–1886. DOI: 10.1093/jn/133.6.1882.
- Sandhu SK, Kaur G. 2002. Alterations in oxidative stress scavenger system in aging rat brain and lymphocytes. Biogerontology 3(3):161–173. DOI: 10.1023/A:1015643107449.
- Sheridan JA, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nature Climate Change 1(8):401–406. DOI: 10.1038/nclimate1259.
- Skrip MM, McWilliams SR. 2016. Oxidative balance in birds: An atoms-to-organisms-to-ecology primer for ornithologists. Journal of Field Ornithology 87:1–20. DOI: 10.1111/jofo.12135.
- Smith EK, O’Neill J, Gerson AR, Mckechnie AE, Wolf BO. 2017. Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert songbirds. Journal of Experimental Biology 220:3290–3300. DOI: 10.1242/jeb.161141.
- Smith EK, O’Neill J, Gerson AR, Wolf BO. 2015. Avian thermoregulation in the heat: Resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert doves and quail. Journal of Experimental Biology 218:3636–3646. DOI: 10.1242/jeb.128645.
- Soorim S, Beissinger SR. 2019. Environmental determinants of total evaporative water loss in birds at multiple temperatures. The Auk 137:1–12. DOI: 10.1093/auk/ukz069.
- Stillman JH. 2019. Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34:86–100. DOI: 10.1152/physiol.00040.2018.
- Swanson DL. 2010. Seasonal metabolic variation in birds: functional and mechanistic correlates. Current Ornithology 17:75–129.
- Swanson DL, Agin TJ, Zhang Y, Oboikovitz P, Dubay S. 2020. Metabolic flexibility in response to within-season temperature variability in house sparrows. Integrative Organismal Biology 2(1). DOI: 10.1093/iob/obaa039.
- Swanson DL, Mckechnie AE, Vézina F. 2017. How low can you go? An adaptive energetic framework for interpreting basal metabolic rate variation in endotherms. Journal of Comparative Physiology B 187(8):1039–1056. DOI: 10.1007/s00360-017-1096-3.
- Swanson D, Zhang Y, Liu JS, Merkord CL, King MO. 2014. Relative roles of temperature and photoperiod as drivers of metabolic flexibility in dark-eyed juncos. Journal of Experimental Biology 217:866–875. DOI: 10.1242/jeb.096677.
- Talbot WA, Gerson AR, Smith EK, Mckechnie AE, Wolf BO. 2018. Avian thermoregulation in the heat: Metabolism, evaporative cooling and gular flutter in two small owls. The Journal of Experimental Biology 221:12. DOI: 10.1242/jeb.171108.
- Tieleman BI, Williams JB. 1999. The role of hyperthermia in the water economy of desert birds. Physiological & Biochemical Zoology 72:87–100. DOI: 10.1086/316640.
- Tieleman BI, Williams JB, Buschur ME, Brown CR. 2003. Phenotypic variation of larks along an aridity gradient: Are desert birds more flexible? Ecology 84:1800–1815. DOI: 10.1890/0012-9658(2003)084[1800:PVOLAA]2.0.CO;2.
- Vézina F, Jalvingh KM, Dekinga A, Piersma T. 2006. Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. Journal of Experimental Biology 209(16):3141–3154. DOI: 10.1242/jeb.02338.
- Villarin JJ, Schaeffer PJ, Markle RA, Lindstedt SL. 2003. Chronic cold exposure increases liver oxidative capacity in the marsupial Monodelphis domestica. Comparative Biochemistry & Physiology Part A Molecular & Integrative Physiology 136:621–630. DOI: 10.1016/S1095-6433(03)00210-1.
- Walters JW, Amos D, Ray K, Santanam N. 2016. Mitochondrial redox status as a target for cardiovascular disease. Current Opinion in Pharmacology 27:50–55. DOI: 10.1016/j.coph.2016.01.006.
- Wang JM, Zhang YM, Wang DH. 2006. Seasonal thermogenesis and body mass regulation in plateau pikas (Ochotona curzoniae). Oecologia 149:373–382. DOI: 10.1007/s00442-006-0469-1.
- Wen J, Qiao QG, Zhao ZJ, Wang DH, Zheng WH, Wang ZX, Liu JS. 2020. Effects of thyroid hormones and cold acclimation on the energy metabolism of the striped hamster (Cricetulus barabensis). Journal of Comparative Physiology B, Biochemical, Systemic, and Environmental Physiology 189:153–165. DOI: 10.1007/s00360-018-1197-7.
- Williams JB, Muñoz-Garcia A, Champagne A. 2012. Climate change and cutaneous water loss of birds. Journal of Experimental Biology 215(7):1053–1060. DOI: 10.1242/jeb.054395.
