Abstract
Ant mandibles perform different functions (e.g. feeding, hunting, and nest building), and previous studies on workers of some species revealed that they are enriched in transition metals (Zn and Mn), which improve cuticle hardness. Whether this trait is adaptive in all ant species is still unclear. One way to test for such adaptation is to compare sexes and castes within species as well as species with different life-histories. Here, we evaluated the transition metal enrichment in workers, queens, and males of 12 species of the Holarctic genus Lasius Fabricius 1804. The genus is adequate to test for the evolution of this trait because of two reasons. First, males show small, often toothless mandibles and do not contribute to nest activities, hence leading to the prediction of lower metal-enrichment compared with workers and queens. Second, this genus includes both socially parasitic (of other Lasius species) and non–parasitic species, leading to the prediction that females of the former, because they engage in fights with the host, have higher metal-enrichment compared with females of the latter. Our Scanning Electron Microscopy/Energy Dispersive X-ray analysis provided evidence for an adaptive role of Zn and Mn in Lasius mandibles. At intra-specific-level, males had lower amount of Zn and Mn in their mandibles compared with workers and queens (which did not differ). Although parasitic and non-parasitic queens had similar metal amounts, parasitic workers showed higher Mn content and marginally higher Zn content than workers of non-parasitic species. Overall, our results strongly suggest that both colony activities and parasitic life-style promoted greater metal-enrichment in Lasius females. Given the huge biological diversity of ants, large comparative studies are needed to assess the generality of our findings.
1. Introduction
Arthropod exoskeleton has been widely studied and highly appreciated for its mechanical properties (Vincent & Wegst Citation2004; Dirks & Taylor Citation2012; Parle et al. Citation2016; Li et al. Citation2020). Despite chitin – the main cuticle component – is known for its properties of lightness and elasticity (Vincent & Wegst Citation2004), exoskeleton hardness is frequently connected with elemental enrichments, which has evolved in arthropods as improvement of cuticular performance (Cribb et al. Citation2008). While some crustaceans possess calcium carbonate within certain parts of their cuticle, some insects have evolved an alternative strategy to improve cuticle hardness and abrasion resistance: the inclusion of transition metals such as zinc (Zn) and manganese (Mn), likely as metal cross-link proteins (Schofield et al. Citation2021). Such transition metals have been found in several clades of insects, particularly in the mandibles and ovipositors (Hillerton & Vincent Citation1982; Broomell et al. Citation2008; Cribb et al. Citation2008; Lehnert et al. Citation2019), i.e. organs prone to be frequently used during the life to chew, cut, and drill hard objects. The presence of transition metals mandible cuticle is connected to a greater hardness and wear limitation, thus increasing their efficient use (Hillerton & Vincent Citation1982; Hillerton et al. Citation1984; Edwards et al. Citation1993; Schofield et al. Citation2002).
In the hyper-diverse insect order Hymenoptera, Zn is the transition metal more commonly found in mandibles, while Mn has been seldom found in a few families often not phylogenetically related (Schofield Citation2001; Schofield et al. Citation2003; Jorge et al. Citation2017; Polidori et al. Citation2020). The presence of transition metals is also common in the ovipositor and sting of Hymenoptera (Quicke et al. Citation1998; Polidori et al. Citation2013; Baumann et al. Citation2018).
In the hymenopteran family Formicidae (Hymenoptera: Aculeata), metal enrichment in mandibles was previously detected in a limited number of species, with Zn always present (Hillerton & Vincent Citation1982; Edwards et al. Citation1993; Schofield et al. Citation2002; Larabee & Suarez Citation2010; Brito et al. Citation2017; Polidori et al. Citation2020; Chowdhury & Rastogi Citation2021) and was also studied in terms of its mechanical effects on the cuticle (Schofield et al. Citation2002). The relationship between force applied and Zn content was recently assessed, pointing out the importance of metal enrichment in improving mechanical properties of cuticule (Birkenfeld et al. Citation2024). Furthermore, it has been demonstrated, across several ant species, how cuticular enrichment, together with mandible shape and position/number of teeth, is linked with feeding habits (granivorous, predatory, necrophagous, nectivorous), territoriality, type of nesting (arboreal, psammophilous, lithophilous), and foraging methods (solitary or by recruitment) (Chowdhury & Rastogi Citation2021).
The evolution of incorporation of transition metals into the ants’ mandibles has allowed the reduction of the force applied, as well as the energy consumed for their use (Schofield et al. Citation2021; Klunk et al. Citation2024; Birkenfeld et al. Citation2024). Ants seem, as all other studied Hymenoptera to date (Polidori et al. Citation2020), to have essentially evolved this strategy to increase cuticle hardness, with the only, striking exception of Acromyrmex echinatior Forel 1899, which have high-magnesium calcite crystals as an additional coating in the exoskeleton which improves its biomechanical properties (Li et al. Citation2020). Despite transition metals are apparently ubiquitous in the mandible teeth of ants, their adaptive role is still not fully clear. Indeed, metal enrichment could merely be a consequence of phylogenetic heritance (Polidori et al. Citation2020) and not necessarily an evolutionary response to ecological or behavioural pressures in all ants. While comparison between sub-castes within one species was performed (Birkenfeld et al. Citation2024), comparative analysis between males, queens, and workers within species, as well as between species with different life-histories, have been never carried out to date. For instance, no evaluation of metals in the mandibles of either queens or males of any ant species was performed to date, and only free-living, non-parasitic ant species were studied from this point of view to date.
