 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The species-specific and age-related autumn migration strategies of the sedge, reed and great reed warbler were investigated at stopover sites between central Europe and north-eastern Africa during autumn. Adult reed warblers accumulated larger fat reserves than juveniles and consequently were able to cover longer distances from most stopover sites. Juvenile sedge warblers, on the other hand, left Europe with significantly larger fat reserves than adults as opposed to the populations migrating along the western route. Both juvenile and adult great reed warblers were potentially able to make long flights without refuelling from the northern part of the Mediterranean region onwards. It was the proximity of large ecological barriers that had the most prominent influence on the potential flight distances of adult and juvenile Acrocephalus warblers during their southward migration. In reed and sedge warblers, the differences in mean flight distances covered by adults and juveniles were the largest (ca. 600 km) just before the crossing of the Mediterranean Sea, but smaller (ca. 200 and 400 km, respectively) in front of the Sahara Desert. Juvenile and adult great reed warblers were potentially able to cover 1660 km from Turkey, which was also supported by very low numbers of individuals caught at the Egyptian ringing sites. The results of this study carried out in the key regions of the eastern European–African flyway documented that migration strategies differed between adults and juveniles of three long-distance migrant species, yet this variation depended on the migration stage and the proximity of natural ecological barriers.
1. Introduction
The accumulation of energy reserves which fuel the flight bouts is one of the most important adaptations to long-distance migration in birds (Bairlein Citation1991; Barboutis et al. Citation2011), and the flight range of migrants depends on the amount of accumulated fat and proteins (Klaassen Citation1996). Accumulated lipids constitute the primary energy source for migrating birds (Bairlein Citation1991; Jenni & Jenni-Eiermann Citation1998), and long-distance species are known to experience an intensive process of energy accumulation (Schaub & Jenni Citation2000; Wojciechowski et al. Citation2014). The potential distances that migrants are able to cover on their way may be estimated based on the size of their fat stores. In general, the flight range estimations are based on the one-step flight assumption in still air, and thus do not consider the real wind conditions that the migrants encounter en route (Zehtindjiev & Liechti Citation2003). Despite these simplifications, such estimations are widely used in the studies on avian migration strategies, as the knowledge of the distance an individual can cover is crucial to unravel migratory strategies adopted by a species or population (e.g., Arizaga et al. Citation2011; Meissner et al. Citation2011; Ożarowska Citation2015; Stępniewska et al. Citation2018; Sander et al. Citation2020; Mazur et al. Citation2021).
Adult birds usually accumulate larger fat reserves and have higher body masses than juveniles (Ellegren Citation1991; Merom et al. Citation1999; Jones et al. Citation2002; Jakubas & Wojczulanis–Jakubas Citation2010; Meissner et al. Citation2011), as they are more efficient in foraging and searching for abundant feeding sites (Wunderle Citation1991; Hockey et al. Citation1998; Meissner Citation2007; Kozik et al. Citation2022). Due to larger fat reserves, adults may cover longer distances without refuelling en route compared to their first-year conspecifics (Bibby & Green Citation1981; Jakubas et al. Citation2014). Extra fat load, however, induce mass-dependent predation risk due to lower manoeuvrability - therefore young individuals often carry relatively less fat than adults to decrease such risk (Woodrey & Moore Citation1997; Lind et al. Citation1999). There are also morphological differences in wing shape in several species, with adults having more pointed and longer wings than juveniles (Nowakowski Citation2000; Ożarowska et al. Citation2021). Such morphological adaptations allow adults to cover migration distances with lower energy expenditure, whereas more rounded and less pointed wings of juveniles are an anti-predatory adaptation, which is crucial for inexperienced juveniles (Alatalo et al. Citation1984). In general, due to these adaptations, as well as experience from previous migrations, adult individuals are more efficient than juveniles in preparing for migration, covering the migration distances, and competing for resources at stopover sites (Moore et al. Citation2003; McKinnon et al. Citation2014). All this finally increases their survival chances (Berthold Citation1996; Åkesson et al. Citation2021). Thus, it may be assumed that various elements of migration strategies of adult and juvenile birds may differ, and these differences may be expressed to various extents in different species. One of the elements shaping migration strategy may be ecological barriers encountered by birds en route.
There are two large ecological barriers in the Western Palaearctic-African avian migration system, i.e., the Mediterranean Sea and the Sahara Desert (Schaub & Jenni Citation2000; Rubolini et al. Citation2002; Barboutis et al. Citation2014). These natural barriers influence the spatiotemporal pattern of accumulation and utilisation of energy stores by birds along migratory routes at inter- and intraspecific levels (Bibby & Green Citation1981; Bairlein Citation1991; Schaub & Jenni Citation2000; Ożarowska Citation2015; Stępniewska et al. Citation2018). Our knowledge of passerine migration strategies in this region is mainly based upon the studies from the western and central routes used by the populations/species wintering in western and central Africa (e.g., Bibby & Green Citation1981; Schaub & Jenni Citation2000; Rubolini et al. Citation2002; Andueza et al. Citation2014). The studies along the eastern migration route, leading to the eastern and south African wintering grounds, are still limited (e.g., Bairlein Citation1991; Ożarowska Citation2015; Procházka et al. Citation2017; Stępniewska et al. Citation2020). The western and eastern routes differ in abiotic and biotic conditions, like in the width of sea and desert regions, wind conditions, vegetation phenology etc. (Biebach et al. Citation1986; Zehtindjiev & Liechti Citation2003; Erni et al. Citation2005; Briedis et al. Citation2020) which influence the spatiotemporal pattern of energy store accumulation in birds, also within the species (Bairlein Citation1991). During seasonal migrations, the eastern route is used by an impressive number of Palaearctic species (Frumkin et al. Citation1995; Biebach et al. Citation2000), which have to cross extensive ecological barriers, which makes this flyway a perfect choice for the studies on intra- and interspecific variability of bird migration strategies.
In this paper, we studied three common Western Palaearctic species: the reed warbler Acrocephalus scirpaceus, the sedge warbler A. schoenobaenus, and the great reed warbler A. arundinaceus. They are insectivorous, prefer wet habitats (mainly reedbeds), and cover thousands of kilometres when migrating between their European breeding grounds and African wintering quarters (Cramp & Brooks Citation1992; Kennerley & Pearson Citation2010). The reed and sedge warblers are similar small-sized passerines, whereas the great reed warbler is about two-fold larger (Cramp & Brooks Citation1992). Apart from similar biology and ecology, these species differ in their autumn migration strategies. Juvenile sedge and great reed warblers are known to cover longer distances without refuelling than reed warblers when moving southwards (Bibby & Green Citation1981; Schaub & Jenni Citation2000; Stępniewska et al. Citation2018, Citation2020). The autumn migration strategy of adult birds has not been well-studied to date, due to the insufficient number of individuals captured at stop-over sites (Schaub & Jenni Citation2000).
