Abstract
We provide data on the biology of Zercon hamaricus Kaczmarek et al. 2021 with regard to duration of egg and juvenile stages, analysis of change of body size during ontogeny, population parameters and morphological analysis of egg and all postembryonic stages using scanning electron microscopy. We confirmed the effectiveness of using hexamethyldisilazane in drying of Z. hamaricus individuals for SEM (Scanning Electron Microscopy) analysis. The overall egg duration was 3.7 ± 1.8 days on average. Duration of postembryonic stages ranged from 4.1 to 4.9 days and was the longest in deutonymphs. Change in length was statistically insignificant in larvae and significant in nymphs. At the species’ type locality juveniles (protonymphs) predominated, females were more abundant than males and juveniles were more abundant than adults. The exochorion of eggs of Z. hamaricus consists of dense areola with long processes and a fine polygonal pattern. We report on the presence and arrangement of metapodal plates in protonymphs and deutonymphs, and illustrate the post-coxal cuticular spines in adults. The influence of preparation method on the shape of the peritreme, position of seta r1 and shield and body size are discussed.
Introduction
The family Zerconidae includes nearly 500 species that inhabit the Holarctic Region (reviewed by Sellnick Citation1944; Błaszak Citation1975; Halašková Citation1977; Mašán & Fenďa Citation2004; Sikora Citation2014) and the number of known taxa is growing quickly (for Zercon, e.g. Bulut et al. Citation2021; Bilki et al. Citation2022; Mohammad-Doustaresharaf et al. Citation2023; Urhan & Karaca Citation2023) but their biology at the species level is still insufficiently studied. We recently described Zercon hamaricus Kaczmarek et al. Citation2021 from Norway. We described all postembryonic stages and compared the ontogeny of Z. hamaricus with that of some similar species. We also discussed the phenomenon of sexual dimorphism in Zerconidae, as Z. hamaricus and at least five other species show clearly visible differences in opisthonotal setation between females and males (Sellnick Citation1958; Błaszak Citation1970, Citation1974; Błaszak & Skorupski Citation1992; Ujvári Citation2011a; Faleńczyk-Koziróg et al. Citation2018; Kaczmarek et al. Citation2021). Sexual dimorphism is expressed in the J-series of dorsal shield setae.
We now expand that study using laboratory-reared specimens of Z. hamaricus and new material collected from the type locality of the species. The aim of this study is to provide information on the ontogenetic development of the species, including the duration and morphology of all life cycle stages, which makes Z. hamaricus the most fully studied species of Zercon so far. It will also allow further morphological, genetic, and biogeographical investigations into the peculiar species group of Zercon with opisthonotal sexual dimorphism (unusual within the genus), which inhabits the higher latitudes of the Northern Hemisphere, and the higher altitudes farther south.
Material and methods
Six samples (250 cm3 each) were collected at the type locality of Zercon hamaricus (Hamar, Norway; 60°47′33.7′′N, 11°2′9.2′′E; 150 m. a.s.l.) on 24 March 2022 by Bogna Kaczmarek-Dhaliwal. Mites were extracted using Tullgren funnels for 7 days and preserved in 70% ethyl alcohol. Living specimens were placed in a collection box filled with a 4 cm thick layer of plaster of Paris and charcoal mixture (8:1) and provided with nematodes as food. For scanning electron microscopy (SEM), individuals in alcohol were dehydrated using alcohols of increasing concentration (in %: 70–80–90–96–absolute, 30 min each) and then were air-dried with the use of hexamethyldisilazane (≥99%, 1 hour in HMDS and then air-dried overnight), placed on Al-stubs with double-sided adhesive tape and coated with Au in an Agar Scientific AGB7340 sputter coater. A Phenom Pure (Thermo Fisher Scientific) scanning electron microscope was used for SEM microphotography. Transmitted light photomicrographs (light microscopy - LM) were made using a Leica DM3000 light microscope with a Leica DFC420 camera and Leica Application Suite 3.8.
Ontogenetic stages were examined using a Nikon SMZ-1270 stereomicroscope with Delta DLT-Cam 1080 camera. SEM and transmitted light micrographs were prepared with HeliconFocus 8.2.2 Pro software. Stereomicroscopic measurements were made using ImageJ 1.54d software (Schneider et al. Citation2012) calibrated with a calibration slide (Polish Optical Industries, 1 mm in 0.01 mm divisions). All measurements are given in µm. The thickness of each stage was determined using the lateral SEM view as the distance from the top of the posterior edge of the podonotum to the bottom edge of the coxa IV.
For the study of egg morphology, freshly laid eggs were collected from an established mass culture, placed in 70% alcohol and prepared the same way as the individuals for the SEM study. Hatched egg shells were prepared the same way. For the egg incubation study, the mass culture was inspected twice a day and freshly laid eggs were observed to the hatching stage without moving them. Four females from the mass culture were placed into small cages (1.5 mL Eppendorf tubes with plaster of Paris and charcoal substrate). Cages were inspected daily, and freshly laid eggs were transferred with a fine brush to new cages and observed daily. The duration of developmental stages was observed daily in these cages. During each observation, the individual was measured dorsally to record the ontogenetic change in size (method as in Marquardt & Kaczmarek Citation2017). To determine change in size (length and width of the body, width measured at half-length) during each developmental stage we used recordings of the first day’s (after moult) and the last day’s (before the moult to the next stage) observations. In calculations of body size change we included all observed stages with both the first- and last-day measurements (the first-day measurements of stages that died before the next moult were excluded). The statistical significance of changes in length/width and egg duration between eggs observed in mass rearing and eggs individually transferred was calculated with the Mann-Whitney test. The statistical significance in different durations of juvenile stages was calculated with the Kruskall-Wallis test using XLStat (Addinsoft Citation2023). In all tests we used alpha = 0.05. All observations were made at the Department of Evolutionary Biology, Faculty of Biological Sciences, Kazimierz Wielki University, Poland.
Results
Egg incubation
We observed the incubation of 38 eggs, 16 within mass rearing and 22 in single-individual cages. In mass rearing 87.5% of eggs hatched (14), while in single-individual cages it was 72.7% (16). A clear change in egg surface morphology and fungal growth on the egg surface was considered to indicate egg death. Egg incubation within mass rearing lasted from 1 to 7 days (N = 14, 4.2 ± 2.1 days on average) and in individual cages it lasted from 1 to 5 days (N = 16, 3.3 ± 1.5 days on average). The overall egg duration was 3.7 ± 1.8 days on average (N = 30). There were no statistically significant differences in duration of egg development between the two types of observation (Mann Whitney, p = 0.208). Out of 16 hatched eggs (single-individual cages) only 50% reached the adult stage (6 ♀♀ and 2 ♂♂) and the others died at larval (1 individual), protonymphal (3) and deutonymphal (4) stage.
Postembryonic development
The duration of the deutonymphal stage was clearly longer than those of larvae and protonymphs, which were comparable (see also ). The larva to adult development lasted about 13 days on average (about 17 days on average for egg–adult time including data on egg incubation from mass rearing and single-individual observations). There were no statistically significant differences in duration between the juvenile stages. The average growth (in µm) during larval stage was 41 (18.9%) in length and 11 (7.7) in width, in protonymphs 59 (21.5) and 26 (15.7) and in deutonymphs 48 (13.3) and 12 (5.3), respectively (). The change in width in all juvenile stages was statistically insignificant. The change in length was statistically non-significant in larvae and significant in nymphs. We found no significant change in length and width between the larvae in the last day before the next moult and the protonymphs in the first day after moult. In other moults (protonymph to deutonymph and deutonymph to female) we found significant changes in length and width.