Here, we tested for the possible adaptive role of mandibles’ metal enrichment in the ant genus Lasius Fabricius 1804, a member of Formicinae with Holarctic distribution (Wilson Citation1955), through intra-specific and inter-specific comparisons. Lasius includes 115 known extant species occurring in a wide range of habitats including grasslands, woodlands, and meadows (Seifert Citation2018). Nests can be built in wood or in the soil. Queens and workers differ in body size, with an overall little marked caste dimorphism (Bolton Citation2018). Lasius mandibles possess from six to ten (queens and workers) or from zero to seven (males) teeth on their inner margin, according to the fact that only females are engaged in feeding, hunting, nest building, and all the other colony activities. Hence, the genus is adequate for intra-specific comparisons of mandible cuticular features. Within this genus, furthermore, both temporary socially parasitic species and autonomous, nest-founding species, occur (Seifert Citation2018). Temporary parasitism involves queens decapitating the host species’ queens (frequently a non-parasitic species) and exploiting workers of the target nest in order to found their own colony (Buschinger Citation2009). Mandibles of parasitic workers of Lasius are known to possess a slightly different mandible shape compared with workers of non-parasitic species (Seifert Citation2018). Hence, this ant genus is also adequate for inter-specific comparisons of mandible chemical composition.
In particular, our predictions were the following two. First, males, not involved in colony activities, are predicted to have lower metal-enrichment in mandibles compared with workers and queens. Second, females (both castes) of socially parasitic species, because they engage in fights with the host, are expected to have higher metal-enrichment in mandibles compared with females (both castes) of the non-parasitic species.
2. Material and methods
2.1. Sample origin and species synopsis
Ninety-one individuals belonging to 12 species of Lasius and spanning queens, workers, and males were collected from different Italian localities in spring and summer from 2014 to 2021 (Table S1). Specimens were identified through dichotomous key provided in Seifert (Citation2018), Seifert (Citation2020) and Lebas and Galkowski (Citation2021). Because males are particularly difficult to identify morphologically, they were gathered together with females from nests. Nests were detected on sight, waiting for nuptial flights or otherwise searching under stones. Samples were preserved in ethanol 95% until their preparation for the microscopy investigations.
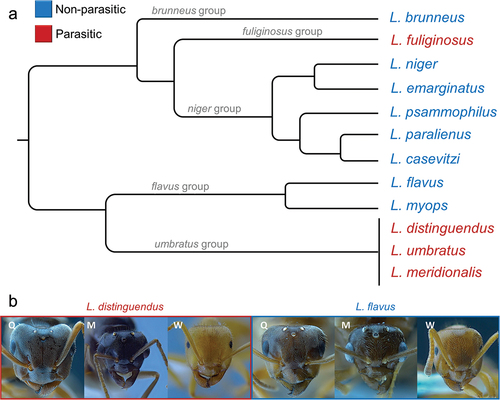
The 12 studied species encompass five different species-group and included both free-living, colony-founding species (here after non-parasitic species) as well as socially parasitic species (here after parasitic species) (). Males, workers, and queens were studied in all species, except for Lasius brunneus, for which males were not available.
Figure 1. a, Phylogeny of the studied Lasius species, manually re-drawn based on Seifert (Citation2020), Maruyama et al. (Citation2008), Blatrix et al. (Citation2020) and Boudinot et al. (Citation2022). b, Pictures of the head of individuals (Q = queen, M = male, W = worker) from two of the studies species (one parasitic and one non-parasitic).
2.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
Specimens were dissected under light microscopy in a way to gently separate the heads from the rest of the body. Mandibles were open up to achieve exposition. The heads were then mounted on adhesive carbon pads attached to aluminium stubs (Polidori et al. Citation2013; Jorge et al. Citation2017) and introduced in the scanning electron microscopy (SEM) without previous gold or platinum coating. From two to four individuals per species and caste were analysed (Tables S1-S2). Pictures of the specimens` heads were taken at convenient magnification to show their entire mandibles. For the Energy Dispersive X-ray Spectroscopy (EDS) analysis, we focused on the occurrence and abundance (weight percentage, wt%) of the transition metal zinc (Zn) and manganese (Mn). A point-analysis was performed on each specimen, i.e. the X-ray was applied to a point on the distal portion of one tooth of the inner side of the mandible (or, in case mandibles lacked inner teeth, on the mandible apical tooth). Some point-analyses were also carried out in the proximal part of the mandible, far from the teeth, to confirm that transition metals lack in such cuticle area as already reported in other hymenopterans (e.g. Polidori et al. Citation2013).
A JEOL JSM-IT500 LV Scanning Electron Microscope (JEOL Ltd., Tokyo, Japan), equipped with Backscattered (BSE) and Secondary Electrons (SE) detectors, coupled with energy dispersive X-ray spectrometry, was employed to perform the chemical micro-analysis at the Department of Earth Sciences “Arditio Desio‶ of the University of Milan (ESD-UniMI). The operating conditions were: vacuum mode, 20 kV accelerating voltage, 10 mm working distance. The ZAF correction was applied to the chemical data, as implemented in the JEOL suite of programs. The wt% of the sigma value (i.e. the error in the weight percent concentration at the one sigma level) was used to determine whether the element is below the detection limits of the sample analysis (Duncumb Citation1994). As in previous studies (Polidori et al. Citation2013, Citation2020; Jorge et al. Citation2017), we considered an element as actually occurring in the cuticle, its wt% had to be greater than three times the wt% of the sigma value (Polidori et al. Citation2013, Citation2020).
