Abstract
This paper provides comprehensive data on the life history of Oxybelus trispinosus (Fabricius, 1787). Female individuals of this species supply approximately 3–4 flies per cell as a food source for their larvae. The collected prey includes specimens of four Diptera families: Calliphoridae, Muscidae, Sarcophagidae, and Tachinidae. Nests are constructed in various soil types, featuring a primary burrow measuring 6–7 cm in length that terminates in a single cell. Both male and female specimens were observed on plants of the Apiaceae family. Notably, these nests faced attacks from kleptoparasitic flies, specifically Metopia argyrocephala (Meigen, 1824) and Senotaina conica (Fallén, 1810) (Diptera: Sarcophagidae). The mature larva of Oxybelus trispinosus exhibits similarities to the larva of Oxybelus variegatus Wesmael, 1852, with a distinguishing characteristic of two straight horizontal lines of four bristles in the frontal region between the antennae. The parietal region is characterised by multiple setae: five setae from the pleurostomal ridge to the anterior tentorial fossa, three more on the sides, and six above the antennae.
Introduction
The genus Oxybelus Latreille, 1797 belongs to the monophyletic family Crabronidae and comprises 267 species globally (Pulawski Citation2023). These wasps are distributed across all regions except Antarctica and Australasia, with their radiation occurring in the late Palaeogene (Sann et al. Citation2018).
Europe hosts 25 described Oxybelus species (Barbier Citation2023). Females of Oxybelus exhibit a feeding behaviour wherein they supply their larvae with paralysed adult flies, particularly from the Anthomyiidae, Calliphoridae, Muscidae, and Sarcophagidae families (Iwata Citation1976).
The extent of prey specialisation varies among species (Bohart & Menke Citation1976). Following prey paralysis, females transport them into the nest impaled on her sting (abdominal transport A1) or using her legs (pedal transport P1 or P2 – only the middle or hind legs are used) (Evans Citation1962). The pedal transport mechanism is regarded as evolutionarily older (Evans Citation1962), with some species having been observed transitioning from P1 to A1 based on nesting conditions or prey size (Peckham & Hook Citation1980; Hook & Matthews Citation1980; Tanaka Citation1985; Tormos et al. Citation2000; Andrietti et al. Citation2013). Certain Oxybelus prey species are economically significant crop pests (Grandi Citation1929) or vectors of important diseases (Bohart & Schlinger Citation1957).
Most Oxybelus species prefer sandy, sun-exposed areas (Lomholdt Citation1984). Oxybelus trispinosus, a widespread species in the Western Palearctic (Pulawski Citation2023), predominantly preys on flies from Anthomyiidae, Muscidae, Calliphoridae, Sarcophagidae, and Tachinidae families (Minkiewicz Citation1931; Bonelli Citation1952; Lomholdt Citation1984; Andrietti et al. Citation2013). Nest structures typically consist of main burrows that may branch, each culminating in a single cell, with an average canal length of approximately 5 cm (Lomholdt Citation1984). In Southern Europe, O. trispinosus exhibits two generations (Bonelli Citation1952).
This study aims to provide a comprehensive description of O. trispinosus nesting biology, including (1) female breeding behaviour, (2) larval description, (3) nest structure, (4) prey range, (5) phenology, and (6) accompanying kleptoparasites.
Material and methods
The research was carried out in Kowalewo Pomorskie (53°10’05.7“N; 18°52’15.5”E) and Sierakowo (53°10’19.0“N; 18°52’21.8”E) in northern Poland throughout the period from early June to late August 2020–2022, specifically on sunny and warm days with temperatures exceeding 18°C.
The site in Kowalewo Pomorskie was located on agricultural, heavily compacted soil, currently uncultivated, overgrown with herbaceous vegetation (Olszewski et al. Citation2021) and on rubble in a soft material of white dolomite grit.