Table I. Duration (in days, average ± SD [range]), length and width (in µm, average ± SD [range]) of larvae (L), protonymphs (P), deutonymphs (D) and females (F) and the length:width ratio ([range]:1) in the first day (after moult) and the last day (before moult to the next stage, juveniles) in Z. hamaricus; statistical significance of length/width change before/after moult between L and P, P and D and D and female.
Table II. Share (in % per sample) of larvae (L), protonymphs (P), deutonymphs (D), females (F) and males (M), total abundance (A) of sample and stage, female:male (F:M) and juvenile:adult (juv:ad) ratios of Z. hamaricus in samples collected at its type locality.
Observation of the Zercon hamaricus population at the type locality
We collected 781 individuals of Z. hamaricus (213 adults and 568 juveniles), which was the only species of Zerconidae at the studied location. The average abundance was 130.2 individuals per 250 cm3 (40–370, standard deviation [SD] = 121.4), 35.5 (14–82, SD = 22.2) for adults and 94.7 (26–288, SD = 90.3) for juveniles (). In most samples the protonymphs predominated within juveniles and all individuals, females were more abundant than males and juveniles were more abundant than adults.
Morphology
Egg morphology ()
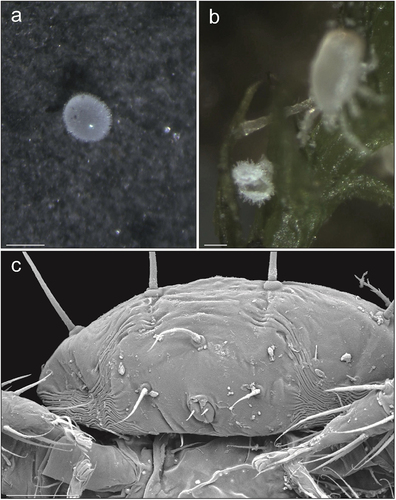
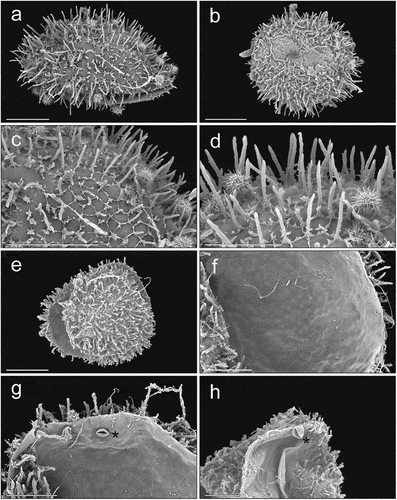
The eggs of Z. hamaricus are spherical (120–130 µm in diameter) and covered with dense areolae with numerous processes perpendicular to the egg surface (). The areolar egg processes are of varying length (6–14 µm) and finely sculptured. On the egg surface there is also a polygonal pattern consisting of fine outgrowths, much shorter than the areolar processes. This structure is always visible but sometimes it does not cover the whole egg surface. It is clear that the long processes grow out from the intersections of lines that form the polygonal pattern. The egg shell is 0.4–0.7 µm thick. The inner surface of the egg shell is smooth, with lighter areas separated by darker bands (). There are also clearly visible irregular veins. In two different egg shells, we also found an inner structure resembling a plant stoma close to the opened edge of the egg ().
Figure 1. Zercon hamaricus SEM (Scanning Electron Microscopy) micrographs of eggs. (a) air-dried egg; (b) egg dried with the use of HMDS (hexamethyldisilazane); (c,d) exochorion; (e) typical view of hatched egg; (f) inner surface of egg chorion; (g,h) stoma-like structure on the inner egg surface (asterisk). Scale bars (µm): a–c, e = 50; d, f–h = 25.
Morphology of instars
Larva ()
On the podonotal shield of the larva setae j1 are horizontal and directed anteriorly, and all the other setae are raised (). Although the podonotal shield of the larva is positioned dorsally, the opisthonotal shield is instead dorso-posteriorly positioned and more vertical than horizontal (). Therefore, only the anterior pair of posterodorsal cavities is directed more upwards and the posterior pair is directed more posteriorly (). All the long setae of the opisthonotum (Z3, Z4, J5) are directed posteriorly and upwards (). The setae of the anal shield (para-anal setae and postanal seta) are directed posteriorly and downwards. The anal shield is suboval and separated from the opisthonotal shield by narrow belts of soft cuticle around its postero-lateral edges. The posterior edge of the anal shield is not well separated from the posterior edge of opisthonotal shield; there are some striations that differ from the other parts of soft cuticle; there is no cribrum (). Setae Z5 and S5 are located on the lateral sides of the body, on the edges of the opisthonotal shield. Only setae Jv1, Jv2, Zv2 and Jv5 are located on the soft cuticle. The epistome is typical for the genus with four processes (), except that one of the studied larvae had five processes. Thickness of larva: 120.
Figure 3. SEM (Scanning Electron Microscopy) micrographs of a Zercon hamaricus larva. (a) dorsal habitus; (b) lateral habitus; (c) anterior part of idiosoma, lateral view; (d) posterior part of idiosoma, lateral view; (e) sternal and anal region; (f) epistome; (g) opisthonotum, dorsal view; (h) opisthonotum, posterodorsal cavities. Scale bars (µm): a, b, g = 100; c, d, e = 50; f, h = 25.
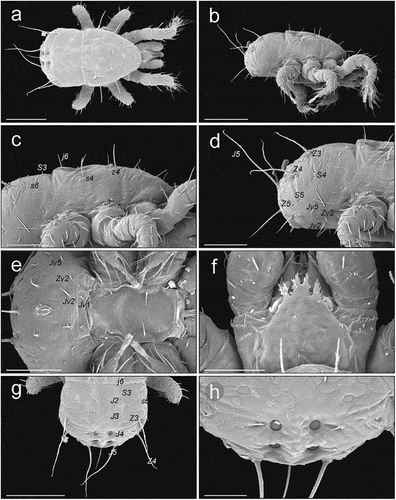
Protonymph ()
On the podonotal shield setae j1 are horizontal and directed anteriorly; r2 are horizontal and directed laterally outward, and the other setae are raised (). The podonotal and opisthonotal shields are horizontal with the edges bent ventrally (). All the long setae of the opisthonotum (S3–5, Z4–5) are directed posteriorly and raised; S3, S4 and S5 are usually directed more laterally (). Setae r5, s6 and R1 are on the lateral parts of the soft cuticle directed postero-laterally, and r3 is directed similarly to r2 (). The inguinal region is surrounded with smooth, soft cuticle (). A small metapodal plate is present on each side in the soft cuticle between the stigma and the ventrianal shield, posterolaterally to coxa IV (). The peritrematal shield has narrow edges; the peritrematal groove is directed laterally, with fine and dense pilae inside (). All the protonymphs we examined had a typical epistome (). Thickness of protonymph: 150.
Figure 4. SEM (Scanning Electron Microscopy) micrographs of a Zercon hamaricus protonymph. (a) dorsal habitus; (b) lateral habitus; (c) anterior part of idiosoma, lateral view (*metapodal plate); (d) posterior part of idiosoma, lateral view; (e) sternal and anal region; (f) epistome; (g) opisthonotum, dorsal view; (h) peritreme, lateral view. Scale bars (µm): a, b = 150; c–e = 50; f, h = 25; g = 100.