2.3. Statistical analysis
Since the used analytical method of metal detection is semi-quantitative, we ranked all the obtained wt% values. For Mn, we ranked values as follows: 0 = absent, 1 = 0.01–0.10%, 2 = 0.11–0.20%, 3 = 0.21–0.30%, 4 = 0.31–0.40%, 5 = 0.41–0.50%, 6 ≥ 0.51%. For Zn, we ranked values as follows: 0 = absent, 1 = 0.01–1.00%, 2 = 1.01–2.00%, 3 = 2.01–3.00%, 4 = 3.01–4.00%, 5 = 4.01–5.00%, 6 = 5.01–6.00%, 7 = 6.01–7.00%, 8 = 7.01–8.00%, 9 = 8.01–9.00%, 10 = 9.01–10.00%, 11 = ≥10.01%. All raw data of %wt recorded during the study can be found in Table S2.
Once ranked, we calculated the average values of wt% for Zn and Mn across individuals per each species (). Then, we averaged the values per species across groups (caste, sex, parasitic or non-parasitic life-style) to apply the statistical analyses and test for our hypotheses. To evaluate the differences in elements’ concentrations we first apply simple, univariate non-parametric statistics. To compare the medians of two groups, Mann–Whitney test was used, while the Kruskal–Wallis test was used for comparisons among more than two groups. In case of overall significance, the Kruskal–Wallis test was followed by Bonferroni paired comparisons. Then, we used Generalized Linear Models (GLMs) to evaluate which of the two explanatory variables (caste/sex and biology (parasitic or non-parasitic)) had greater effect on the variability of element concentrations. One model was performed for each of the two considered elements (Zn and Mn). All statistics were performed in R through the RStudio Software v 2022.02.2–485, R v 4.1.3 (R Core Team Citation2022) (for GLM, using the R package lme4 (Bates et al. Citation2015)) and in PAST 3.04 (Paleontological Statistics Software Package) Hammer et al. (Citation2001 (for the non-parametric univariate tests and to create the graphs). In text and tables, average values are reported ± standard error (SE).
Table I. Mean values ± SE of the ranked % of Zn and Mn, per species and caste.
3. Results
The SEM pictures revealed that Lasius males of the studied species possess much fewer (often none) and smaller teeth on the inner side of the mandibles, compared with workers and queens (). The EDS analysis revealed that Zn and Mn largely occur in Lasius mandible teeth (). When occurring, the presence of Zn and Mn was visible in the EDS spectra by the presence of peaks at 1.2 (ZnL) and 8.8 KeV (ZnKα) and 5.9 KeV (MnKα) (). The metal-enriched mandible teeth appeared whiter than the rest of the cuticle in the SEM pictures (). On the other hand, none of the EDS analyses performed in the proximal part of the mandibles gave spectra with Zn or Mn peaks (Fig. S1). Apart from the obvious high abundance of Carbon (C), Oxygen (O), and Nitrogen (N) (the main components of chitin), other elements were detected at small concentrations and/or were not associated with a relevant function in insects, and thus were not further considered here (Table S2). Chlorine (Cl) was recorded at often relatively high abundances (up to 1%, Table S2) because it is known to co-occur with Zn in metal-enriched insect cuticle (e.g. Jorge et al. Citation2017), being incorporated in the ant mandibles at the same time as Zn (i.e. after pre-ecdysial tanning) (Schofield et al. Citation2003) and being probably involved in the protein complex including Zn (Jorge et al. Citation2017; Schofield et al. Citation2021).
Figure 2. Examples of SEM images of male, queen and worker mandibles of L. distinguendus (parasite) (a–c) and L. psammophilus (not parasite) (d–f). g–l, the EDS spectra for the mandibles of the individuals shown in a–f. Zn and Mn peaks are indicated and drawn from zoomed images in grey circles.
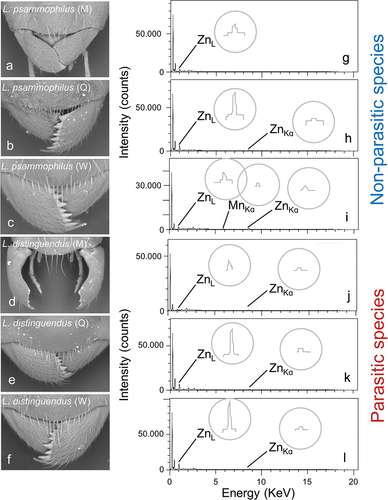
Important difference emerged between castes, sexes, and life-histories in the transition metals. From a general inspection of the raw concentrations obtained from the EDS analysis, males had mandibles with 0 wt% to 8 wt% of Zn (<3 wt% in most individuals) and from 0 wt% to 0.4% of Mn (with no Mn in most individuals) (Table S2). Workers and queens, on the other hand, possessed mandibles with teeth enriched with 1 wt% to almost 15 wt% of Zn, with most of the individuals having >3 wt%. Mn in females was rarely absent and was >0.5 wt% in several individuals (Table S2). Once ranked, these values showed statistically significant variations among the tested groups.