This study aimed to compare the autumn migration strategies of adults and juveniles of the three common Acrocephalus species along the eastern European–African flyway, while taking into account also the species-specific differences. We used the ringing data collected at six distant stopover sites, from Central Europe to north-eastern Africa and focused on the potential flight ranges of adults and juveniles at different stages of their southward journey, analysing the energy reserves of migrants.
2. Materials and methods
2.1. Study area and fieldwork
We used the data collected at six ringing stations from Poland to Egypt, working within the South-East European Bird Migration Network (SEEN): Rakutowskie in Poland, Kalimok in Bulgaria, Kuscenneti in Turkey, Burullus, Wadi El Rayan, and Saluga Ghazal in Egypt (). The total distance between the northernmost and southernmost stations was ca. 3400 km. To enlarge the sample size from Saluga Ghazal, the data from the nearby Wadi Allaqi ringing station (ca. 30 km distant), which operated during a single season, were also included.
Table I. Ringing stations (country, geographic coordinates in brackets, geographical characteristics and location), years of field studies, catching dates, numbers of ringed adults (nad) and juveniles (njuv) of the reed, sedge and great reed warbler. First captures only.
At each station, birds were captured with mist nets, which were placed mainly in reedbeds composed mostly of Phragmites australis. This habitat is optimal for the studied species on migration (Bairlein Citation1983; Cramp & Brooks Citation1992). The fieldwork was carried out in different years, from 2002 to 2015 (). The standard SEEN methodology was applied during the fieldwork, with constant mist-netting effort and a standard set of biometric measurements, including the maximum flattened wing length (1 mm accuracy), body mass (0.1 g accuracy), and fat score based on visual inspection of subcutaneous fat deposits with a nine-step Busse scale, ranging from 0 (no fat visible under the skin in furculum and on the belly) to 8 (furculum and belly completely covered by fat) (Busse & Meissner Citation2015). Captured individuals were aged according to the plumage abrasion (fresh feathers – juveniles, worn – adults) and presence of dark tongue spots (juveniles). All studied species are monomorphic and cannot be sexed in field conditions during autumn migration (Svensson Citation1992).
2.2. Species and age structure
The species composition was presented at each station as the percentage of the first captures of reed, sedge, and great reed warblers among these three species. To show the latitudinal changes in the proportions of age groups, the share of adult and juvenile individuals of each species was presented within the three regions: central Europe, hereafter “C Europe” (Rakutowskie), south-eastern Europe and Asia Minor, hereafter “SE Europe” (Kalimok, Kuscenneti) and north-eastern Africa, hereafter “NE Africa” (Burullus, Wadi El Rayan, and Saluga Ghazal) ().
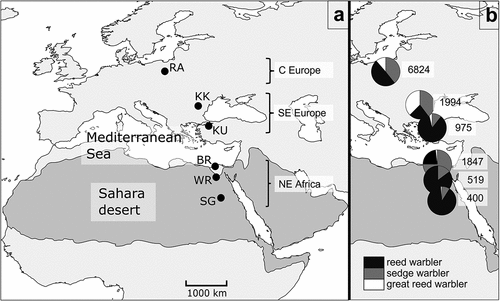
Figure 1. Locations of the ringing stations (black dots): RA – Rakutowskie, KK – Kalimok, KU – Kuscenneti, BR – Burullus, WR – Wadi El Rayan, SG – Saluga Ghazal, grouped into three regions (A) and shares of captured reed (black), sedge (dark grey), and great reed warblers (white) at each station with total number of caught birds of all species at each station (B). The Sahara and the deserts of the Arabian Peninsula are shown as shaded areas (after Rappole & Jones Citation2003; Fransson et al. Citation2006; modified).
2.3. Energy stores and estimation of potential flight range
We used the package “FlyingR” in R environment ver. R 4.1.2 (https://cran.r-project.org/web/packages/FlyingR/index.html, R Core Team Citation2020) to calculate the potential flight ranges of individual migrants (Masinde Citation2020). This is the first package available in the R environment allowing for such estimates and it is based on a time-marching simulation. The theoretical approach is based on the “Pennycuick model” (Pennycuick Citation2008). In our study, the ratio of true airspeed and minimum power speed was held constant, and the muscle mass adjustment criterion was set as “the constant specific work” (CSW), which refers to the work done in each wing-beat contraction by a unit mass of muscles. During migration flight birds consume also energy derived from proteins of their flight muscles and other organs, but proteins are used as a supplementary fuel (Jenni & Jenni-Eiermann Citation1998). CSW adjustment criterion determines how much muscle tissue is taken in each calculation cycle, and it seems to be better than other criteria that have been tested (Pennycuick Citation1998). Flight range estimations were calculated for each individual having wing length and body mass measured, yet only for those captured for the first time in the season (“first captures”), as in other studies (Schaub & Jenni Citation2000; Ożarowska Citation2015; Stępniewska et al. Citation2018, Citation2020).
The parameters used for the estimation of flight ranges were calculated as follows:
Fat mass (kg) – derived from equation:
Lean body mass (LBM) was calculated as the individual size-specific predicted lean body mass (iLBM) by determining the linear regression on body mass with a wing length of birds with fat score = 0, for each species and age group separately (Andueza et al. Citation2014). As the preliminary analysis showed a decrease in mean lean body mass in adults and juveniles along with the latitude (Supplementary Table S1), to calculate fat mass we distinguished two clusters of stations separated by the Mediterranean Sea, i.e., “northern” (Rakutowskie, Kalimok and Kuscenneti) and “southern” (Burullus, Wadi El Rayan and Saluga Ghazal). Due to very low sample sizes of adult reed and sedge warblers with fat score = 0 captured in Egypt (Supplementary Table S2) it was impossible to calculate iLBM for them at “southern” stations. Instead, we used the mean lean body mass (mLBM) of adult reed and sedge warblers with fat score = 0 captured at these locations (Neto et al. Citation2010). The individuals of negative or equal to zero estimated fat masses were incapable of covering any distance according to the “FlyingR”, and thus were excluded from further estimations. The sample of individuals included in these analyses was lower than the total number of captures, as in some adult birds wing length was not measured due to the primaries’ abrasion, particularly in individuals captured at Egyptian stations. Due to the insufficient number of adult great reed warblers captured and measured in Egyptian stations (, Supplementary Table S1), this species was not included in the flight range estimations in NE Africa.