Deutonymph ()
On the podonotal shield setae j1, r3, r4, r5, s3 and s6 are horizontal; j1 is directed anteriorly, and the other setae are directed laterally (). The other podonotal setae are raised. All the long setae of the opisthonotum (S3–5, Z4–5) are directed posteriorly and raised, and S3, S4 and S5 are directed more horizontally and more laterally than the Z-setae (). The ventral idiosoma, except the sternal, ventrianal and peritrematal shields, is covered with soft and striated cuticle. The ventrianal shield is convex (). The cuticle between the R-setae and the lateral edges of ventrianal shield is more horizontal. The belt of cuticle between the posterior edges of coxae IV and the anterior edge of the ventrianal shield is ventrally convex. The inguinal region has clearly striated soft cuticle. The metapodal plates are located close to the anterolateral edges of the ventrianal shield, clearly recessed in the surrounding soft cuticle, and more dorsal than the ventrianal shield (). The peritrematal shield has broad edges; the peritrematal groove is directed laterally, with fine and dense pilae inside (). Most of the studied deutonymphs had a typical epistome, except in one deutonymph the medial process was tripartite (). Thickness of deutonymph: 180.
Figure 5. SEM (Scanning Electron Microscopy) micrographs of a Zercon hamaricus deutonymph. (a) dorsal habitus; (b) lateral habitus; (c) anterior part of idiosoma, lateral view; (d) posterior part of idiosoma, lateral view (*metapodal plate); (e) sternal region; (f) epistome; (g) opisthonotum, dorsal view; (h) peritrematal region, lateral view. Scale bars (µm): a, b = 200; c, e, h = 50; d = 100; f = 25; g = 150.
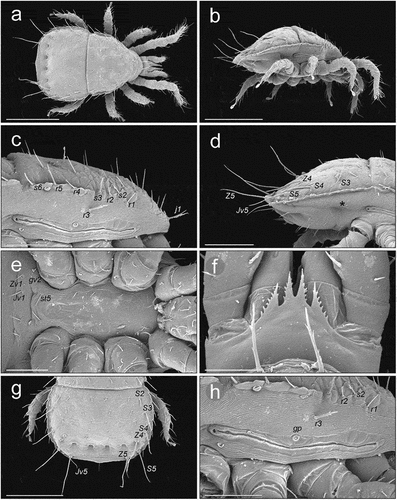
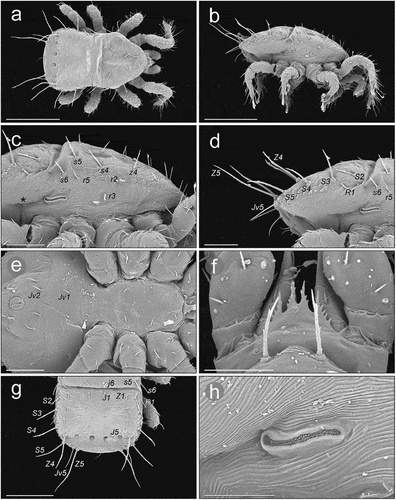
Adults ()
On the podonotal shield setae j1, r1, s2, r2, s3, r3, r4, r5 and s6 are horizontal, j1 is directed anteriorly, and the other setae are directed more laterally (). The other podonotal setae are raised. The dorsal part of the podonotal shield is horizontal (convex-arched) and anteriorly connected to pleural (more vertical) parts without a visible boundary (). The pleural parts of the anterior idiosoma have a scale-like sculpture only in the anterior part (up to r3 and gp), while the posterior part (peritrematal) only has some striations. The pleural parts of the anterior idiosoma on each side are connected to the peritrematal region without a clearly visible border, located ventrally to a line r4–r5–s6. The pleural part of the anterior idiosoma between line r4–r5–s6 and dorsal (anti-axial) edge of peritrematal shield is more horizontal (). The peritrematal shield has visible antiaxial and posterior edges that pass diagonally from its dorsal edge towards coxae III–IV. The ventrianal shield is ventrally convex (). The cuticle between the R-setae and the lateral edges of the ventrianal shield is more horizontal. The belt of cuticle between the posterior edges of coxae IV and the anterior edge of the ventrianal shield is ventrally convex. The inguinal region has clearly visible, dense cuticular spines directed antero-ventrally (towards the coxae) (). The peritrematal groove is directed laterally, with fine and dense pilae inside (). Some of the long setae of the opisthonotum (J5, Z4, Z5) are directed posteriorly and raised; S5 is directed more horizontally and more laterally (). Setae S3 and S4 in some individuals were more vertical, in some more horizontal and raised posteriorly. The long, hooked part of the peritreme of both sexes in its ventral (adaxial) part has three (rarely two) visible short arched sections (). Except for the sexual dimorphism seen on the sterno-genital shields, the morphology of cheliceral digits differs between sexes. In the male the fixed digit is straighter and bifurcate and the mobile digit is curved distally, while in the female both digits are curved distally (). All the adults we studied had a typical epistome (). Thickness of adults: 180 (female), 160 (male).
Figure 6. SEM (Scanning Electron Microscopy) micrographs of a female Zercon hamaricus. (a) dorsal habitus; (b) lateral habitus; (c) anterior part of idiosoma, lateral view; (d) posterior part of idiosoma, lateral view; (e) sternal region; (f) epistome; (g) opisthonotum, dorsal view; (h) peritrematal region, lateral view (*post-coxal cuticular spines). Scale bars (µm): a, b, g = 200; c–e = 100; f = 25; h = 50.
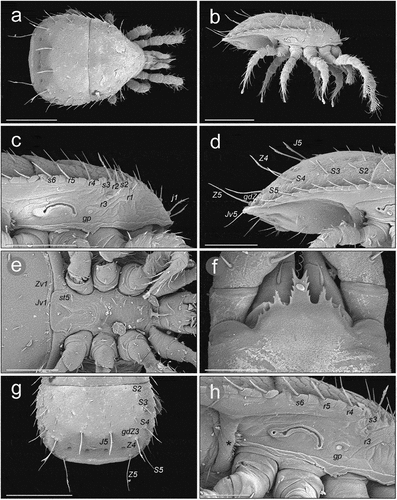
Figure 7. SEM (Scanning Electron Microscopy) micrographs of a male Zercon hamaricus. (a) dorsal habitus; (b) lateral habitus; (c) anterior part of idiosoma, lateral view; (d) posterior part of idiosoma, lateral view; (e) sternal region; (f) epistome; (g) opisthonotum, dorsal view; (h) peritrematal region, lateral view (*post-coxal cuticular spines). Scale bars (µm): a, b, g = 200; c–e = 100; f = 25; h = 50.
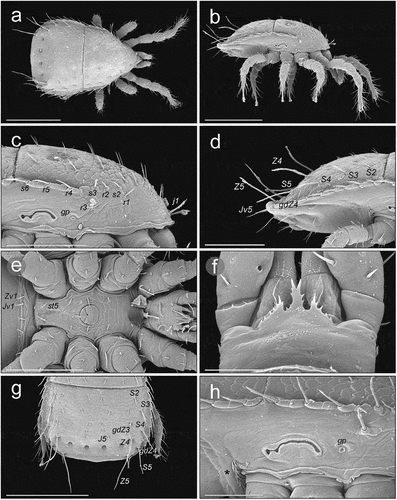
Figure 8. SEM (Scanning Electron Microscopy) micrographs of Zercon hamaricus. (a) female, anterior view; (b) female, posterior view; (c) chelicera of male; (d) chelicera of female; (e) post-coxal region of male, ventral view; (f) post-coxal region of deutonymph, ventral view (*metapodal plate); (g) post-coxal region of a deutonymph, lateral view (*metapodal plate); (h) post-coxal region of protonymph, ventral view (*metapodal plate). Scale bars (µm): a, b = 100; c–f, h = 25; g = 50.