Males, workers, and queens differed in the ranked wt% of Zn (Kruskal–Wallis test: χ2 = 8.04, N = 35, p = 0.017), with significant paired differences between males and both workers (Bonferroni paired comparison: p = 0.007) and queens (p = 0.033), but no differences between workers and queens (p = 0.55) (, ). Males, workers, and queens also differed in the ranked wt% of Mn (Kruskal–Wallis test: χ2 = 10.02, N = 35, p = 0.006) (, ), with significant paired differences between males and both workers (Bonferroni paired comparison: p = 0.002) and queens (p = 0.034), again with no differences between workers and queens (p = 0.28). Since males had much lower concentrations of both Zn and Mn, the comparison between parasitic and non-parasitic species was carried out only for workers and queens. Parasitic queens and non-parasitic queens did not differ in the ranked wt% of both Zn (Mann–Whitney test: U = 12.5, N = 12, p = 0.59) and Mn (Mann–Whitney test: U = 15, N = 12, p = 0.93) (). However, workers of parasitic species possessed a significantly higher wt% of Mn compared with those of non-parasitic species (Mann–Whitney test: U = 0, N = 12, p = 0.008) and a marginally significantly higher wt% of Zn (Mann–Whitney test: U = 6, N = 12, p = 0.10) compared with those of non-parasitic species (, ).
Figure 3. Box-and-whisker plots showing medians (horizontal lines within boxes), 1° and 3° quartile (horizontal lines closing the boxes), and maximum and minimum values (ends of the whiskers) for the ranked values of Zn (a, c) and Mn (b, d), calculated across species, in males (M), queens (Q) and workers (W) (a-b) and in workers of parasitic and non-parasitic species (c-d). * = [0.1 ≥ p ≥0.01], ** = P < 0.01.
![Figure 3. Box-and-whisker plots showing medians (horizontal lines within boxes), 1° and 3° quartile (horizontal lines closing the boxes), and maximum and minimum values (ends of the whiskers) for the ranked values of Zn (a, c) and Mn (b, d), calculated across species, in males (M), queens (Q) and workers (W) (a-b) and in workers of parasitic and non-parasitic species (c-d). * = [0.1 ≥ p ≥0.01], ** = P < 0.01.](/cms/asset/1af1bad9-4c88-445d-89a3-c9a8f1b800de/tizo_a_2355308_f0003_oc.jpg)
The GLMs largely confirmed these results. The caste/sex affected the ranked wt% of both Zn (Adjusted R2 = 0.247, F = 4.718, df = 3, 31, p = 0.008) and Mn (Adjusted R2 = 0.3022, F = 5.909, df = 3, 31, p = 0.003), with males possessing the lowest values compared with females, and the species biology affected the ranked wt% of Mn and marginally of Zn, with parasitic species possessing higher values than non-parasitic species (). The statistical parameters of the GLMs and the associated P-values seem to give more importance to the differences between females and males than to those between parasitic and non-parasitic species ().
Table II. Results of the General Linear Model (GLM) analysis for Zn, Mn, and Cl. Explanatory variables were caste (W = worker, Q = queen) and biology (p = parasitic). SE = Standard Error. In bold, P-values <0.05.
Across workers and queens, Zn ranked wt% was correlated with Mn ranked wt% (Pearson test: workers: r = 0.89, N = 12, p < 0.001; queens: r = 0.60, N = 12, p = 0.038). However, a correlation between Zn and Mn did not appear in males (Pearson test: r = 0.09, N = 11, p = 0.79), since they often had no Mn even if Zn occurs in appreciable concentrations.
4. Discussion
Our study provided new information on the enrichment of mandible teeth by transition metals in ants, a group for which few species and only workers were previously investigated from this point of view. More in particular, workers of a total of 21 species were previously studied (Hillerton & Vincent Citation1982; Edwards et al. Citation1993; Schofield et al. Citation2002; Larabee & Suarez Citation2010; Brito et al. Citation2017; Polidori et al. Citation2020; Chowdhury & Rastogi Citation2021; Klunk et al. Citation2024) (Table S3) spanning only five out of the 16 extant subfamilies of ants. Furthermore, the leaf-cutter genus Atta, with five species studied, is overrepresented in cuticular composition investigations (Table S3). In these studied ant species, workers have always Zn-enriched mandibles (roughly in a range of 3–16 wt%), with this metal often co-occurring with Mn (always <1 wt%).