The second site Sierakowo was located on sandy loam, overgrown with grassland vegetation, with some segetal and ruderal species. Helichrysum arenarium (L.), Peucedanum oreoselinum (L.), Sedum sp. and Senecio vernalis Waldst. & Kit. dominated among herbaceous plants (Olszewski et al. Citation2022). The nests were located on bare patches of soil. Field observations were made through direct observation of both sexes, supplemented by photographs and videos captured using a EOS M50 camera. Additionally, a Raynox M-250 macroscopic lens was used for photography. The analysis of nest structures involved careful excavation. Prey preferences were based on the materials collected during nest excavation. Part of the larval specimens for description were transferred to Pampel solution (30 volumes of distilled water, 15 volumes of 96% ethanol, 6 volumes of formaldehyde, and 4 volumes of glacial acetic acid) as described by Švácha and Danilevsky (Citation1987). The remaining larvae were allowed to develop into the adult stage. The intact larvae were photographed, and their sclerotised parts were examined. To achieve this, larvae were immersed in a 10% solution of hot (60°C) KOH for 12 hours to dissolve non-integument parts. Subsequently, the integument was stained with a 5% solution of Chlorazol Black E (Sigma Aldrich) for 2 seconds before being transferred to 96% ethanol. Species-specific characteristics were observed by placing the integument in glycerol and examining the head, mouthparts, spiracles, and other parts under a light microscope. The same specimens were used to study small structures such as setae, sensillae, or mouthparts. Figures of (1) the head, emphasizing the clypeus, labrum, maxillae, and labium; (2) the mandibles in anterior view; and (3) the spiracles of the larvae were drawn. Kleptoparasitic fly larvae were reared in Eppendorf tubes until they reached the adult stage. However, attempts to rear digger wasps under laboratory conditions from egg stage to pupa stage were unsuccessful. Specimens of kleptoparasitic flies and prey are deposited in the Department of Ecology and Biogeography of Nicolaus Copernicus University, Poland, and larvae are deposited in the University of Hradec Králové, Czech Republic.
Results
Environmental preferences and nesting behaviour of Oxybelus trispinosus
Behavioural data. Single nests were found at sandy sites (N = 6) and in wasteland (N = 4) in Sierakowo and on white stone rubble (N = 1) in Kowalewo Pomorskie, of which six complete nests were active (). On one occasion, a female was observed digging a second nest at a distance of 6 cm on the following day. Adults, both females and males, were mainly observed between 10 a.m. and 4 p.m. Males mainly flew low () to the ground near the nests, exhibiting territorial behaviour characterised by frequent perching in specific locations. When another male was too close, a quick contact occurred, after which both males flew away. No copulation was observed. Before digging the nest, the female first used her mandibles and then her front legs, or flew away in search of a better place. During digging the nest, the female created a small mound near the entrance to the main burrow ().
Figure 1. Adult specimen of Oxybelus trispinosus. (a) Male; (b–d) Female; (e) Female with prey at nest entrance; (f) Female with prey in flight.

Figure 2. Nest of Oxybelus trispinosus. (a–c) Top view of nest entrances; (d) Lateral view of the nest and brood cell.
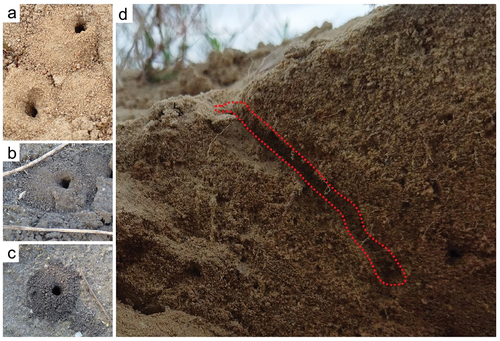
Table I. Prey of Oxybelus trispinosus.
Oxybelus trispinosus constructs single-cell nests (n = 5) in approximately 50–60 minutes, with the cell positioned at the end of the burrow. The length of the main burrow was approximately 7 cm, the diameter of the tubule was approximately 4 mm, and the main burrow was slightly oblique and terminated in a single cell (N = 2; ).
Females (; ) typically constructed their nests on a flat surface (N = 5) but occasionally on a steep surface (N = 1) composed of white stones. An average of 3 to 4 flies were stored in each cell. The minimum distance between the nest entrances was about 6 cm. The entrance to the burrow remained open throughout the provisioning period. Returning females with their prey near the nest supported it using their hind pair of legs (). The female with her prey always landed in front of the nest entrance or immediately entered the nest. One case was recorded in which the prey got stuck in the nest opening, which was used by the female S. conica to deposit its larvae (). A single observation was of a female returning with her prey, followed at a short distance of about 20–30 cm by two Senotaina conica (Fallén, 1810) (Diptera: Sarcophagidae) (). The frequency with which the female carried her prey to the nest ranged from 10 to 18 minutes, typically about 12 minutes. The preimaginal stage of O. trispinosus is shown in . An elongated egg of O. trispinosus was found attached to the ventral side of the prey’s neck (). Kleptoparasitic flies observed near the nest (N = 5; ) and those entering the nests in the absence of the female (two observations in Kowalewo Pomorskie were identified as the Metopia argyrocephala (Meigen, 1824) (Diptera: Sarcophagidae) species. Of all the nests analysed, two larvae of M. argyrocephala were reared as adult insects and subsequently identified as this species. No more than one kleptoparasitic fly larva was observed in each nest cell.