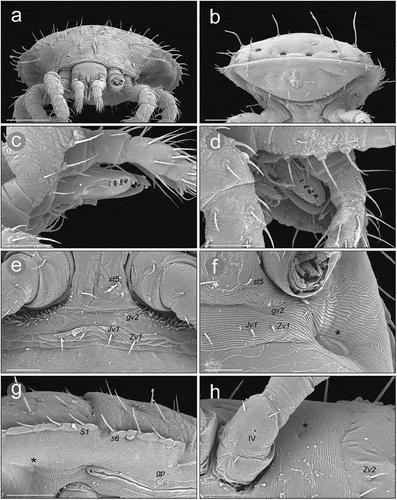
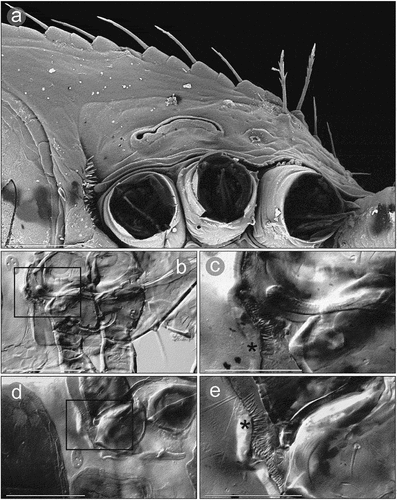
Figure 9. Micrographs of a female Zercon hamaricus. (a) peritrematal shield, SEM (Scanning Electron Microscopy) micrograph, ventral view; (b, d) peritrematal shield and post-coxal region, transmitted light microscopy, ventral view; (c, e) magnifications of the areas marked with rectangles on “b” and “d”, respectively. *post-coxal cuticular spines. Scale bars (µm): a, c, e = 50; b, d = 100.
Discussion
Ontogeny and egg morphology
Here we show the first data on the duration of ontogenetic stages in Zerconidae. The lack of similar data at the family level means we can only compare our results on Z. hamaricus with distant relatives that we have studied so far, i.e. Trichouropoda ovalis (Koch Citation1839), Sejus togatus Koch Citation1836 and Holaspulus tenuipes (Berlese Citation1904; Marquardt & Kaczmarek Citation2017, Citation2019a, Citation2019b). In all those species we found that the larva was the shortest and the deutonymph was the longest lasting stage. In Z. hamaricus the duration of larval and protonymphal stages was comparable and clearly shorter than the last nymphal stage. Our results show that, at least under laboratory conditions, the overall reproductive success (offspring reaching the adult stage) in Z. hamaricus is rather low as only 50% of observed eggs hatched and completed the full ontogeny. In Z. hamaricus we found statistically significant change in size only in nymphal stages and only with regard to length, while in the previously studied taxa we observed significant growth in the length and width of nymphs. The body size of individuals on the last day before a moult (e.g. the larva in the last day before moult to protonymph) and the next instar (e.g. the first day after moult in protonymph) did not differed significantly in Z. hamaricus only during the first moult (larva to protonymph). In T. ovalis and H. tenuipes we found significant changes in body size during all moults (with some exceptions in case of width) while in S. togatus all changes of body size during moult were non-significant.
In our previous works we also studied the behaviour of oviposition and egg protection strategy, egg morphology and egg incubation time (Marquardt et al. Citation2013a, Citation2013b, Citation2015, Citation2016; Marquardt & Kaczmarek Citation2018, Citation2019c). In the case of Z. hamaricus we were unsuccessful in recording oviposition behaviour. All eggs we observed in the previously studied species were ellipsoidal, while those of Z. hamaricus are spherical. In Z. hamaricus there is a clearly visible sculptured exochorion, which resembles relatively dense thin projections perpendicular to the egg surface. In all the species that we observed so far, the exochorion formed after the egg was pushed out of the genital opening of the female, and the female waited for the exochorion to be formed, holding the egg beneath the gnathosoma before depositing the egg. Even if the exochorion was not clearly visible [e.g. in Lasioseius ometes (Oudemans Citation1903)], the prolonged time that the female spent holding the egg before deposition proved the presence of some external protecting structures on the egg surface. In some species (e.g. L. ometes and H. tenuipes) the exochorion formation was accompanied by a specific behaviour, i.e. covering the egg with substrate particles (even if the exochorion was clearly visible as in H. tenuipes). Sometimes only the exochorion formation was observed and the egg was not additionally buried, e.g. in Pergamasus brevicornis Berlese Citation1903. In Z. hamaricus we did not observe covering the egg with substrate particles and eggs were usually placed in confined spaces (e.g. between moss leaves), but some eggs were also laid the on flat (open) areas. We do not have data on oviposition behaviour in Z. hamaricus but we predict that this process is similar to that previously described in P. brevicornis, i.e. with exochorion formation after pushing the egg out of the genital opening, with the phase of female waiting for the exochorion formation and without the egg covering.
The morphology of egg surface in Mesostigmata has been mostly studied in Parasitidae, most of which possess a clearly visible exochorion (Rapp Citation1959; Berry Citation1973; Witaliński Citation1977, Citation1987; Korn Citation1982; Alberti et al. Citation1999). In most cases this structure resembles a network in stereoscopic view, and is actually a system of outgrowths that form a polygonal pattern consisting of lamellae on the egg surface. In Z. hamaricus a similar pattern is visible on the egg surface, but the lamellae are not very tall and most of the exochorion consists of areolae made up of many relatively long processes that grow out from the intersections of the lamellae. A similar and very regular structure of the external egg envelope was observed by Lee (Citation1974) in Ologamasidae (formerly Rhodacaridae). In all studied species [Acugamasus semipunctatus (Womersley Citation1942), Athiasella dentata (Womersley Citation1942), Euepicrius filamentosus Womersley Citation1942, Gamasellus concinus (Womersley Citation1942), Gamasiphis fornicatus Lee Citation1970 and Gamasiphoides propinquus (Womersley Citation1956)] the authors observed the polygonal structure on the egg surface. In the latter two species, there were also clearly visible, quite long, additional processes projecting from the egg surface, and in E. filamentosus they were present but very short (conspicuous pile and rudimentary pile, respectively, according to the original description). Interestingly, Lee (Citation1974) emphasized that the presence of clear processes on the egg surface was correlated with the absence of egg burying behaviour, which supports our previous and present observations. Currently we do not know if the external egg morphology in Zerconidae is species (or genus) specific – this issue needs further study. To the best of our knowledge, there are no previous data on the morphology of the internal surface of egg shells in Mesostigmata.
In our work on reproductive behaviour of Macrocheles glaber (Müller Citation1860) we observed ovoviviparity and the female assisted the hatching of the larva, most often by chewing the egg at the section adjacent to the ventral surface of the offspring (Marquardt et al. Citation2015). We did not observe the hatching behaviour in Z. hamaricus, but this kind of female assistance may also occur in the case of oviparity. In each of the opened eggs that we observed in Z. hamaricus, they were opened along the equatorial plane which suggests the presence of a seam-like structure, but this was not visible on the outer or inner surfaces of the egg shell. We do not know if the egg opening is self-initiated by the egg shell or induced by the larva. If the latter hypothesis is valid, the inner stoma-like structure (if not an artefact) could be regarded as an opening trigger activated by the larva. We of course cannot exclude that it could be also some other kind of structure, e.g. for respiration. Similarly, the lighter areas separated by darker bands on the inner surface of the egg shell correspond, in our opinion, to the polygonal structure of the exochorion. Nevertheless, further study of internal structures of eggs in Zerconidae is needed to clarify their true origin and function.