Our study largely confirmed such general features for ants. Most importantly, our study is the first performed in a comparative framework within one single ant genus and that provided data on queens, workers, and males, as well as on socially parasitic species. This allowed us to evidence the adaptive role of metals in Lasius. A previous large comparative analysis of Zn and Mn enrichment in Hymenoptera mandibles, together with the above-cited studies on ants, revealed that phylogenetic inertia (Blomberg & Garland Citation2002) is the main responsible for metal-enrichment in the mandibles of ants as a group, which re-acquired Zn- and Mn-enrichment within Aculeata after having loss them at the ancestral node from which both Formicoidea and Apoidea arose (Polidori et al. Citation2020). However, at a smaller scale (i.e. within lineages) the possible evolutionary pressures driving the abundance of these metals were still not fully understood for ants. Nonetheless, such pressures were recognized, for other body parts (ovipositors), in other hymenopteran lineages. For example, within fig wasps (Agaonidae), species that lay eggs in hard fig syconia have more often high concentrations of transition metals in the ovipositor, and in gall wasps (Cynipidae) species that oviposit in hard plant tissues are more prone to have higher concentrations of metals (Polidori et al. Citation2013; Kundanati & Gundiah Citation2014). Even though mandibles evolution in ants still needed to be deepened, our results suggest that within the genus Lasius, behavioural specialization (caste/sex and parasitism) appeared from our study as a likely driver for the evolution of greater abundance of Zn and Mn in the mandibles.
Our first prediction was that males have lower metal-enrichment in mandibles compared with workers and queens. The limited use of the mandibles by males compared with queens and workers seems a reasonable and probable explanation for a reduction of transition metals in male mandibles’ cuticle. This is in clear accordance with the striking morphological difference in mandibles between sexes in these, as well as other, ants (Wilson Citation1955; Dlussky & Pisarski Citation1971), with a notable reduction in mandible size and in teeth size and number in males. In males of the genus Lasius, both species with toothless mandibles and others with few ones have evolved (Kutter Citation1977; Seifert Citation1988). Despite the low autonomy of males and the relatively short lifespan (Hölldobler & Wilson Citation1990; Wilson Citation2020), an exceptional case of mandibles use has been reported in males of Lasius meridionalis, which are capable to move objects and feeding themselves independently (Collingwood Citation1979). Accordingly, males of this species had one of the highest value of Zn recorded here for Lasius males (though not Mn) and teeth on the inner side of the mandibles, emphasizing that there could be special, still unknown behaviors which might deserve further studies.
However, no differences emerged in either Zn or Mn between queens and workers. Workers live from a few months to a couple of years (Parker Citation2010) and make continuous use of their mandibles, requiring greater resistance to limit abrasion, while queens are much longer-lived, reaching 20–30 years (Keller Citation1998; Quque & Bles Citation2020) using the mandibles almost exclusively during the colony foundation phase. As the incorporation of transition metals is directly connected with mandibles’ function efficiency, a positive relationship between hardness and the individual fitness was identified in insects (Laiolo et al. Citation2021). Therefore, we assume that queens with poorly enriched mandibles may have difficulties in the construction phase of claustrum and in its defense. Furthermore, given the longevity of queens, mandible hardness may have evolved to provide durability over time. As a consequence, it can be hypothesized that the similarity in the percentages of Zn and Mn between workers and queens may depend on a trade-off between longevity of individuals and use of mandibles. Our intra-specific comparison opens to a still unexplored question related with how, within a single species, metals concentrate more in the mandibles of a sex over the other. The larval diet seems to account for variation in hymenopteran mandibles’ metal concentration (Polidori et al. Citation2020), but it is currently unknown if Lasius males and females are fed differently in the nest. Alternatively, evolution limited – because no more necessary – the biochemical pathway responsible for metal accumulation in males’ mandible teeth, even in case of identical larval diet for both sexes.
Our second prediction was that females of socially parasitic species have higher metal-enrichment in mandibles compared with females of the non-parasitic species. We found a partial confirmation to this hypothesis, perhaps also because of the small sample of parasitic species. From one side, workers of parasitic species were significantly more enriched in Mn and marginally more enriched in Zn, than workers of non-parasitic species. This result can be explained by the behavior and interaction between host and parasite in young parasitized colonies: after the success of parasitic queen during colony foundation, workers of the two species coexist in the same nest for a long period, until the natural death of the host workers (Stukalyuk et al. Citation2021). While nests are mixed, the host species are able to recognize the workers of the parasitic one (Liu et al. Citation2000; Pulliainen et al. Citation2019; Schultner & Pulliainen Citation2020) and acts of aggression are reported in several ant genera (Buschinger Citation2009). Moreover, once increased sufficiently in number, parasitic workers kill the remains of the host ones (Hölldobler Citation1953). Consequently, workers of parasitic species may benefit from possessing stronger mandibles through higher metal-enrichment, as a response to their need to maintain control on the hosts.
On the other hand, while parasitic queens invade the host nests and decapitate the resident queen to establish as the new colony owner, we have found no significant difference between parasitic and non-parasitic queens in both Zn and Mn. An alternative hypothesis is that parasitic queens benefit from their larger body size and mandible shape, rather than from an increased concentration of metals, during host nest invasion. This hypothesis might find support by looking indeed at the difference in mandible inner (i.e. cutting) surface in parasitic Lasius species compared to the non-parasitic ones (Seifert Citation1988, Citation2018). In the former, the cutting surface seems visibly larger and the mandibles overall more robust. Micro-modification in shape may lead to a change in mandibular functioning in ants (Klunk et al. Citation2021), and further morpho-functional studies in parasitic and non-parasitic Lasius may highlight interesting difference in cutting performance.