Wing span (m) and wing area (m2) – we used mean values for reed and sedge warblers based on the data published by Hedenström and Møller (Citation1992). In the great reed warbler, we calculated these values based on 20 individuals measured at Rakutowskie station and the Operation Baltic stations in northern Poland (J. K. Nowakowski, unpubl. data). These values were as follows: wing span: 0.1946 m (reed and sedge warbler) and 0.2836 m (great reed warbler); wing area: 0.00773 m2 (reed warbler), 0.00746 m2 (sedge warbler), and 0.01422 m2 (great reed warbler).
To relate the potential flight range (the response variable) with the age of an individual and the location of the stop-over site (“station”) where it was captured (categorical variables), we used the generalized linear model (GLM) with the normal error distribution and identity link function (McCullagh & Nelder Citation1989). The model was run for each species separately. In most cases, the estimated flight ranges did not follow a normal distribution. To normalize these data, they were transformed using the Box-Cox parametric power transformation technique (Sakia Citation1992).
Mean flight distances were calculated for all individuals besides those with negative or equal to zero estimated fat masses, so the data set included individuals with fat reserves large enough to commence migration but also those unready for the long flight without refuelling. To illustrate and analyse this complex pattern, we calculated the share of individuals able to cross the distance from the stations located just in front of the main geographical barriers to their southern edge, i.e., from Kuscenneti in Turkey to the southern edge of the Mediterranean Sea and the Sahara Desert; then from Burullus in the Nile Delta, located just after sea crossing, and from Saluga Ghazal in southern Egypt, located about the half of the desert crossing, to the southern edge of the Sahara Desert (). We assumed a one-step flight without refuelling (Pennycuick Citation2008), and southward direction of the flight (Valkama et al. Citation2014). The width of the eastern part of the Mediterranean Sea was calculated as a linear distance of ca. 600 km (Stępniewska et al. Citation2018), whereas the width of the eastern part of the Sahara Desert is ca. 1800 km (Fransson et al. Citation2006).
Except flight range calculations, all other analyses were performed with STATISTICA 13.1 software (TIBCO Software Inc., Palo Alto, USA).
3. Results
3.1. Species abundance and age structure
The reed warbler was the most numerous species at most ringing stations, except Kalimok in Bulgaria (22.8%) and Burullus in northern Egypt (22.9%) (). It was distinctly dominant in Kuscenneti in Turkey (85.6%) and along the Nile Valley, i.e., in Wadi El Rayan and Saluga Ghazal (85–90% of captured birds). The sedge warbler was the most abundant in the Nile Delta and constituted up to 75% of all warblers captured there. The great reed warbler was the least abundant species, and its share did not exceed 12% at most stations, except Kalimok in Bulgaria, where it accounted for 37.5% of all captured birds.
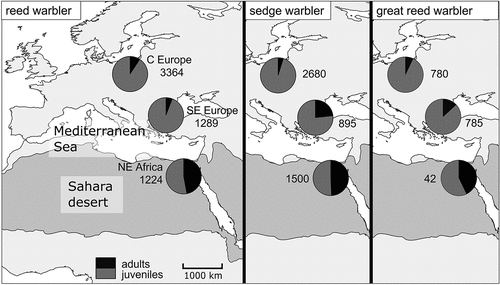
Juveniles of each species constituted 91–95% of all birds captured in C Europe (). In SE Europe, the percentage of adult sedge and great reed warblers was higher (up to 23.1% and 13.1%, respectively) than in C Europe, which was not the case in the reed warbler (5.8%). The proportions of age groups were approximately even in NE Africa, where the percentage of juveniles was 51–57%.
Figure 2. Share of adults (black) and juveniles (dark grey) of the reed, sedge, and great reed warbler in three regions: C Europe, SE Europe, and NE Africa. The total number of individuals caught in each region has been added. The Sahara and the deserts of the Arabian Peninsula are shown as shaded areas (after Rappole & Jones Citation2003; Fransson et al. Citation2006; modified).
3.2. Flight range estimations
In general, adults and juveniles differed in flight range, which was also significantly different at the stations compared. However, the differences between age groups varied between the stations, i.e. the interaction effect was significant in all the studied species (GLM; ). Thus, due to these significant interactions we cannot make a general assessment of changes in migratory distance within a species departing from subsequent stopovers (Rowe Citation2007). Hence, each age class within the species was treated separately using one-way ANOVA, with the “station” as the factor (Ott & Longnecker Citation2001; McDonald Citation2009) (Supplementary Table S3). In order to check the differences in distances covered by adults and juveniles from given station, t-test was applied within each station.
Table II. Results of generalised linear model (GLM) testing the effect of age, station location, and their interaction on potential flight ranges of the reed, sedge, and great reed warbler. Birds with fat mass = 0 were excluded.
In Europe, mean potential distances covered by individuals increased while heading southwards (). We found that the average flight distance covered from the southernmost station Kuscenneti in Turkey was significantly higher than that from northernmost Rakutowskie in Poland – within both age groups in reed and sedge warbler, and within juveniles in great reed warbler. Only for adult great reed warblers such statistically significant differences were not found (Supplementary Table S3). The lowest mean values were noted at Rakutowskie (ca. 500 km, reed and sedge warbler; ), whereas the highest at Kuscenneti (up to approx. 2200 km, sedge warbler; ). The largest differences in mean flight distances between age groups were noted in reed and sedge warblers at Kuscenneti (approx. 600 km; ), where adult reed warblers were able to cover significantly longer distances than juveniles (t-test, t = 4.66, p < 0.001), while it was opposite in the sedge warbler (t-test, t = 2.69, p = 0.008). In the great reed warbler, the largest difference in mean flight distances (approx. 1000 km; ) between the age groups was noted at Kalimok in Bulgaria (t-test, t = 5.53, p < 0.001). At Kuscenneti in Turkey, adult and juvenile great reed warblers were able to cover similarly long distances of approx. 1700–1800 km (; t-test, t = 0.24, p = 0.81), yet a low sample size should be considered.
Figure 3. Mean potential flight ranges of adults (black dots) and juveniles (white dots) of the reed, sedge, and great reed warbler covered from ringing stations, arranged from the northernmost (left) to the southernmost (right). RA – Rakutowskie, KK – Kalimok, KU – Kuscenneti, BR – Burullus, WR – Wadi El Rayan, SG – Saluga Ghazal. Birds with fat mass = 0 were excluded. Grey rectangles indicate the position of two large ecological barriers: the Mediterranean Sea and the Sahara desert. Average values are based on untransformed data to illustrate the potential ability to overcome the distance and barriers presented. Vertical lines show the 95% confidence intervals. The results of the statistical comparison with t-test between mean flight distances (after Box-Cox transformation) covered by adults and juveniles from each station are given at the bottom of each chart: *significance at p < 0.05; ns - not significant (t-test).