Ecology
Most descriptions of new Zerconidae species do not include all developmental stages. Some descriptions include only the female, and those that include juveniles often lack some particular ontogenetic stage. Our field-collected material used for the description of Z. hamaricus lacked larvae so we obtained them from the laboratory culture to make the description complete (Kaczmarek et al. Citation2021). In the current material we found 87 larvae and 481 nymphs. The difference could be caused by collecting at a different time of year – early spring 2022, compared with the holotype and paratypes collected at the end of July 2017.
Usually two abundance peaks are found in mesostigmatid mite communities, in the spring and the autumn. For most populations of Zercon triangularis and Z. peltatus studied by Kaczmarek (Citation2000) in young Scots pine forests, species abundance was higher in spring than in autumn. The same may be true for Z. hamaricus in Norway, but the autumn analysis is still required. Kaczmarek (Citation2000) found similar sex ratios in Z. peltatus and Z. triangularis but a different age structure when compared to the present species. In unpolluted areas of young Scots pine forests (Leucobryo-Pinetum, 20 years old), the female:male ratio was 1.9:1–5.7:1 and 1.2:1–3.5:1 for Z. peltatus and Z. triangularis, respectively. In these populations adults were usually more abundant than juveniles (ad:juv ratio 1:1–12.4:1 for Z. peltatus and 0.9:1–1.7:1 in Z. triangularis, average annual values) (Kaczmarek Citation2000). Kaczmarek (Citation1989) found that adults of Z. triangularis were more abundant than juveniles during both spring and autumn at the control plot located 25 km from a Nitrogen Fertilizer Plant at Włocławek (Poland). For Z. hamaricus we found that juveniles were over 2.5 times more abundant than adults, but a comparison with congeners is impossible with the limited available data.
Zercon peltatus and Z. triangularis showed clear and opposite responses as bioindicators. In Z. peltatus it was found that the percentage of males increased with decreasing distance from the pollutant source, while for Z. triangularis, this trend was shown by the females (Kaczmarek Citation2000). The abundance and biomass of both Z. peltatus and Z. triangularis decreased significantly with decreasing distance from the pollutant source. The same trend was visible at the level of species presence or absence – Z. peltatus was not found even in control plots close to areas of nitrogen/sulphuric and calciferous pollutions but was present in other areas, while Z. triangularis was present at most control areas and in the minority of heavily polluted plots studied by Kaczmarek (Citation1989, Citation2000). Interestingly, Z. peltatus occurred at all the plots polluted only with sulphur, suggesting it is sensitive to nitrogen pollution. Kaczmarek (Citation1998) showed that in the soils contaminated with low and medium doses of heavy metals, Z. zelawaiensis Sellnick Citation1944 was replaced by Z. peltatus. Moreover, significant negative correlations were found between the density of Z. peltatus and lead and copper concentrations; and for the density of Z. triangularis and calcium dust concentration as well as the general density of Zercon and sulphuric pollution (Kaczmarek Citation1998). Zercon hamaricus was the only representative of the genus at the locality surveyed in the present study. Considering previous findings on the bioindicative potential of Zercon, the numerous populations of Z. hamaricus could possibly be used for biomonitoring of environmental quality in southern Norway. We also need further data on the distribution of Z. hamaricus at other localities.
Postembryonic stage morphology
In our work regarding S. togatus (Marquardt & Kaczmarek Citation2017) we observed and discussed the three-dimensional (3D) arrangement of selected setae in living larvae and protonymphs using data from light microscopy. Here we present the data on 3D setal arrangement and other morphological characters in all instars of Z. hamaricus based on a SEM study. To obtain high-quality data from SEM imaging, it is crucial to avoid deformation of individuals during drying. Here we proved that air-drying using HMDS preserves the morphology of eggs and postembryonic stages of Z. hamaricus very effectively, and can be used instead of the much more expensive critical point drying as previously shown in the case of e.g. insect eggs, plant rhizomes and associated mychorizal fungi (Ubero-Pascal et al. Citation2005; Thomasson & Thomasson Citation2011; Bhattacharya et al. Citation2020). We did not perform a comparison between individuals that were air-dried, slide-mounted, and SEM-mounted; however, we presume that the latter category is the most precise in terms of body shape, body size, and ultrastructure, whereas the setal length is slightly affected regardless of the preparation method.
It is worth emphasizing here that LM (Light Microscopy) and SEM (Scanning Electron Microscopy) studies supplement each other and do not compete. The classical description of chaetotaxy is possible but would require a different approach with the use of only SEM. Due to preparation techniques, individuals prepared for LM are flattened, which allows the convenient measurement of lengths of setae observed dorso-ventrally. The SEM preparations allow 3D imaging of the setal arrangement but make measurements of setae length much problematic, unless we observe them laterally and they are precisely upright.
Interestingly, if we compare lengths and widths of juveniles from the previous paper (Kaczmarek et al. Citation2021) with the current data we can see some regularity. In the case of length of larvae and protonymphs the range presented here as in vivo measurements is wider than that in the previous paper. Other words, we did not have very short (young) and very long (old) larvae and protonymphs during the original species description. In the case of deutonymphs the ranges of length in our previous and present material overlap, but most of the individuals measured previously were longer than those measured in the present study. In the case of width we can see that all juveniles used for the species description were wider than those from the present study. This could be explained by the difference in the observation method. For the species description we used mites mounted on slides, so these measurements should be more accurate than those made under the stereomicroscope. However, pressing down on the coverslip, which is good for setae measurement using LM, can influence the apparent body size, as found by Gwiazdowicz (Citation2010) in Sejus sejiformis (Balogh Citation1938).
The thickness of individual stages of Z. hamaricus in this study ranges from 120 µm in larvae to 150–180 µm in nymphs and adults. During preparation of mites for taxonomic study using LM the individuals are undoubtedly flattened. Considering the 3D body plan of Z. hamaricus, the preparation for LM study can influence the interpretation of the shape of the peritreme, the position of seta r1, and the shield size as well as the body size.
Shape of the peritreme
The morphology of the peritreme using LM in dorso-ventral aspect strongly resembles its shape in lateral aspect using SEM, so we predict that the ventral part of the idiosoma, in particular most of pleural parts beneath the line r-R setae, becomes horizontal when compressed with a coverslip for LM. Without such deformation the ventral aspect of the peritreme should be different (compare for the female). In some drawings of Zercon (e.g. Zercon (Zercorientalia) sinensis Petrova & Tascaeva Citation1968, Z. parivus Moraza Citation1991, and Z. spinosus Ujvári Citation2011b) the peritreme is presented much straighter than usual for the genus. We assume that the use of flat or concave slides for LM can cause differences in the visible morphology of the peritreme. The amount of influence that preparation has on the shape of the peritreme in the above-mentioned species is unknown; however, in Z. sinensis depicted laterally by Petrova (Citation1977) (the only lateral picture of Zercon to our knowledge) the peritreme is also much straighter than is usual in the genus.