Further considerations support an adaptive role linked with parasitism in Lasius. Indeed, the highest values of transition metals were measured in the workers of Lasius fuliginosus (Latreille 1798), the only known hyperparasite species in Europe (Seifert Citation2018). This species establishes a nest through social parasitism on another Lasius species which previously parasitized a nest of a non-parasitic species. Hence, L. fuliginosus may have evolved a higher mandible hardness than that – already greater than non-parasitic species – of its hosts. The high abundance of metals in the mandible of this species supports our hypothesis also for another reason. In fact, it is the only parasitic species analysed here belonging to a clade otherwise entirely composed of non-parasitic species (the niger-group) (). Similarly, the non-parasitic Lasius flavus and L. myops, both closely related with the completely parasitic umbratus group, have lower concentrations of metals than the species of the latter. At last, Lasius brunneus, a non-parasitic species, and the parasitic L. fuliginosus, both digging in wood (Seifert Citation2018), do not have similar concentrations of metals, thus further supporting the role of parasitism in shaping cuticular structure.
A larger species sample of both parasitic and non-parasitic Lasius species, analysed in a formally phylogenetically corrected framework, may further confirm our findings. In addition, many other ant genera have evolved social parasitism, showing a wide range of behaviors (Buschinger Citation2009; Rabeling Citation2020) and phenotypes (Hölldobler & Wilson Citation1990; Fischer et al. Citation2020) and the evaluation of the metal content in the mandibles of species from many ant lineages might support the generality of our results.
Authors’ contributions
C.P. conceived the study, designed the methodology, collected the SEM/EDS data, performed the statistical analysis, and drafted the manuscript. E.N. carried out the field sampling, collected the SEM/EDS data, and drafted the manuscript.
Availability of data and material
Data analysed in this study are available in the Supplementary Table S2. The studied specimens are preserved at E.N. personal collection and at the University of Milan.
Supplemental Material
Download MS Word (18.6 KB)Supplemental Material
Download MS Word (25.8 KB)Supplemental Material
Download MS Word (27.7 KB)Supplemental Material
Download TIFF Image (1 MB)Acknowledgments
We thank Stefania Crespi (Department of Earth Sciences “Ardito Desio”, University of Milan) for technical assistance with the SEM images and the EDS analysis. We thank Fabrizio Rigato (Museo di Storia Naturale di Milano) for helping with species identification.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2024.2355308
Additional information
Funding
References
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1):1–48. DOI:10.18637/jss.v067.i01.
- Baumann K, Vicenzi EP, Lam T, Douglas J, Arbuckle K, Cribb B, Seán BG, Fry BG. 2018. Harden up: Metal acquisition in the weaponized ovipositors of aculeate hymenoptera. Zoomorphology 137(3):389–406. DOI: 10.1007/s00435-018-0403-1.
- Birkenfeld V, Gorb SN, Krings W. 2024. Mandible elemental composition and mechanical properties from distinct castes of the leafcutter ant Atta laevigata (Attini; Formicidae). Interface Focus 14(2):20230048. DOI: 10.1098/rsfs.2023.0048.
- Blatrix R, Aubert C, Decaëns T, Berquier C, Andrei-Ruiz MC, Galkowski C. 2020. Contribution of a DNA barcode to an assessment of the specificity of ant taxa (Hymenoptera: Formicidae) on Corsica. European Journal of Entomology 117:420–429. DOI: 10.14411/eje.2020.046.
- Blomberg SP, Garland T. 2002. Tempo and mode in evolution: Phylogenetic inertia, adaptation and comparative methods. Journal of Evolutionary Biology 15(6):899–910. DOI: 10.1046/j.1420-9101.2002.00472.x.
- Bolton B. 2018. Lasius. In AntWeb. https://www.antweb.org/.
- Boudinot BE, Borowiec MI, Prebus MM. 2022. Phylogeny, evolution, and classification of the ant genus Lasius, the tribe Lasiini and the subfamily Formicinae (Hymenoptera: Formicidae). Systematic Entomology 47(1):113–151. DOI: 10.1111/syen.12522.
- Brito TO, Elzubair A, Araújo LS, Camargo SADS, Souza JLP, Almeida LH. 2017. Characterization of the mandible Atta laevigata and the bioinspiration for the development of a biomimetic surgical clamp. Materials Research 20(6):1525–1533. DOI: 10.1590/1980-5373-mr-2016-1137.
- Broomell CC, Zok FW, Waite JH. 2008. Role of transition metals in sclerotization of biological tissue. Acta Biomaterialia 4(6):2045–2051. DOI: 10.1016/j.actbio.2008.06.017
- Buschinger A. 2009. Social parasitism among ants: A review (Hymenoptera: Formicidae). Myrmecological News 12:219–235.
- Chowdhury R, Rastogi N. 2021. Comparative analysis of mandible morphology in four ant species with different foraging and nesting habits. 10.1101/2021.08.26.457866.
- Collingwood CA. 1979. The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomology Scandinavica 8:1–174.
- Cribb BW, Stewart A, Huang H, Truss R, Noller B, Rasch R, Zalucki MP. 2008. Insect mandibles–comparative mechanical properties and links with metal incorporation. Naturwissenschaften. Jan 951:17–23. Epub 2007 Jul 24. PMID: 17646951. 10.1007/s00114-007-0288-1.
- Dirks JH, Taylor D. 2012. Fracture toughness of locust cuticle. Journal of Experimental Biology 215(9):1502–1508. DOI: 10.1242/jeb.068221.