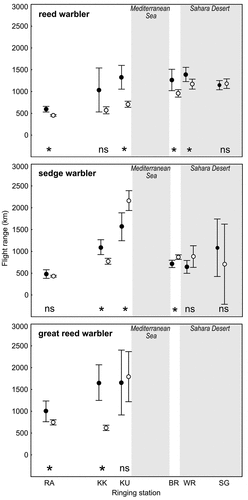
In NE Africa, the differences in mean flight distances covered by adults and juveniles were lower and did not differ significantly between the outermost stations, Burullus and Saluga Ghazal (ANOVA, Tuckey post-hoc test, p > 0.05 in all species). In Burullus in the Nile Delta, adult reed warblers were able to cover on average longer distances than juveniles (t-test, t = 2.61, p = 0.009), but at Saluga Ghazal in the southern part of the Nile Valley, these distances were similar (t-test, t = 0.21, p = 0.83). In the sedge warbler, mean flight distances of both age groups were lower at Burullus (Egypt) than at Kuscenneti (Turkey), but juveniles were still able to cover longer distances than adults (t-test, t = 3.06, p = 0.002). Due to the low sample size, the potential migration distances of the great reed warbler were not analysed in NE Africa.
The percentage of individuals potentially able to cross either the Mediterranean Sea or both the Mediterranean Sea and the Sahara Desert varied among the species (). At Kuscenneti in Turkey, about one-third of juvenile and two-thirds of adult reed warblers were ready to cross the Mediterranean Sea (Chi-square test, χ2 = 30.71, p < 0.001), whereas in the two other species, these proportions were similarly high in both age groups, reaching more than two-thirds (Chi-square test, p > 0.49 in both species). Among these individuals, some had energy reserves high enough to reach the southern edge of the Sahara. At Kuscenneti (Turkey) and Burullus (Egypt), the proportions of such birds were significantly higher in adult reed warblers (Fisher exact test, χ2 = 6.72, p = 0.030 and χ2 = 4.97, p = 0.031, respectively), whereas in the sedge warbler in juveniles (Fisher exact test, χ2 = 3.64, p = 0.046 and Chi-square test, χ2 = 6.94, p = 0.008, respectively). At Saluga Ghazal in the southern part of the Nile Valley, however, such proportion of adult and juvenile reed warblers was similar (Chi-square test, χ2 = 0.79, p = 0.37), whereas the number of captured sedge warblers was insufficient to make a similar comparison.
Table III. Fractions of juveniles and adults of the reed, sedge, and great reed warbler potentially able to reach the southern border of the Mediterranean Sea and the Sahara desert from stopover sites. These fractions refer to the numbers (N ad, N juv) of individuals included in the flight range estimations in FlyingR (birds with negative or equal to zero estimated fat masses were excluded). The statistically significant differences between the proportion of adults and juveniles at a given ringing station within the species are marked with an asterisk (Chi-square test or Fisher exact test, p < 0.05). The distances from the stations to the southern edge of the Mediterranean Sea were as follows: Kuscenneti −1000 km, and to the southern edge of the desert: Kuscenneti −2850 km, Burullus −1900 km, Saluga Ghazal −1000 km.
4. Discussion
Trans-Saharan migrants adopt different strategies to cover the long distances between the European breeding grounds and the African wintering quarters. They either accumulate high fat reserves already on the European continent or just before the Sahara Desert, steadily increase fuel stores before reaching this barrier or feed and fuel while crossing the Sahara (Schaub & Jenni Citation2000). Adults and juveniles of the same species may differ in their migration strategy as juvenile individuals travel for the first time crossing unfamiliar regions, guided by inherited information, whereas adults may rely on their experience from previous seasons (Bairlein Citation1991; Woodrey & Moore Citation1997; Berthold Citation1999; Moore et al. Citation2003; Barboutis et al. Citation2014; Åkesson et al. Citation2021).
Our results showed that the amount of fat deposits increased southwards in both age groups, as individuals needed to accumulate energy reserves to fly across the sea, deserts, and other ecological barriers (Bairlein Citation1991; Schaub & Jenni Citation2000; Fransson et al. Citation2006; Ożarowska Citation2015). In the case of adult reed warblers, such a trend also appears to be evident in Europe. However, the number of adult birds captured in Turkey is insufficient to confirm this hypothesis. Regardless of the variation in the amount of accumulated fat reserves between age groups at particular stages of the journey, the overall trends of changes in potential flight distances from the northernmost to southernmost stopover sites were roughly similar within the species. The sedge warbler and great reed warbler represent the “early fuelling” strategy, as they accumulate fat reserves well before crossing the Sahara. The reed warbler, on the other hand, adopts the “late fuelling” strategy, as it accumulates the energy stores to cross the desert just at its northern borders, in northern Africa (Schaub & Jenni Citation2000; Stępniewska et al. Citation2018, Citation2020). Still, this increase in fat reserves with declining latitude, varied with respect to the stage of the journey and progressed differently in age groups within each species. In central Europe (Rakutowskie), i.e., during the early stage of autumn migration, migrants do not need to accumulate large fat stores due to many feeding opportunities in this region and the lack of large ecological barriers to cross (Jones et al. Citation2002; Fransson et al. Citation2006). Migrants tend to avoid carrying large fat reserves to reach the next suitable feeding site, as overloading is energetically expensive (Gudmundsson et al. Citation1991). While moving southwards (Kalimok) migrants gradually increased their fat reserves, but this increase was lower in juveniles than in adults. At these sites, still far from the ecological barriers, juvenile warblers usually had lower fat reserves than adults, which supports the results of other studies (Bibby & Green Citation1981; Woodrey & Moore Citation1997; Jakubas & Wojczulanis–Jakubas Citation2010). This pattern was different when migrants approached the Mediterranean Sea region (Kuscenneti). At this stage of migration, we found the largest disproportions in fat loads, and consequently potential flight distances, between adults and juveniles. These differences were species-specific, and the potential distances covered by juveniles were approx. 600 km longer than in adults (sedge warbler), approx. 600 km shorter than in adults (reed warbler) or similarly long in these two age groups (great reed warbler). When compared between species, the variation in mean flight distances covered by adults from Kuscenneti was lower than by juveniles and did not exceed 400 km. In juveniles, however, the differences were distinctly higher and reached 1500 km between the reed and sedge warbler.