Seta r1
According to Lindquist and Moraza (Citation1998), dorsal seta r1 is positioned laterally or ventrolaterally to z3. In Z. hamaricus it is located ventrolaterally to z3 and we predict that this is true for all Zercon species. Moreover, Lindquist and Moraza (Citation1998) distinguished two possible locations of r1, namely: (1) on the peritrematal shield or (2) in the area of confluence of the peritrematal and podonotal shields. Sikora (Citation2014) stated that peritrematal shields are anteriorly connected with the podonotum. From the SEM study, we assume that although we can distinguish the visible lateral (adaxial and anti-axial) and posterior borders of the peritrematal shield, in the anterior part it is fused with pleural parts of the podonotum with no visible edge. Consequently, the lateral parts of the podonotum merge smoothly into the peritrematal shields, as pictured by Petrova (Citation1977) in Z. sinensis. If the above-mentioned flattening along the r-R line is considered using LM, the r1 can be observed dorsally in specific species if it is located more dorsally and, in other species, can be oppositely regarded as ventral seta if it is positioned more ventrally to z3. In the lateral picture of Z. sinensis by Petrova (Citation1977) the seta r1 (p1 acc. to original designation) is presented in this species relatively high, at the level of the r-R setae, and is designated as a dorsal seta, which supports our hypothesis.
Shield and body size
In all the postembryonic stages of Z. hamaricus the dorsal shields are arc-shaped in a transverse plane, so it is possible that pressing during LM preparation can influence the length and width of the shield, depending on the pressing force and elasticity of the shield and its surroundings. We assume that the relative change will increase towards the younger stages. Therefore, especially for larvae and nymphs, our measurements given in the species description are probably overestimated. For example, in larvae the lateral edges of the podonotal shield are visible along the line z2–z4–s4 in SEM, while in LM the edge is much more to the sides in case of z2–z4 setae. Also, the opisthonotal shield of the larvae is modified in LM preparation, as the shape of anterior and posterior postdorsal cavities is the same and insertions of J5 setae are not on the posterior edge of the body in our drawings, while the SEM data differ in these characters. All our drawings of the nymphs of Z. hamaricus include the soft cuticle visible from the dorsal aspect, which is not true when compared with the SEM data, as in all nymphs the soft cuticle is located more ventrally and more adaxially when compared to lateral edges of dorsal shields. Of course, as long as we use the same preparation technique the measurements should be still generally comparable between species; however, we cannot exclude that in Zercon the amount of soft cuticle visible from the dorsal aspect and also body thickness increases with the age of juvenile stage. The increasing amount of soft cuticle protruding laterally more and more beyond the dorsal shield over time along with increase in body thickness has been observed in several Uropodina deutonymphs e.g. Leiodinychus krameri, Trichouropoda ovalis, Uroobovella marginata and Uropoda orbicularis (Radinovsky Citation1965; Faasch Citation1967; Marquardt & Kaczmarek Citation2019b).
Dimorphic chelicerae, metapodal plates and post-coxal cuticular spines
Here we also confirmed our previous observations on dimorphism visible in the morphology of cheliceral digits between sexes of Zerconidae. These observations are supplemented here in Z. hamaricus with SEM micrographs (for a discussion see Kaczmarek et al. Citation2021).
All ventral drawings of deutonymphs of Zercon contain the pair of metapodal plates near the antero-lateral edges of the ventrianal plate. When compared to the ventrianal plate, these smaller plates are pressed down in ventral aspect and surrounded with raised soft cuticle. We also found similar plates in protonymphs. They are smaller and less compressed than those of deutonymphs. They were visible using SEM but were not found using LM, probably because in all our protonymphs the area of interest was covered by legs IV and the plates were not visible. Also, in drawings of the ventral surface of the protonymph in Halašková (Citation1970), Błaszak (Citation1974) and Lindquist and Moraza (Citation1998), this character was not included. Interestingly in two species of Halozercon – H. barguzin Marchenko et al. Citation2018 and H. tigerek Marchenko Citation2019 – metapodal plates are present in females, and in the former species also visible but fused with ventrianal shield in males. The other seven known species of Halozercon do not have metapodal plates in adults and there are no metapodal plates reported in any known nymphs of this genus (Marchenko et al. Citation2018, Citation2019, Citation2021).
The posterior edges of coxa IV in Z. hamaricus carry cuticular spines that are clearly visible in the SEM micrographs. During the LM study on Z. hamaricus these structures were visible as very short lines parallel to the axis of the body. We have also seen them in Z. utemisovi Kaczmarek et al. Citation2020 in both sexes and they have been similarly shown in males and females of Z. parivus (Moraza Citation1991; Lindquist & Moraza Citation1998; Kaczmarek et al. Citation2020, Citation2021) as well as all species of Zercon (Zercorientalia) described by Ujvári (Citation2011b) excluding Z. sinensis. Petrova and Tascaeva (Citation1968) did not report these short parallel lines along the posterior edge of coxae IV of this species [absent also in Ujvári (Citation2011b) who only used original drawings]; however, on the original lateral drawing in Petrova (Citation1977) some spiked structures were marked posteriorly to the last coxae in Z. sinensis, which suggests the presence of this character in this species.
Because in most cases the detailed drawings of Zerconidae are focused on the dorsal side (mainly opisthonotum), it is difficult to determine whether this character is exclusive to Zercon. Interesting postcoxal structures have been found in other Mesostigmata such as Asternoseiidae (Trigynaspida: Cercomegistina) and Parholaspididae (Monogynaspida: Gamasina), although in both groups these are not so close to coxae IV (i.e. they are moved posteriorly) and in the latter group are rather internal structures with only post-coxal openings. The most similar to those of Z. hamaricus in terms of appearance are cuticular spine organs in e.g. Asternoseius which, along with spiky depressions close to coxae IV in Allothyrus (Parasitiformes: Holothyrida), are called peridia (singular: peridium). Pictures of peridia in Holothyrida can be found in Van der Hammen (Citation1983) and Krantz and Walter (Citation2009), and those of Asternoseius e.g. in the online key for Parasitiformes at lucidcentral.org (Walter Citation2023). Van der Hammen (Citation1983) defined peridia of Holothyrida as glandular structures, and Krantz and Walter (Citation2009) emphasized the defensive function of this structure. In Z. hamaricus cuticular spines are located much closer to coxae IV, and the relation of this structure to peridia and its function remain unknown. Our preliminary hypothesis is that it could be regarded as protecting the depressions at the posterior edges of coxae IV against fine soil particles that could limit or even block the leg movements, as the grooves along the posterior edges of these coxae are much wider than those of other legs. To test our hypothesis, more studies are required on the anatomy of Zerconidae.
Acknowledgements
We thank Dr Bruce Halliday (Australian National Insect Collection, CSIRO, Canberra) for his constructive feedback on an earlier draft of this paper, thorough reading of the manuscript and valuable linguistic suggestions. We are grateful to Dr Mehmet Karaca (Pamukkale Üniversitesi, Denizli, Türkiye) for his help with access to relevant publications and Bogna Kaczmarek-Dhaliwal, MSc for her help with collection of samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Addinsoft (2023). XLSTAT Statistical and Data Analysis Solution. New York, USA. https://www.xlstat.com
- Alberti G, Gegner A, Witaliński W. 1999. Fine structure of the genital system in the females of Pergamasus mites (Acari: Gamasida: Pergamasidae). Journal of Morphology 240(3):195–223. DOI: 10.1002/(SICI)1097-4687(199906)240:3<195:AID-JMOR1>3.0.CO;2-E.
- Balogh J. 1938. Systematische Studien über eine neue Milbengattung: Willmannia gen. nov. Zoologischer Anzeiger 123:259–265.
- Berlese A. 1903. Acari nuovi. Manipulus II. Redia, Firenze 1:258–280.
- Berlese A. 1904. Spicilegia zoologica. Acari nuovi. Manipulus II. Redia 1:258–280.