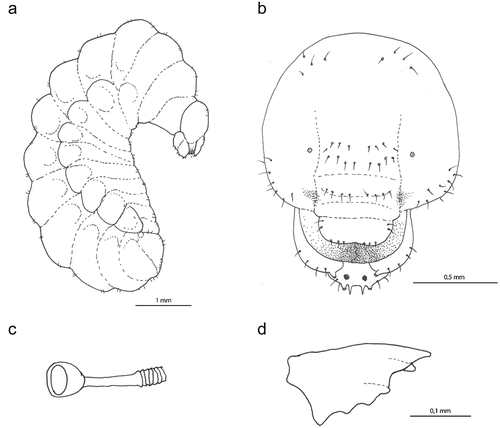
Description of the mature larva
Material: one specimen examined (Sierakowo, 2.08.2022)
Diagnosis
The mature larva of O. trispinosus () is quite large and differs from other species by the blunt apical teeth of the lower jaw (as in O. variegatus and O. argentatus Curtis, 1833). The lower jaw has three teeth, the upper teeth are the longest and blunt, the inner teeth are blunt, and the outer teeth are small. Antenna base ring diameter is less than 1/4 of the distance from the nearest point of the ring to the centre of the anterior tentorium fossa; antennal papilla without pigment. The distinguishing feature of the mature larva is the two linear rows of four setae in the frontal region between the antennae. The parietal region has multiple setae – five setae from the pleurostomal ridge to the anterior tentorial fossa, three more on the sides, and six more on the sides above the antennae.
Body length: 9.1 mm (N = 1). Body vestiture with numerous spicules and only several slender pale setae, tapering to fine points, arising from small but distinct alveoli; these setae are not elongated. Several setae on the mouthparts, only the mouthparts brownish. Setae sparse, not abundant on elevated dorsal surfaces of the thorax and on the anterior ventral surface of the thorax; metasomal tergites T1–T7 have fewer setae than T8–T10. Body form of the postdefecating larva wide and slightly dorsoventrally flattened and robust; body segments similarly wide along their entire length (). Paired body tubercles present and well developed in all mesosomal and metasomal segments except the metathorax and T10, similarly large in all segments and only on T9 very small. Dorsal tubercles wide, flat, and well developed in all three thoracic segments and abdominal tergites, while less conspicuous in T9. Body shape of the predefecating larva in lateral outline with the first abdominal segments having the largest diameter and outline slightly tapering anteriorly and posteriorly. Abdominal segment 9 more hirsute than the previous one, segment 10 attached to the middle of segment 9 in lateral view; anus positioned medially and laterally. Spiracles () unpigmented, subequal in diameter; atrium globular, slightly wider than deep, projecting slightly above body wall, with rim; ratio of atrial opening diameter to peritreme width 0.75; atrial inner surface with rows of concentric wrinkles with primary tracheal opening; primary tracheal opening without collar; subatrium short, with about 10 chambers of approximately equal size, except one or two next to atrium of slightly larger diameter. Sex characters unknown.