After crossing the Mediterranean Sea, the differences in mean flight distances covered by the two age groups decreased. Yosef and Chernetsov (Citation2005) reported from Israel, that the difference between the condition index of adults and juveniles was not significant among reed warblers caught during autumn migration there. We found that adult and juvenile sedge warblers were able to cover distinctly shorter distances from Burullus in the Nile Delta than from Kuscenneti in Turkey, and the proportion of birds able to reach the southern edge of the Sahara dropped three times. It was not the case in reed warblers, as they were able to cover longer distances than from Kuscenneti and the higher shares of individuals of both age groups were able to reach the southern edge of the Sahara Desert, similar to the pattern reported from the western route (Hilgerloh & Wiltschko Citation2000). The results of different studies show that sedge warblers adopt the long-step migration strategy, i.e., accumulate large fat reserves (Bibby & Green Citation1981; Schaub & Jenni Citation2000; Stępniewska et al. Citation2018). It is therefore probable that birds with very high reserves captured in Kuscenneti simply overfly Egypt and do not stop in NE Africa (Stępniewska et al. Citation2018). It could be supported by catching results from Israel, where the spring/autumn numbers ratio was 6.0/1 in sedge warblers, whereas 1.8/1 in reed warblers (Yosef & Chernetsov Citation2004). On the other hand, individuals with lower energy reserves, which accumulated their fat stores well before the Mediterranean basin, must land and stop in the Nile Delta to refuel, as willow warblers Phylloscopus trochilus do in NW Africa (Hilgerloh & Wiltschko Citation2000). Then this may be the reason for the sudden drop in the potential flight range documented in sedge warblers captured in Burullus. Our estimates indicate that more than 90% of these sedge warblers would not be able to fly beyond the southern edge of the Sahara without refuelling. The large numbers of sedge warblers captured in Burullus and almost no birds recorded along the Nile Valley suggest that these birds probably replenish fat reserves in their optimal habitats, i.e., in the vast reedbeds of the Nile Delta on the Mediterranean coast, and then continue their passage across the Sahara Desert. Reed warblers, on the other hand, had higher energy reserves compared to sedge warblers before crossing the Sahara desert, as their energy reserves increased gradually (Ożarowska et al. Citation2011). This species adopts a short-step migration strategy before reaching the desert, which means that it does not need large fat depots to reach the next stop-over (Schaub & Jenni Citation2000). Moreover, some birds probably do not cross the Mediterranean Sea in a single non-stop flight, they rather migrate with short steps through Cyprus or circumvent the Sea along the eastern coast (Ożarowska et al. Citation2011; Stępniewska et al. Citation2018). These individuals do not face any major barriers on their way. Consequently, they arrive in the Nile Delta with higher energy reserves and potentially are able to cover longer distances across the desert than sedge warblers.
In the present study, we observed a decrease in the share of juveniles in all species when moving southwards. The number of juveniles in the population may decrease with time as inexperienced individuals are predicted to undergo stronger selection pressures (Cheng et al. Citation2019). In another long-distance migrant, the barn swallow Hirundo rustica, however, the survival of juveniles shortly after fledgling was low, whereas during the rest of the nonbreeding season, including the passage, it was similarly high as in adults (Grüebler et al. Citation2014). Therefore, age-related mortality, connected with individual experience, may not account for the observed decrease in the share of juvenile birds. Instead, this decrease may reflect different migration strategies of both age groups (Cresswell & Bauer Citation2014). The latitudinal disproportion in age groups could be the result of different timings of migration or distances they cover (Cristol et al. Citation1999). Almost equal proportions of age groups in Egypt in all the species may reflect, e.g., a definite need to stopover before the largest barrier, as all birds will encounter harsh conditions of the Sahara desert ahead. In the case of reed and sedge warblers, this hypothesis may be supported by almost no differences in mean flight ranges between adults and juveniles at African stopover sites.
Obtained results showed that juvenile reed warblers consequently adopted a strategy of shorter migration steps than adults – regardless of the distance to ecological barriers. In the sedge warbler, however, juveniles carried larger fat reserves compared to adults just in front of the Mediterranean Sea and the Sahara Desert. Hence, they were able to perform longer flights without refuelling en route. The arrival at the next stopover site with extra fat is a kind of reassurance, as the birds are less vulnerable to the quality of stopover sites and the potential lack of food supplies (Nielsen & Rees Citation2013). Such a strategy increases the “margin of safety” of inexperienced individuals (Woodrey & Moore Citation1997) in the face of unpredictable food supplies and deteriorating habitat conditions while moving southwards (Bibby & Green Citation1981; Bensch et al. Citation1991). Our results, however, were in contradiction with the studies on the sedge warblers flying along the western route, i.e., through France, Portugal (Bibby & Green Citation1981), and Italy (Spina & Bezzi Citation1990), where juveniles had smaller energy reserves than adults. We suppose that different environmental and geographical conditions of western and eastern migratory routes may cause this variation at the intraspecific level, as it was found in garden warblers Sylvia borin migrating along these routes (Bairlein Citation1991). In the great reed warbler, both juveniles and adults were able to cover similarly long distances in one flight in front of the ecological barriers (Stępniewska et al. Citation2020), which was also found in the barn swallow Hirundo rustica (Grüebler et al. Citation2014) and the wood thrush Hylocihla mustelina (McKinnon et al. Citation2014). The number of captured individuals of each species varied along the SE migration route, which resulted in the variation in the species composition of Acrocephalidae at each station. The reed warbler was the only species which was captured in high numbers at all stations being the most numerous in more arid areas. This results from its habitat and diet preferences on migration, as the reed warbler exploits a broader range of habitats than the other two species, and it is a food opportunist feeding on a wide range of arthropods (Bibby & Green Citation1981; Cramp & Brooks Citation1992). The sedge warbler and great reed warbler are strongly associated with wet reedbeds on migration compared to the reed warbler (Cramp & Brooks Citation1992), which is supported by our results, as these species were the most numerous at the stations with the most extensive reedbeds, i.e., Rakutowskie in Poland, Kalimok in Bulgaria, and Burullus in the Nile Delta. The sedge warbler is a food specialist preferring plum aphids Hyalopterus pruni, which are a temporarily and spatially restricted food source. The superabundance of these arthropods enables sedge warblers to accumulate large energy reserves in a short time for a long journey without refuelling en route (Bibby et al. Citation1976; Bibby & Green Citation1981; Cramp & Brooks Citation1992). Molecular analyses of subcutaneous fat reserves and blood metabolites confirm these differences in migration strategies of the sedge and reed warblers (Araújo et al. Citation2022). Very low numbers of great reed warblers in Egypt suggest that in autumn the species rather crosses the area of NE Africa without stopping over, while during the spring passage it was regularly captured there (Zaniewicz & Chruściel Citation2011). Moreover this hypothesis is further supported by the results of the present study as about two-thirds of adult and juvenile great reed warblers were able to cross the Mediterranean Sea from Turkey. Indeed, data on adult great reed warblers tagged with geolocators revealed that birds from Bulgarian and Turkish local populations flew almost directly from the breeding grounds to the African wintering quarters (Koleček et al. Citation2016). A similar strategy was reported from the western and central migration routes, where autumn concentrations of great reed warblers were scarce in south-eastern France and in Italy, whereas south of the Sahara the species was common (Cramp & Brooks Citation1992).