- Berry RE. 1973. Biology of the predaceous mite, Pergamasus quisquiliarum, on the garden symphylan, Scutigerella immaculata, in the laboratory. Annals of the Entomological Society of America 66(6):1354–1356. DOI: 10.1093/aesa/66.6.1354.
- Bhattacharya R, Saha S, Kostina O, Muravnik L, Mitra A. 2020. Replacing critical point drying with a low-cost chemical drying provides comparable surface image quality of glandular trichomes from leaves of Millingtonia hortensis L. f. in scanning electron micrograph. Applied Microscopy 50(1):15. DOI: 10.1186/s42649–020–00035–6.
- Bilki K, Urhan R, Karaca M. 2022. Mites of the family Zerconidae (Acari: Mesostigmata) from Southwestern Turkey, with description of three new species. Acarological Studies 4(2):89–103. DOI: 10.47121/acarolstud.1129248.
- Błaszak C. 1970. Zercon polonicus sp. n. (Acari, Zerconidae) a new species of mite from Poland. Bulletin de L’Academie Polonaise des Sciences – Série des Sciences Biologiques 18:265–268.
- Błaszak C. 1974. Zerconidae (Acari, Mesostigmata) Polski. Monografie Fauny Polski. Warszawa: PWN. p. 315.
- Błaszak C. 1975. A revision of the family Zerconidae (Acari, Mesostigmata) (systematic studies on family Zerconidae – I). Acarologia 17:553–569.
- Błaszak C, Skorupski M. 1992. Zercon wisniewskii sp. n. A new species of mite from Russia (Acari: Mesostigmata: Zerconidae). Genus 3:201–210.
- Bulut DR, Urhan R, Karaca M. 2021. Zerconid mites (Acari, Zerconidae) from eastern parts of Aydın Province (Turkey), with description of Zercon karacasuensis sp. nov. Acarological Studies 3(2):73–81. DOI: 10.47121/acarolstud.911415.
- Faasch H. 1967. Beitrag zur Biologie der einheimischen Uropodiden Uroobovella marginata (C.L. Koch 1839) und Uropoda orbicularis (O.F. Müller 1776) und experimentelle Analyse ihres Phoresieverhaltens. Zoologische Jahrbücher Abteilung für Systematik, Geographie und Biologie der Tiere 94:521–608.
- Faleńczyk-Koziróg K, Shevchyk VL, Pylypenko V, Kaczmarek S. 2018. A new species of zerconid mite Zercon shevtchenkoi n. sp. (Acari: Mesostigmata: Zerconidae) from Ukraine. Acarologia 58(4):837–844. DOI: 10.24349/acarologia/20184288.
- Gwiazdowicz DJ. 2010. Sejoidea, Antennophoroidea, Celaenopsoidea, Microgynioidea (Acari: Mesostigmata) of Poland. Poznań (Poland): Wydawnictwo Naukowe Bogucki. p. 142.
- Halašková V. 1970. Zerconidae of Czechoslovakia (Acari: Mesostigmata). Acta Universitatis Carolinae – Biologica 1969:175–352.
- Halašková V. 1977. A revision of the genera of the family Zerconidae (Acari: Gamasides) and descriptions of new taxa from several areas of Nearctic Region. Czechoslovakia: Academia, Studie ČSAV, Prague. p. 74.
- Kaczmarek S. 1989. Soil acarofauna of Scots pine forest in the area of influence of the nitrogen plant in Włocławek, with particular emphasis on Gamasida. Department of Agricultural and Forest Biology of the Polish Academy of Sciences in Poznań, PhD dissertation.
- Kaczmarek S. 1998. Effect of industrial pollution emitted to the atmosphere on soil mites of Zercon genus (Acari, Gamasida) in the young Scots pine forests. Zeszyty Naukowe Akademii Techniczno-Rolniczej w Bydgoszczy Ochrona Środowiska 214:215–220.
- Kaczmarek S. 2000. The soil gamasid mites (Acari) of young Scots pine forests in some regions polluted by factories. Poland: Wydawnictwo Wyższej Szkoły Pedagogicznej, Bydgoszcz. p. 121.
- Kaczmarek S, Marquardt T, Jangazieva B. 2020. Zercon utemisovi sp. n. – a new species of Zerconidae (Parasitiformes: Mesostigmata) from Kazakhstan with notes on Zercon karadaghiensis Balan, 1992. International Journal of Acarology 46(1):52–59. DOI: 10.1080/01647954.2019.1704867.
- Kaczmarek S, Marquardt T, Seniczak A. 2021. A new species of Zercon (parasitiformes: Mesostigmata) from Norway, with notes on sexual dimorphism in Zerconidae. Systematic & Applied Acarology 26:1676–1702. DOI: 10.11158/saa.26.9.5.
- Koch CL. 1836. Deutschlands Crustaceen, Arachniden und Myriopoden, ser. Deutschlands Insecten (Panzer). In: Herrich-Schäffer, editor. Regensburg: Pustet, Vol. 4, p. 17.
- Koch CL. 1839. Deutschlands Crustaceen, Myriapoden und Arachniden. Ein Beitrag zur Deutschen Fauna. Regensburg: Herrich-Schäffer, Vol. 27, p. 22.
- Korn W. 1982. Zur Fortpflanzung von Poecilochirus carabi G. u. R. Canestrini 1882 (syn. P. necrophori Vitzt.) und P. austroasiaticus Vitzthum 1930 (Gamasina, Eugamasidae). Spixiana 5:261–288.
- Krantz GW, Walter DE. 2009. A manual of acarology. 3rd ed. Lubbock (TX): Texas Tech University Press. p. 807.
- Lee DC. 1970. The Rhodacaridae (Acari: Mesostigmata); classification, external morphology and distribution of genera. Records of the South Australian Museum (Adelaide) 16:1–219.
- Lee DC. 1974. Rhodacaridae (Acari: Mesostigmata) from near Adelaide, Australia. III. Behaviour and development. Acarologia 16:21–44.
- Lindquist EE, Moraza ML. 1998. Observations on homologies of idiosomal setae in Zerconidae (Acari: Mesostigmata), with modified notation for some posterior body setae. Acarologia 39:203–226.
- Marchenko II. 2019. Three new species of Halozercon (Acari: Mesostigmata: Zerconidae) from Altai Mountains in South Siberia (Russia). Zootaxa 4568(3):401–434. DOI: 10.11646/zootaxa.4568.3.1.
- Marchenko II. 2021. Four new species of Halozercon (Acari: Mesostigmata: Zerconidae) from South Siberia Mountains (Russia) with a key to all known species. Zootaxa 4941(2):151–185. DOI: 10.11646/zootaxa.4941.2.1.
- Marchenko II. 2018. A new species of Halozercon (Acari: Zerconidae) from South Siberia (Russia) with additional information on Halozercon karacholana Wiśniewski et al. 1992. Zootaxa 4394(3):347–370. DOI: 10.11646/zootaxa.4394.3.2.
- Marquardt T, Faleńczyk-Koziróg K, Kaczmarek S. 2013a. Oviposition behaviour of the soil mite Pergamasus brevicornis (Acari: Parasitidae). Experimental and Applied Acarology 60(3):403–409. DOI: 10.1007/s10493-013-9677-7.
- Marquardt T, Faleńczyk-Koziróg K, Kaczmarek S. 2013b. Oviposition behaviour of the soil mite Veigaia cerva (Acari: Veigaiidae). Experimental and Applied Acarology 60(3):395–402. DOI: 10.1007/s10493-013-9676-8.
- Marquardt T, Kaczmarek S. 2017. Postembryonic development of Sejus togatus CL Koch, 1836 (Parasitiformes: Mesostigmata: Sejida) with notes on moulting behaviour. International Journal of Acarology 43(7):557–562. DOI: 10.1080/01647954.2017.1370012.