Head: moderately small in relation to body size; oriented in normal hypognathous position relative to thorax. Setae long but sparse on the upper part of head capsule; those of maxillary and labial apices large, straight, and conspicuous. Head capsule unpigmented except for points of articulations with mandibles; mandibles moderately pigmented, except for mandibular apices and areas of articulation with head capsule strongly pigmented; maxillary sclerites faintly pigmented; salivary lips not projecting, unpigmented; antennal papilla, maxillary, and labial palpi, all uniformly moderately pigmented (). Spiculation apparently absent even on hypopharynx, not on maxilla. Coronal and post-occipital ridges absent. Tentorium mostly absent due to impending ecdysis. Parietal bands absent. In lateral view, clypeus slightly projecting beyond frons, antenna arising from ill-developed prominence, and labrum protruding well beyond clypeus. Diameter of basal ring of antenna less than 1/4 of distance from the closest point on ring to the centre of anterior tentorial pit; antennal papilla unpigmented, moderately large and not elongated, bearing perhaps two sensilla apically. Frontal area between antennae with two linear rows of four setae. Parietal region with multiple setae – five setae from pleurostomal ridge to anterior tentorial pit and three more setae on the sides, six more setae on both sides above the antennae. Clypeus wide with poorly -developed basal and apical margins, eight sensillae basally on both sides. Labrum very shallowly emarginated apically in the middle, with a group of five conspicuous setae and several sensillae on each side apically; labral sclerite not defined and only very faintly pigmented. Epipharynx simple with no visible structures. Mandible moderately robust; darkly pigmented, tridentate with apical tooth longest and blunt, medial tooth with blunt apex, lateral tooth small; cuspal area developed, projecting, with surface irregularly uneven; outer mandibular surface without setae (). Maxillary apex strongly bent mesad in frontal view, so that the maxillary palpus is in subapical position; cardo distinct, posterior end directed towards posterior tentorial pit; stipes weakly sclerotised; maxillary palpi elongated, probably more than twice the basal diameter, both pigmented like antennal papilla, but slightly thinner than papilla. Stipes with six conspicuous setae. Labium not divided into prementum and postmentum; apex moderately narrow in frontal view. Two setae on both sides and two smaller on ventral surface. Salivary lips round and well visible, with inner surface bearing parallel longitudinal grooves; width of lips slightly more than double width of maxillary palpus. Labial palpus elongated with three sensillae in the middle.
Discussion
Our data on nest structure, prey, and the behaviour of O. trispinosus females are mostly consistent with the observations of Andrietti et al. (Citation2013) and Nicoli Aldini (Citation2004). Food provisioning started only after nest digging was completed and ended with the final sealing of the nest. The females provisioned the nests with adult specimens of flies from the following families: Calliphoridae, Muscidae, Sarcophagidae, and Tachinidae. The following researchers have already confirmed the presence of the prey families mentioned above: Calliphoridae by Nicoli Aldini (Citation2004), Bonelli (Citation1952) and Minkiewicz (Citation1931), Sarcophagidae by Nicoli Aldini (Citation2004), Muscidae by Bonelli (Citation1952), Tachinidae by Bonelli (Citation1952), and Anthomyiidae by Minkiewicz (Citation1931). The diverse food base reflects well the broad environmental range of inhabited areas manifested by O. trispinosus (Andrietti et al. Citation2013). The food base of females can be affected by their diurnal activity, as has been confirmed for several Nearctic species (Peckham et al. Citation1973; Peckham & Hook Citation1980; Peckham Citation1985).
The structure of the main burrow differs from the description presented by Minkiewicz (Citation1931), being slightly oblique. Oxybelus trispinosus digs single-cell nests, as described by Bonelli (Citation1952). Several years later, the same author reported multicellular nests (Bonelli Citation1969). Blösch (Citation2000) also mentioned two-cell nests; however, in this study did not confirm such trends. It is important to note that we only have five nests, making it insufficient to draw conclusive results.
Senotainia conica was observed on several occasions flying at a short distance (about 10 cm) behind a female of O. trispinosus carrying prey. Such satellite (SAT) flight behaviour is associated with an improved visual system compared to other host species using non-satellite (NON-SAT) strategies (Polidori et al. Citation2022). The information on prey stuck in the burrow reported in our study corroborates the findings by Andrietti et al. (Citation2013) and Nicoli Aldini (Citation2004). This situation may favour the laying of larvae by S. conica ().
Grandi (Citation1961) reports that the number of prey affects future sex and ranges from 3 to 5 flies. Our study confirms this range of prey. A better indicator than the number of prey may be the mass of all prey deposited in the cell (due to diverse food preferences), as suggested by Andrietti et al. (Citation2013). The mode of prey transport observed in this study for O. trispinosus agrees with that reported by Minkiewicz (Citation1931) and Bonelli (Citation1952). No change in transport from P1 to A1 was observed during the tests, as reported by Andrietti et al. (Citation2013).
The breeding behaviour of O. trispinosus shows common characteristics with one of the Nearctic species groups (Peckham et al. Citation1973) in the following aspects: (1) the upper part of the tubule is vertical; (2) the tubule is open when the cell is provisioned (except in Oxybelus sparideus Cockerell, 1895); 3) individuals of these species enter the nest with prey held using their legs (except in O. subulatus Robertson, 1889 – prey impaled on the sting).
Based on the available data, a relationship can be observed between the prey transport strategy and the instances when the female covers the nest entrance, protecting it from kleptoparasites or parasitoids (Andrietti et al. Citation2013; P. Olszewski, unpublished data).