Our results indicate that the analyses based on the data for only one age group or at a single site of a migration route may be insufficient to describe the migratory strategy of the species in detail. The spectrum of migratory behaviour of common Acrocephalidae species seems to be more complex than the pattern presented for juveniles only (e.g., by Hilgerloh & Wiltschko Citation2000 or Schaub & Jenni Citation2000). The age-related migration strategies of the studied species have a characteristic spatio-temporal pattern, which is influenced by large ecological barriers, i.e., the Mediterranean Sea and the Sahara Desert. We conclude that species-specific migration strategy includes age-specific strategy component, which may differ between juvenile and adult individuals.
Authors’ contributions
Design and methodology: K.S., W.M., A.O. Data collection: P.B., G.Z., S.B., M.I., P.Z. Data analysis: K.S., G.Z. Writing original draft: K.S. Writing review and editing: all authors.
Availability of data and material
Data analysed in this study are available from the corresponding author upon reasonable request.
Compliance with ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Ethics approval consent to participate
Bird ringing and data collection were conducted by experienced, professional ringers having valid licenses and permissions of the national ringing centres in Poland, Bulgaria, Turkey, and Egypt. All fieldwork was done according to the ethical standards recommended by those institutions.
Supplemental Material
Download MS Word (70.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2024.2350199
Additional information
Funding
References
- Åkesson S, Bakam H, Martinez Hernandez E, Ilieva M, Bianco G. 2021. Migratory orientation in inexperienced and experienced avian migrants. Ethology Ecology & Evolution 3(3):206–229. DOI: 10.1080/03949370.2021.1905076
- Alatalo RV, Gustafsson L, Lundberg A. 1984. Why do young passerine birds have shorter wings than older birds? Ibis 126(3):410–415. DOI: 10.1111/j.1474-919x.1984.tb00264.x
- Andueza M, Barba E, Arroyo JL, Feliu J, Gómez J, Jubete F, Lozano L, Monrós JS, Moreno–Opo R, Neto JM, Onrubia A, Tenreiro P, Valkenburg T, Arizaga J. 2014. Geographic variation in body mass of first-year Reed Warblers Acrocephalus scirpaceus in Iberia. Ornis Fennica 91(2):88–99. DOI: 10.51812/of.133847
- Araújo PM, Viegas I, Da Silva LP, Lopes PB, Tavares LC, Ramos JA. 2022. Blood metabolites and profiling stored adipose tissue reveal the differential migratory strategies of Eurasian reed and sedge warblers. Birds 3(4):359–373. DOI: 10.3390/birds3040024
- Arizaga J, Sánchez JM, Díez E, Cuadrado JF, Asenjo I, Mendiburu A, Jauregi JI, Herrero A, Elosegi Z, Aranguren I, Andueza M, Alonso D. 2011. Fuel load and potential flight ranges of passerine birds migrating through the western edge of the Pyrenees. Acta Ornithologica 46(1):19–28. DOI: 10.3161/000164511x589875
- Bairlein F. 1983. Habitat selection and associations of species in European passerine birds during southward, post–breeding migrations. Ornis Scandinavica 14(3):239–245. DOI: 10.2307/3676157
- Bairlein F. 1991. Body mass of Garden Warblers (Sylvia borin) on migration: A review of field data. Vogelwarte 36:48–61.
- Barboutis C, Henshaw I, Kullberg C, Nikolopoulou S, Fransson T. 2014. Fuelling in front of the barrier — are there age based behavioral differences in garden warblers Sylvia borin? PeerJ 2:e319. DOI: 10.7717/peerj.319
- Barboutis C, Henshaw I, Mylonas M, Fransson T. 2011. Seasonal differences in energy requirements of garden warblers Sylvia borin migrating across the Sahara desert. Ibis 153(4):746–754. DOI: 10.1111/j.1474-919X.2011.01160.x
- Bensch S, Hasselquist D, Hedenström A, Ottosson U. 1991. Rapid moult among palaearctic passerines in West Africa‐an adaptation to the oncoming dry season? Ibis 133(1):47–52. DOI: 10.1111/j.1474-919x.1991.tb04809.x
- Berthold P. 1996. Control of bird migration. London: Chapman and Hall.
- Berthold P. 1999. A comprehensive theory for the evolution, control and adaptability of avian migration. Ostrich 70(1):1–11. DOI: 10.1080/00306525.1999.9639744
- Bibby CJ, Green RE. 1981. Autumn migration strategies of Reed and Sedge Warblers. Ornis Scandinavica 12(1):1–12. DOI: 10.2307/3675898
- Bibby CJ, Green RE, Pepler GRM, Pepler PA. 1976. Sedge Warbler migration and reed aphids. Brit Birds 69:384–399.
- Biebach H, Biebach I, Friedrich W, Heine G, Partecke J, Schmidl D. 2000. Strategies of passerine migration across the Mediterranean Sea and the Sahara Desert: A radar study. Ibis 142(4):623–634. DOI: 10.1111/j.1474-919x.2000.tb04462.x
- Biebach H, Friedrich W, Heine G. 1986. Interaction of body mass, fat, foraging and stopover period in trans–Sahara migrating passerine. Oecologia 69(3):370–379. DOI: 10.1007/bf00377059
- Briedis M, Bauer S, Adamík P, Alves JA, Costa JS, Emmenegger T, Gustafsson L, Koleček J, Krist M, Liechti F, Lisovski S, Meier CM, Procházka P, Hahn S. 2020. Broad–scale patterns of the Afro–Palaearctic landbird migration. Global Ecology and Biogeography 29(4):722–735. DOI: 10.1111/geb.13063
- Busse P, Meissner W. 2015. Bird ringing station manual. Warsaw: De Gruyter Open Ltd. DOI: 10.2478/9788376560533
- Cheng Y, Fiedler W, Wikelski M, Flack A. 2019. “Closer‐to‐home” strategy benefits juvenile survival in a long‐distance migratory bird. Ecology and Evolution 9(16):8945–8952. DOI: 10.1002/ece3.5395
- Cramp S, Brooks DJ. 1992. Handbook of the birds of Europe, the Middle East and North Africa: The birds of the western Palearctic, vol. VI (Warblers). New York: Oxford University Press.