- Dlussky GM, Pisarski B. 1971. Rewizja polskich gatunków mrówek (Hymenoptera: Formicidae) z rodzaju Formica L. Fragmenta Faunistica (Warsaw) 16:145–224. DOI: 10.3161/00159301FF1971.16.12.145.
- Duncumb P. 1994. Correction procedures in electron probe microanalysis of bulk samples. Mikrochimica acta 114(1):3–20. DOI: 10.1007/BF01244530.
- Edwards AJ, Fawke JD, McClements JG, Smith SA, Wyeth P. 1993. Correlation of zinc distribution and enhanced hardness in the mandibular cuticle of the leaf-cutting ant Atta sexdens rubropilosa. Cell Biology International 17(7):697–698. DOI: 10.1006/cbir.1993.1125.
- Fischer G, Friedman NR, Huang JP, Narula N, Knowles LL, Fisher BL, Mikheyev AS, Economo EP. 2020. Socially parasitic ants evolve a mosaic of host-matching and parasitic morphological traits. Current biology. 3018:3639–3646.e4. ISSN 0960-9822. 10.1016/j.cub.2020.06.078
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Version 2.15. Paleontol Electron 4:9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
- Hillerton JE, Robertson B, Vincent JFV. 1984. The presence of zinc or manganese as the predominant metal in the mandibles of adult stored-product beetles. Journal of Stored Products Research 20(3):133–137. DOI: 10.1016/0022-474X(84)90020-1.
- Hillerton JE, Vincent JFV. 1982. The specific location of zinc in insect mandibles. Journal of Experimental Biology 101(1):333–336. DOI: 10.1242/jeb.101.1.333.
- Hölldobler K. 1953. Beobachtungen über die Koloniengründung von Lasius umbratus umbratus Nyl. Zeitschrift für Angewandte Entomologie. 34 4: January/December. 598–606. 10.1111/j.1439-0418.1953.tb00704.x.
- Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press.
- Jorge A, Polidori C, Garcia-Guinea J, Nieves-Aldrey JS. 2017. Spectral cathodoluminescence analysis of hymenopteran mandibles with different levels of zinc enrichment in their teeth. Science Direct Arthropod Structure & Development 46(1):39–48. DOI: 10.1016/j.asd.2016.07.001.
- Keller L. 1998. Queen lifespan and colony characteristics in ants and termites. Insectes Sociaux 45(3):235–246. DOI: 10.1007/s000400050084.
- Klunk CL, Argenta MA, Casadei-Ferreira A, Economo EP, Pie MR. 2021. Mandibular morphology, task specialization and bite mechanics in Pheidole ants (Hymenoptera: Formicidae). Journal of the Royal Society Interface 18(179):20210318. DOI: 10.1098/rsif.2021.0318.
- Klunk CL, Heethoff M, Hammel JU, Gorb SN, Krings W. 2024. Mechanical and elemental characterization of ant mandibles: Consequences for bite mechanics. Interface Focus. 2024 Apr 12. 142:20230056. 10.1098/rsfs.2023.0056. PMID: 38618235; PMCID: PMC11008963.
- Kundanati L, Gundiah N. 2014. Biomechanics of substrate boring by fig wasps. Journal of Experimental Biology 217(11):1946–1954. DOI: 10.1242/jeb.098228.
- Kutter H. 1977. Hymenoptera, Formicidae. Insecta Helvetiae Fauna 6:1–298.
- Laiolo P, Pato J, Illera JC, Obeso JR. 2021. Selection for functional performance in the evolution of cuticle hardening mechanisms in insects. Evolution. May, 755:1132–1142. Epub 2021 Mar 7. PMID: 33634481. 10.1111/evo.14201.
- Larabee F, Suarez A 2010. Structure and composition of trap-jaw ant mandibles. Conference paper, Entomological Society of America Annual Meeting 2010, San Diego, CA.
- Lebas C, Galkowski C. 2021. Description de la reine et du mâle de Lasius casevitzi Seifert & Galkowski, 2016 (Hymenoptera, Formicidae). Bulletin de la Société Linnéenne de Bordeaux 49:386–390.
- Lehnert MS, Reiter KE, Smith GA, Kritsky G. 2019. An augmented wood-penetrating structure: Cicada ovipositors enhanced with metals and other inorganic elements. Scientific Reports 9(1):19731. DOI: 10.1038/s41598-019-56203-6.
- Li C, Gorb SN, Rajabi H. 2020. Cuticle sclerotization determines the difference between the elastic moduli of locust tibiae. Acta Biomaterialia 103:189–195. February 2020. DOI: 10.1016/j.actbio.2019.12.013.
- Li H, Sun CY, Fang Y, Carlson CM, Xu H, Ješovnik A, Sosa-Calvo J, Zarnowski R, Bechtel HA, Fournelle JH, Andes DR, Schultz TR, Gilbert PUPA, Currie CR. 2020. Biomineral armor in leaf-cutter ants. Nature Communications 11(1):5792. DOI: 10.1038/s41467-020-19566-3.
- Liu Z, Yamane S, Yamamoto H, Wang Q. 2000. Nestmate discrimination and cuticular profiles of a temporary parasitic ant Lasius sp. and its host L. fuliginosus (Hymenoptera: Formicidae). Journal of Ethology 18(2):69–74. DOI: 10.1007/s101640070002.