- Marquardt T, Kaczmarek S. 2018. Pre-ovipositional and ovipositional behaviour of Holaspulus tenuipes (Berlese) (Parasitiformes: Mesostigmata: Parholaspididae) with notes on egg chorion, incubation, and hatching. International Journal of Acarology 44(2–3):111–114. DOI: 10.1080/01647954.2018.1450443.
- Marquardt T, Kaczmarek S. 2019a. Postembryonic development of Holaspulus tenuipes (Berlese, 1904) (Parasitiformes: Mesostigmata: Parholaspididae). International Journal of Acarology 45(6–7):356–360. DOI: 10.1080/01647954.2019.1651765.
- Marquardt T, Kaczmarek S. 2019b. Postembryonic development of Trichouropoda ovalis (C. L. Koch, 1839) (Parasitiformes: Mesostigmata: Trematuridae) with notes on factors influencing the ontogeny in Uropodina. International Journal of Acarology 45(1–2):48–55. DOI: 10.1080/01647954.2018.1540657
- Marquardt T, Kaczmarek S. 2019c. Pre-ovipositional and ovipositional behaviour of Trichouropoda ovalis (C.L. Koch) and Uroobovella marginata (C.L. Koch) (Parasitiformes: Uropodina: Trematuridae, Urodinychidae) with notes on egg incubation and hatching behaviour. Journal of Natural History 53(15–16):991–1000. DOI: 10.1080/00222933.2019.1608328.
- Marquardt T, Kaczmarek S, Halliday B. 2015. Ovoviviparity in Macrocheles glaber (Müller) (Acari: Macrochelidae), with notes on parental care and egg cannibalism. International Journal of Acarology 41(1):71–76. DOI: 10.1080/01647954.2014.990511.
- Marquardt T, Kaczmarek S, Krantz GW. 2016. Pre-ovipositional and ovipositional behaviour of Lasioseius ometes (Oudemans) and Hypoaspis kargi Costa (Acari: Dermanyssiae: Ascidae, Laelapidae) with notes on egg protection strategies in Mesostigmata. Journal of Natural History 50(23–24):1473–1482. DOI: 10.1080/00222933.2015.1117672.
- Mašán P, Fenďa P. 2004. Zerconid mites of Slovakia (Acari, Mesostigmata, Zerconidae). Bratislava: Institute of Zoology, Slovak Academy of Sciences. p. 238.
- Mohammad-Doustaresharaf M, Karaca M, Bagheri M, Urhan R. 2023. A taxonomic study on the zerconid mites (Acari: Zerconidae) in northwestern Iran: Descriptions of three new species with three new records. Systematic & Applied Acarology 28:429–460. DOI: 10.11158/saa.28.3.3.
- Moraza ML. 1991. Zercon parivus sp. n. una nueva especie de Acari (Mesostigmata: Zerconidae). Boletín de la Asociación española de Entomología 15:79–90.
- Müller J. 1860. Insekten Epizoen der mährischen Fauna. Jahrbuch der Königlich-Kaiserlichen Mährisch-Schlesischen Gesellschaft für Ackerbrau, Naturkunde und Landeskunde. Brünn: R. Rohrer’s Erben. pp. 157–184.
- Oudemans AC. 1903. Notes on Acari. 8. Entomol Bericht 1–24:100–103.
- Petrova AD. 1977. Family Zerconidae Canestrini, 1891. In: Gilyarov MS, Bregetova NG, editors. A Key to the Soil Inhabiting Mites, Mesostigmata. Leningrad: Nauka. pp. 577–621.
- Petrova AD, Tascaeva AZ. 1968. Gamasoid mites (Parasitiformes, Gamasoidea) from Southern China. Zoologicheskii Zhurnal 46:1179–1191.
- Radinovsky S. 1965. The biology and ecology of granary mites of the Pacific Northwest. III. Life history and development of Leiodinychus krameri (Acarina: Uropodidae). Annals of the Entomological Society of America 58(3):259–267. DOI: 10.1093/aesa/58.3.259.
- Rapp A. 1959. Zur Biologie und Ethologie der Käfermilbe Parasitus coleoptratorum L. 1758. Ein Beitrag zum Phoresie-Problem. Zoologische Jahrbücher Abteilung für Systematik, Geographie und Biologie der Tiere 86:303–366.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nature Methods 9(7):671–675. DOI: 10.1038/nmeth.2089.
- Sellnick M. 1944. Zercon C. L. Koch. Acari – Blätter für Milbenkunde 5:30–41.
- Sellnick M. 1958. Die Familie Zerconidae Berlese. Acta Zoologica Hungaricae 3:313–368.
- Sikora B. 2014. Mites of the Family Zerconidae (Acari: Mesostigmata) of the Nearctic Region. Annales Zoologici 64(2):131–250. DOI: 10.3161/000345414x682463.
- Thomasson SA, Thomasson JR. 2011. A comparison of CPD (Critical Point Drying) and HMDS (Hexamethyldisilazane) in the preparation of Corallorhiza spp. rhizomes and associated mycorrhizae for SEM (scanning electron microscopy). Transactions of the Kansas Academy of Science 114(1 & 2):129–134. DOI: 10.1660/062.114.0113.
- Ubero-Pascal N, Fortuño JM, De Los Ángeles Puig M. 2005. New application of air-drying techniques for studying Ephemeroptera and Plecoptera eggs by scanning electron microscopy. Microscopy Research and Technique 68(5):264–271. DOI: 10.1002/jemt.20248.
- Ujvári Z. 2011a. First records of Zerconidae (Acari: Mesostigmata) south of the Tropic of Cancer, Mexico, with description of five new species. International Journal of Acarology 37(3):201–215. DOI: 10.1080/01647954.2010.502907.
- Ujvári Z. 2011b. A new subgenus and two new species of Zercon C. L. Koch, 1836 (Acari: Zerconidae) from Southeast Asia. Zootaxa 2995(1):45–54. DOI: 10.11646/zootaxa.2995.1.3.
- Urhan R, Karaca M. 2023. Contributions to the Zerconidae (Acari: Mesostigmata) fauna of Dilek Peninsula-Büyük Menderes Delta National Park, Türkiye. Acarological Studies 5(1):21–33. DOI: 10.47121/acarolstud.1226687.
- Van der Hammen L. 1983. New notes on Holothyrida (anactinotrichid mites). Zoologische Verhandelingen 207:1–48.
- Walter DE. 2023. Families of Parasitiformes in Soil. Interactive On-Line Key. Available: https://keys.lucidcentral.org/keys/v3/parasitiformes_in_soil/. Accessed May 30 2023.
- Witaliński W. 1977. Scanning microscopy investigations of egg surface of some mesostigmatic Acari. Pedobiologia 17(2):97–101. DOI: 10.1016/s0031-4056(23)00153-1.
- Witaliński W. 1987. Egg-Shells in mites: Cytological aspects of vitelline envelope and chorion formation in Pergamasus barbarus berlese (Gamasida, Pergamasidae). International Journal of Acarology 13(3):189–196. DOI: 10.1080/01647958708683766.
- Womersley H. 1942. Additions to the Acarina-Parasitoidea of Australia. Transactions of the Royal Society of South Australia 66:142–171.
- Womersley H. 1956. On some new Acarina-Mesostigmata from Australia, New Zealand and New Guinea. Journal of the Linnean Society of London, Zoology 42(288):505–599. DOI: 10.1111/j.1096-3642.1956.tb02218.x.