- Cresswell W. 2014. Migratory connectivity of Palaearctic–African migratory birds and their responses to environmental change: The serial residency hypothesis. Ibis 156(3):493–510. DOI: 10.1111/ibi.12168
- Cristol DA, Baker MB, Carbone C. 1999. Differential migration revisited: Latitudinal segregation by age and sex classes. Current Ornithology 15:33–88.
- Ellegren H. 1991. Stopover ecology of autumn migrating Bluethroats Luscinia s. svecica in relation to age and sex. Ornis Scandinavica 22(4):340–348. DOI: 10.2307/3676506
- Erni B, Liechti F, Bruderer B. 2005. The role of wind in passerine autumn migration between Europe and Africa. Behavioral Ecology 16(4):732–740. DOI: 10.1093/beheco/ari046
- Fransson T, Jakobsson S, Kullberg C, Mellroth R, Pettersson T. 2006. Fuelling in front of the Sahara desert in autumn—an overview of Swedish field studies of migratory birds in the eastern Mediterranean. Ornis Svecica 16(1–2):74–83. DOI: 10.34080/os.v16.22723
- Frumkin R, Pinshow B, Kleinhaus S. 1995. A review of bird migration over Israel. Journal für Ornithologie 136(2):127–147. DOI: 10.1007/bf01651235
- Grüebler MU, Korner‐Nievergelt F, Naef‐Daenzer B. 2014. Equal nonbreeding period survival in adults and juveniles of a long‐distant migrant bird. Ecology and Evolution 4(6):756–765. DOI: 10.1002/ece3.984
- Gudmundsson G, Lindström Å, Alerstam T. 1991. Optimal fat loads and long-distance flights by migrating Knots Calidris canutus Sanderlings C. alba and Turnstones Arenaria interpres. The Ibis 133(2):140–152. DOI: 10.1111/j.1474-919x.1991.tb04825.x
- Hedenström A, Møller AP. 1992. Morphological adaptations to song flight in passerine birds: A comparative study. Proceedings of the Royal Society of London, Series B: Biological Sciences 247:183–187. DOI: 10.1098/rspb.1992.0026
- Hilgerloh G, Wiltschko W. 2000. Autumn fat load and flight range of passerine long-distance migrants in southwestern Spain and northwestern Morocco. Ardeola 47(2):259–263. DOI: 10.1080/03078698.2006.9674344
- Hockey PAR, Turpie JK, Velásquez CR. 1998. What selective pressures have driven the evolution of deferred northward migration by juvenile waders? Journal of Avian Biology 29(3):325–330. DOI: 10.2307/3677117
- Jakubas D, Wojczulanis–Jakubas K. 2010. Sex– and age–related differences in the timing and body condition of migrating Reed Warblers Acrocephalus scirpaceus and Sedge Warblers Acrocephalus schoenobaenus. Naturwissenschaften 97(5):505–511. DOI: 10.1007/s00114-010-0666-y
- Jakubas D, Wojczulanis–Jakubas K, Foucher J, Dziarska–Pałac J, Dugué H. 2014. Age and sex differences in fuel load and biometrics of Aquatic Warblers Acrocephalus paludicola at an autumn stopover site in the Loire Estuary (NW France). Ardeola 61(1):15–30. DOI: 10.13157/arla.61.1.2014.15
- Jenni L, Jenni-Eiermann S. 1998. Fuel supply and metabolic constraints in migrating birds. Journal of Avian Biology 29(4):521–528. DOI: 10.2307/3677171
- Jones J, Francis CM, Drew M, Fuller S, Ng MWS. 2002. Age-related differences in body mass and rates of mass gain of passerines during autumn migratory stopover. The Condor 104(1):49–58. DOI: 10.1093/condor/104.1.49
- Kennerley P, Pearson D. 2010. Reed and Bush Warblers. London: Christopher Helm.
- Klaassen M. 1996. Metabolic constraints on long–distance migration in birds. The Journal of Experimental Biology 199(1):57–64. DOI: 10.1242/jeb.199.1.57
- Koleček J, Procházka P, El‐Arabany N, Tarka M, Ilieva M, Hahn S, Honza M, de la Puente J, Bermejo A, Görsoy A, Bensch S, Zehtindjiev P, Hasselquist D, Hansson B. 2016. Cross‐continental migratory connectivity and spatiotemporal migratory patterns in the great reed warbler. Journal of Avian Biology 47(6):756–767. DOI: 10.1111/jav.00929
- Kozik R, Meissner W, Listewnik B, Nowicki J, Lasecki R. 2022. Differences in foraging behaviour of a migrating shorebird at stopover sites on regulated and unregulated sections of a large European lowland river. Journal of Ornithology 163(3):791–802. DOI: 10.1007/s10336-022-01984-3
- Lind J, Fransson T, Jakobsson S, Kullberg C. 1999. Reduced take-off ability in robins (Erithacus rubecula) due to migratory fuel load. Behavioral Ecology and Sociobiology 46(1):65–70. DOI: 10.1007/s002650050593
- Masinde B 2020. Birds’ Flight Range – Sensitivity Analysis. Master’s thesis, Department of Computer and Information Science, Linköping University, Sweden. Available: http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva–166248
- Mazur AE, Remisiewicz M, Underhill LG. 2021. Sex‐specific patterns of fuelling and pre‐breeding body moult of Little Stints Calidris minuta in South Africa. Ibis 163(1):99–112. DOI: 10.1111/ibi.12840
- McCullagh P, Nelder JA. 1989. Generalized linear models. London: Chapman and Hall.
- McDonald JH. 2009. Handbook of biological statistics. Vol. 2. Baltimore, MD: Sparky House Publishing.
- McKinnon EA, Fraser KC, Stanley CQ, Stutchbury BJ. 2014. Tracking from the tropics reveals behaviour of juvenile songbirds on their first spring migration. Public Library of Science ONE 9(8):e105605. DOI: 10.1371/journal.pone.0105605
- Meissner W. 2007. Stopover strategy of adult and juvenile Red Knots Calidris c. canutus in the Puck Bay, southern Baltic. Ardea 95(1):97–104. DOI: 10.5253/078.095.0111
- Meissner W, Karlionova N, Pinchuk P. 2011. Fuelling rates by spring-staging Ruffs Philomachus pugnax in Southern Belarus. Ardea 99(2):147–155. DOI: 10.5253/078.099.0204
- Merom K, McCleery R, Yom-Tov Y. 1999. Age-related changes in wing-length and body mass in the Reed Warbler Acrocephalus scirpaceus and Clamorous Reed Warbler A. stentoreus. Bird Study 46(2):249–255. DOI: 10.1080/00063659909461137
- Moore F, Mabey S, Woodrey M. 2003. Priority access to food in migratory birds: Age, sex and motivational asymmetries. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian migration. Berlin Heidelberg: Springer–Verlag. pp. 281–292. DOI: 10.1007/978-3-662-05957-9_19
- Neto JM, Encarnacao V, Fearon P. 2010. Distribution, phenology and condition of Aquatic Warblers Acrocephalus paludicola migrating through Portugal. Ardeola 57:181–189.