Mature larvae have been described for five Palaearctic species and two Holarctic species (Olszewski et al. Citation2021). The research results so far suggest that all known larvae exhibit morphological uniformity (Grandi Citation1929, Citation1954; Maréchal Citation1930; Evans Citation1957; Asis et al. Citation1997). This can be clearly observed in the epipharynx, which is smooth and devoid of spinules, being either bare or papillose (Lomholdt Citation1984; Asis et al. Citation1997). The spinules are mostly distributed on the sides of the base, and the lacinial area is both papillose and spinulose. This character is present in all known larvae of Oxybelus (Asis et al. Citation1997). The mature larva is similar to that of Oxybelus variegatus, which is distinguished by two vertical rows of four hairs in front of the centre of the antennae. The parietal area has many bristles: five bristles from the pleurostomal ridge to the front of the tentorial fossa, three more on the side, and six more on the side above the antennae.
The most distinguishing characteristics of O. trispinosus in terms of breeding behaviour in the conducted research relate to: (1) the presence of a single-cell nest; (2) complete lack of sealing of the nest entrance when bringing prey into the cell; (3) wide access to food resources; (4) plasticity in relation to the habitat; (5) holding the prey during transport with the legs.
Acknowledgments
We thank Karolina and Łukasz Musiał for the opportunity to conduct our research in Kowalewo Pomorskie and Kamila Ludwik for the opportunity to conduct our research in Sierakowo. We are also grateful to two anonymous reviewers for their helpful comments. PO was supported by project 2023/07/X/NZ8/00143 of the Polish National Science Centre and PB was supported by the Excellence Grant of University of Hradec Kralove Nr. 2203/2024.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Andrietti F, Polidori C, Casiraghi M, Bellati A, Passerini E, Martinoli A. 2013. Small–scale sympatric digger wasps Oxybelus argentatus and Oxybelus trispinosus segregate activity, hunt for different prey, and diverge in nesting behaviour. Annales de la Société entomologique de France (N.S.): International Journal of Entomology 49(2):205–221. DOI: 10.1080/00379271.2013.815041.
- Asis JD, Tormos J, Gayubo SF. 1997. Descripción de la larva madura de Oxybelus lamellatus y O. spectabilis Gerstaecker (Hymenoptera: Sphecidae). Miscellánia Zoològica 20:59–64.
- Barbier Y. 2023. Fauna Europaea: Crabronidae. In: Mitriou M, editor. 2013. Fauna Europaea: Hymenoptera. Fauna Europaea. Version 2017.06. Available: https://fauna-eu.org. Accessed 20 November 2023.
- Blösch M. 2000. Die Grabwespen Deutschlands. Keltern: Goecke & Evers. p. 480.
- Bohart RM, Menke AS. 1976. Sphecid wasps of the World. A generic revision. Berkeley, Los Angeles, London: University of California Press. p. 696. DOI: 10.1525/9780520309548.
- Bohart RM, Schlinger EI. 1957. California wasps of the genus Oxybelus (Hymenoptera, Sphecidae, Crabroninae). Bulletin of the California Insect Survey 4:103–134.
- Bonelli B. 1952. Osservazioni biologiche sul “Mellinus arvensis L.” e sul “Oxybelus trispinosus F.” (Hymenoptera, Sphecidae). Bollettino dell’Istituto di Entomologia dell’Università di Bologna 19:137–143.
- Bonelli B. 1969. Observazioni biologiche sugli imenotteri melliferi e predatori della Val di Fiemme XXXI. Bollettino dell’Istituto di Entomologia dell’Università di Bologna 29:165–172.
- Evans HE. 1957. Studies on the larvae of digger wasps (Hymenoptera, Sphecidae). Part III: Philanthinae, Trypoxyloninae, and Crabroninae. Transactions of the American Entomological Society 83:79–117.
- Evans HE. 1962. The evolution of prey-carrying mechanisms in wasps. Evolution 16(4):468–483. DOI: 10.1111/j.1558-5646.1962.tb03237.x.
- Grandi G. 1929. Contributi alla conoscenza biologica e morfologica degli Imenotteri melliferi e predatori. IX. Bollettino del Laboratorio di Entomologia del R. Istituto Superiore Agrario di Bologna 2:255–291, pls. VII–X.
- Grandi G. 1954. Contributi alla conoscenza degli Imenotteri Aculeati. XXVI. Bollettino dell’Istituto di Entomologia della Università di Bologna 20:81–255.
- Grandi G. 1961. Studi di un Entomologo sugli Imenotteri superiori. Bollettino dell’Istituto di Entomologia della Università di Bologna 25:i-xv, 1–659.