- Maruyama M, Steiner FM, Christian S, Toshiharu A, Ross C, Birgit SS. 2008. A DNA and morphology based phylogenetic framework of the ant genus Lasius with hypotheses for the evolution of social parasitism and fungiculture. BMC Evolutionary Biology 8(1). DOI:10.1186/1471-2148-8-237.
- Parker JD. 2010. What are social insects telling us about aging? Myrmecological News 13:103–110.
- Parle E, Larmon H, Taylor D, Doucet D. 2016. Biomechanical factors in the adaptations of insect tibia cuticle. PLOS ONE 11(8):e0159262. DOI: 10.1371/journal.pone.0159262.
- Polidori C, Jorge A, Keller A, Ornosa C, Tormos J, Asís JD, Nieves-Aldrey JL. 2020. Strong phylogenetic constraint on transition metal incorporation in the mandibles of the hyper-diverse Hymenoptera (Insecta). Organisms Diversity & Evolution 20(3):511–526. ISSN 1439-6092 Volume 20 Number 3 Org Divers Evol. DOI: 10.1007/s13127-020-00448-x.
- Polidori C, Jorge A, Nieves-Aldrey JL, Fontaneto D. 2013. Breaking up the wall: Metal enrichment in ovipositors, but not in mandibles, co-varies with substrate hardness in gall-wasps and their associates. PLOS ONE 8(7):1–7. DOI: 10.1371/journal.pone.0070529.
- Pulliainen U, Helanterä H, Sundström L, Schultner E. 2019. The possible role of ant larvae in the defence against social parasites. Proceedings of the Royal Society B: Biological Sciences 286(1898):20182867. DOI: 10.1098/rspb.2018.2867.
- Quicke DLJ, Wyeth P, Fawke JD, Basibuyuk HH, Vincent JFV. 1998. Manganese and zinc in the ovipositors and mandibles of hymenopterous insects. Zoological Journal of the Linnean Society 124(4):387–396. DOI: 10.1111/j.1096-3642.1998.tb00583.x.
- Quque M, Bles O. 2020. Lasius. In: Encyclopedia of Social Insects. Springer. DOI: 10.1007/978-3-319-90306-4_169-1.
- Rabeling C. 2020. Social Parasitism. In: Starr C, editor. Encyclopedia of Social Insects. Cham: Springer. DOI:10.1007/978-3-319-90306-4_175-1.
- R Core Team. 2022. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. URL. https://www.R-project.org/.
- Schofield RMS. 2001. Metals in cuticular structures. In: Brownell P, Polis G, editors Scorpion Biology and Research. Oxford, UK: Oxford University Press. pp. 234–256.
- Schofield RMS, Bailey J, Coon JJ, Devaraj A, Garrett RW, Goggans MS, Hebner MG, Lee BS, Lee D, Lovern N, Ober-Singleton S, Saephan N, Seagal VR, Silver DM, Som HE, Twitchell J, Wang X, Zima JS, Nesson MH. 2021. The homogenous alternative to biomineralization: Zn-and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Scientific Reports 11(1):17481. DOI: 10.1038/s41598-021-91795-y.
- Schofield RMS, Nesson MH, Richardson KA. 2002. Tooth hardness increases with zinc content in mandibles of young adult leaf-cutter ants. Naturwissenschaften 89(12):579–583. DOI: 10.1007/s00114-002-0381-4.
- Schofield RMS, Nesson MH, Richardson KA, Wyeth P. 2003. Zinc is incorporated into cuticular “tools” after ecdysis: The time course of the zinc distribution in “tools” and whole bodies of an ant and a scorpion. Journal of Insect Physiology 49(1):31–44. DOI: 10.1016/S0022-1910(02)00224-X.
- Schultner E, Pulliainen U. 2020. Brood recognition and discrimination in ants. Insectes sociaux 67(1):11–34. DOI: 10.1007/s00040-019-00747-3.
- Seifert B. 1988. A revision of the European species of the ant subgenus Chthonolasius (Insecta, Hymenoptera, Formicidae). Entomology Abhandlungen Staatl Museum Tierkdres 51:143–180.
- Seifert B. 2018. The ants of Central and North Europe. Lutra Verlags-und Vertriebsgesellschaft Tauer, Görlitz.
- Seifert B 2020. A taxonomic revision of the Palearctic members of the subgenus Lasius s.Str. (Hymenoptera, Formicidae). Soil organisms, 92 (1) May 2020. DOI: 10.25674/so92iss1pp15.
- Stukalyuk S, Radchenko Y, Gonchar O, Akhmedov A, Stelia V, Reshetov A, Shymanskyi A. 2021. Mixed colonies of Lasius umbratus and Lasius fuliginosus (Hymenoptera, Formicidae): When superparasitism may potentially develop into coexistence: A case study in Ukraine and Moldova. 10.5281/zenodo.5753121.
- Vincent JFV, Wegst UGK. 2004. Design and mechanical properties of insect cuticle. Arthropod Structure & Development. 33:187–199. 1 July 2004. 10.1016/j.asd.2004.05.006.
- Wilson EO. 1955. A monographic revision of the ant genus Lasius. Bulletin of the Museum of Comparative Zoology 113:1–201.
- Wilson EO. 2020. Tales from the Ant World. ISBN 9781631495564. New York, N.Y.: Liveright Publishing Corp.