- Nielsen B, Rees J. 2013. Fat accumulation and autumn migration strategy of Reed Warblers Acrocephalus scirpaceus and Sedge Warblers A. schoenobaenus in southern Sweden. Ringing & Migration 28(2):69–76. DOI: 10.1080/03078698.2013.869904
- Nowakowski JJ. 2000. Long-term variability of wing length in a population of the Reed Warbler Acrocephalus scirpaceus. Acta Ornithologica 35(2):173–182. DOI: 10.3161/068.035.0210
- Ott L, Longnecker M. 2001. An introduction to statistical methods and data analysis. Boston: Cengage Learning.
- Ożarowska A. 2015. Contrasting fattening strategies in related migratory species: The blackcap, garden warbler, common whitethroat and lesser whitethroat. Annales Zoologici Fennici 52(1–2):115–127. DOI: 10.5735/086.052.0210
- Ożarowska A, Stępniewska K, Ibrahim WAL. 2011. Autumn and spring migration of the Reed Warbler Acrocephalus scirpaceus in Egypt — some interesting aspects and questions. Ostrich 82(1):49–56. DOI: 10.2989/00306525.2010.541502
- Ożarowska A, Zaniewicz G, Meissner W. 2021. Sex and age-specific differences in wing pointedness and wing length in blackcaps Sylvia atricapilla migrating through the southern Baltic coast. Current Zoology 67(3):271–277. DOI: 10.1093/cz/zoaa065
- Pennycuick CJ. 1998. Computer simulation of fat and muscle burn in long-distance bird migration. Journal of Theoretical Biology 191(1):47–61. DOI: 10.1006/jtbi.1997.0572
- Pennycuick CJ. 2008. Modelling the flying bird. Elsevier. DOI: 10.1016/s1875-306x(08)x0002-4
- Procházka P, Hahn S, Rolland S, Van der Jeugd H, Csörgő T, Jiguet F, Mokwa T, Liechti F, Vangeluwe D, Korner–Nievergelt F. 2017. Delineating large-scale migratory connectivity of reed warblers using integrated multistate models. Diversity and Distributions 23(1):27–40. DOI: 10.1111/ddi.12502
- Rappole JH, Jones P. 2003. Evolution of old and new world migration systems. Ardea 90:525–537.
- R Core Team. 2020. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Rowe P. 2007. Essential statistics for the pharmaceutical sciences. Chichester: John Wiley & Sons Ltd. DOI: 10.1002/9781119109075
- Rubolini D, Gardiazabal Pastor A, Pilastro A, Spina F. 2002. Ecological barriers shaping fuel stores in barn swallows Hirundo rustica following the central and western Mediterranean flyways. Journal of Avian Biology 33(1):15–22. DOI: 10.1034/j.1600-048x.2002.330104.x
- Sakia RM. 1992. The Box–Cox transformation technique: A review. The Statistician 41(2):169–178. DOI: 10.2307/2348250
- Sander MM, Heim W, Schmaljohann H. 2020. Seasonal and diurnal increases in energy stores in migratory warblers at an autumn stopover site along the Asian–Australasian flyway. Journal of Ornithology 161(1):73–87. DOI: 10.1007/s10336–019–01701–7
- Schaub M, Jenni L. 2000. Body mass of six long–distance migrant passerine species along the autumn migration route. Journal of Ornithology 141(4):441–460. DOI: 10.1007/bf01651574
- Spina F, Bezzi EM. 1990. Autumn migration and orientation of the Sedge Warbler (Acrocephalus schoenobaenus) in northern Italy. Journal für Ornithologie 131(4):429–438. DOI: 10.1007/bf01639819
- Stępniewska K, Ożarowska A, Busse P, Bobrek R, Zehtindjiev P, Ilieva M, Meissner W. 2020. Autumn migration strategy of juvenile great reed warblers Acrocephalus arundinaceus on the eastern European flyway: A spatiotemporal pattern of accumulation and utilisation of energy stores. The European Zoological Journal 87(1):537–551. DOI: 10.1080/24750263.2020.1814882
- Stępniewska K, Ożarowska A, Busse P, Zehtindjiev P, Ilieva M, Hnatyna O, Meissner W. 2018. Fuelling strategies differ among juvenile Sedge and Reed Warblers along the eastern European flyway during autumn migration. Ornis Fennica 95(3):103–114. DOI: 10.51812/of.133934
- Svensson L. 1992. Identification Guide to European Passerines. Stockholm: British Trust for Ornithology.
- Valkama J, Saurola P, Lehikoinen A, Lehikoinen E, Piha M, Sola P, Velmala W. 2014. The Finnish Bird Ringing Atlas. Volume II — Finnish Museum of Natural History and Ministry of Environment, Helsinki (In Finnish with English Summary).
- Wojciechowski MS, Yosef R, Pinshow B. 2014. Body composition of north and southbound migratory blackcaps is influenced by the lay‐of‐the‐land ahead. Journal of Avian Biology 45(3):264–272. DOI: 10.1111/j.1600-048x.2013.00345.x
- Woodrey MS, Moore FR. 1997. Age-related differences in the stopover of fall landbird migrants on the coast of Alabama. The Auk 114(4):695–707. DOI: 10.2307/4089289
- Wunderle JM. 1991. Age-specific foraging proficiency in birds. Current Ornithology 8:273–324.
- Yosef R, Chernetsov N. 2004. Stopover ecology of migratory sedge warblers (Acrocephalus schoenobaenus) at Eilat, Israel. Ostrich - Journal of African Ornithology 75(1–2):52–56. DOI: 10.2989/00306520409485412
- Yosef R, Chernetsov N. 2005. Longer is fatter: Body mass changes of migrant Reed Warblers (Acrocephalus scirpaceus) staging at Eilat, Israel. Ostrich - Journal of African Ornithology 76(3–4):142–147. DOI: 10.2989/00306520509485486
- Zaniewicz G, Chruściel J. 2011. Burullus ringing station (N Egypt) – Ringing results and seasonal bird migration dynamics in 2005-2007. The Ring 33(1–2):77–87. DOI: 10.2478/v10050-011-0006-4
- Zehtindjiev P, Liechti F. 2003. A quantitative estimate of the spatial and temporal distribution of nocturnal bird migration in south–eastern Europe – a coordinated moon–watching study. Avian Science 3:37–45.