- Hook AW, Matthews W. 1980. Nesting biology of Oxybelus sericeus with a discussion of nest guarding by male sphecid wasps (Hymenoptera). Psyche: A Journal of Entomology 87(1–2):21–37. DOI: 10.1155/1980/14621.
- Iwata K. 1976. Evolution of instinct. Comparative ethology of Hymenoptera. New Delhi: Amerind Publishing.
- Lomholdt O. 1984. The Sphecidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica. 4. 2nd ed. Leiden, Copenhagen: E.J. Brill/Scandinavian Science Press. p. 452.
- Maréchal P. 1930. Sur trois hyménopteres se développant dans un cocon en mosaique (Miscophus spurius Dahlb. Oxybelus bipunctatus Ol. Mutilla rufipes F.). Mémoires de la Société Royale Entomologique de Belgique 23:1–23, pls. I–IV.
- Minkiewicz R. 1931. Nids et proies des sphégiens de Pologne. Deuxième série de fragments éthologiques. Polskie Pismo Entomologiczne 10:196–218.
- Nicoli Aldini R. 2004. Behavioural observations on three species of Oxybelus (Hymenoptera Sphecidae) nesting in syntopy. Redia LXXXVII:253–256.
- Olszewski P, Bogusch P, Klejdysz T, Szpila K. 2022. Nesting behaviour and description of the larva of Alysson spinosus (Panzer, 1801) (Hymenoptera: Bembicidae). The European Zoological Journal 89(1):957–965. DOI: 10.1080/24750263.2022.2100494.
- Olszewski P, Bogusch P, Mięsikowski M, Baños-Picon L, Puchałka R. 2021. Behavioural and ecological data on dryudella stigma (panzer, 1809) (Hymenoptera, astatidae) with the first description of the mature larva. Journal of Hymenoptera Research 82:305–316. DOI: 10.3897/jhr.82.63594.
- Olszewski P, Bogusch P, Szpila K. 2021. Life History of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a Description of the Mature Larva. Insects 12(2):100. DOI: 10.3390/insects12020100.
- Peckham DJ. 1985. Ethological observations on Oxybelus (Hymenoptera: Sphecidae) in southwestern New Mexico. Annals of the Entomological Society of America 78(6):865–872. DOI: 10.1093/aesa/78.6.865.
- Peckham DJ, Hook AW. 1980. Behavioral observations on Oxybelus in southeastern North America. Annals of the Entomological Society of America 73(5):557–567. DOI: 10.1093/aesa/73.5.557.
- Peckham DJ, Kurczewski FE, Peckham DB. 1973. Nesting Behavior of Nearctic Species of Oxybelus (Hymenoptera: Sphecidae)1,2. Annals of the Entomological Society of America 66(3):647–661. DOI: 10.1093/aesa/66.3.647.
- Polidori C, Piwczyński M, Ronchetti F, Johnston NP, Szpila K. 2022. Host‑trailing satellite flight behaviour is associated with greater investment in peripheral visual sensory system in miltogrammine flies. Scientific Reports 12(1):2773. DOI: 10.1038/s41598-022-06704-8.
- Pulawski W 2023. Catalog of Sphecidae. California Academy of Sciences, San Francisco. Available: https://researcharchive.calacademy.org/research/entomology/entomology_resources/hymenoptera/sphecidae/genera/Oxybelus.pdf. Accessed on 2023 November 8.
- Sann M, Niehuis O, Peters RS, Mayer C, Kozlov A, Podsiadlowski L, Banks S, Meusemann K, Misof B, Bleidorn C, Ohl M. 2018. Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evolutionary Biology 18(71):1–15. DOI: 10.1186/s12862-018-1155-8.
- Švácha P, Danilevsky ML. 1987. Cerambycoid larvae of Europe and Soviet Union. Part I.- Acta Universitatis Carolinae. Biologica 30:1–176.
- Tanaka Y. 1985. Alternative manners of prey-carrying in the fossorial wasp, Oxybelus strandi Yasumatsu (Hymenoptera, Sphecoidea). Kontyû 53:277–283.
- Tormos J, Asís JD, Gayubo SF, Portillo M, Torres F. 2000. Nesting Behavior of Oxybelus lamellatus Olivier (Hymenoptera: Sphecidae). Annals of the Entomological Society of America 93(2):326–332. DOI: 10.1603/0013-8746(2000)093[0326:NBOOLO]2.0.CO;2.