- Williams JB, Tieleman BI. 2000. Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures. Journal of Experimental Biology 203:3153–3159. DOI: 10.1242/jeb.203.20.3153.
- Wu YN, Li L, Xiao YC, Zhou LM, Wu MS, Zhang HY, Liu JS. 2014. Effects of temperature acclimation on body mass and energy budget in the Chinese bulbul Pycnonotus sinensis. Zoological Research 35:33–41. DOI: 10.1186/s40657-014-0004-8.
- Wu MX, Zhou LM, Zhao LD, Zhao ZJ, Zheng WH, Liu JS. 2015. Seasonal variation in body mass, body temperature and thermogenesis in the Hwamei, Garrulax canorus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 179:113–119. DOI: 10.1016/j.cbpa.2014.09.026.
- Xia SS, Yu AW, Zhao LD, Zhang HY, Zheng WH, Liu JS. 2013. Metabolic thermogenesis and evaporative water loss in the Hwamei Garrulax canorus. Journal of Thermal Biology 38:576–581. DOI: 10.1016/j.jtherbio.2013.10.003.
- Xuanyuan YJ, Chen R, Xu JH, Zhou JC, Li M, Liu JS. 2023. Seasonal acclimatization and temperature acclimation in small passerine birds is achieved via metabolic adjustments. Avian Research 14:108–118. DOI: 10.1016/j.avrs.2023.100084.
- Xu RP, Yu CW, Mao LY, Jiang MC, Gao LY, Li M, Liu JS. 2022. Antioxidant defense mechanisms and fatty acid catabolism in Red-billed Leiothrix (Leiothrix lutea) exposed to high temperatures. Avian Research 13:100013. DOI: 10.1016/j.avrs.2022.100013.
- Yamauchi C, Fujita S, Obara TL. 1981. Laboratory animals science. Laboratory Animals 31:251–258.
- Yang ZH, Liu JS, Shao SL. 2010. The effect of short-term continuing food restriction on Passer montanus body weight and BMR and its ecological meaning. Chinese Journal of Zoology 45:119–124.
- Zhao LD, Wang RM, Wu YN, Wu MS, Zheng WH, Liu JS. 2015. Daily variation in body mass and thermoregulation in male Hwamei (Garrulax canorus) at different seasons. Avian Research 7:14–21. DOI: 10.1186/s40657-015-0011-4.
- Zheng WH, Li M, Liu JS, Shao SL, Xu XJ. 2014. Seasonal variation of metabolic thermogenesis in Eurasian Tree Sparrows (Passer montanus) over a latitudinal gradient. Physiological & Biochemical Zoology 87:704–718. DOI: 10.1086/676832.
- Zheng WH, Lin L, Liu JS, Pan H, Cao MT, Hu YL. 2013. Physiological and biochemical thermoregulatory responses of Chinese bulbuls Pycnonotus sinensis to warm temperature: phenotypic flexibility in a small passerine. Journal of Thermal Biology 38:240–246. DOI: 10.1016/j.jtherbio.2013.03.003.
- Zheng WH, Lin L, Liu JS, Xu XJ, Li M. 2012. Geographic variation in basal thermogenesis in little buntings: relationship to cellular thermogenesis and thyroid hormone concentration. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 164:483–490. DOI: 10.1016/j.cbpa.2012.12.004.
- Zheng WH, Liu JS, Jiang XH, Fang YY, Zhang GK. 2008. Seasonal variation on metabolism and thermoregulation in Chinese bulbul. Journal of Thermal Biology 33:315–319. DOI: 10.1016/j.jtherbio.2008.03.003.
- Zheng WH, Liu JS, Shao SL. 2008. Seasonal acclimatization of metabolism in Eurasian tree sparrows (Passer montanus). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 151:519–525. DOI: 10.1016/j.cbpa.2008.07.009.
- Zheng WH, Liu JS, Swanson DL. 2014. Seasonal phenotypic flexibility of body mass, organ masses, and tissue oxidative capacity and their relationship to resting metabolic rate in Chinese bulbuls. Physiological & Biochemical Zoology Pbz 87:432–444. DOI: 10.1086/675439.
- Zhou YY, Jing WX, Dahms HU, Hwang JS, Wang L. 2017. Oxidative damage, ultrastructural alterations and gene expressions of hemocytes in the freshwater crab sinopotamon henanense exposed to cadmium. Ecotoxicology & Environmental Safety 138:130–138. DOI: 10.1016/j.ecoenv.2016.12.030.
- Zhou LM, Xia SS, Chen Q, Wang RM, Zheng WH, Liu JS. 2016. Phenotypic flexibility of thermogenesis in the hwamei (Garrulax canorus): Responses to cold acclimation. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 310:330–336. DOI: 10.1152/ajpregu.00259.2015.
