Abstract
Chemical signaling through pheromones is an ancient and widespread mode of communication. Turtles and tortoises (chelonians) secrete pheromones via mental (chin) glands and have superior olfactory capacities rendering them a promising group to study the evolution and function of chemical communication in vertebrates. Here, we use state-of-the art proteomics and lipidomics techniques to identify and explore the possible functions of proteins and lipids secreted by mental glands in turtles. We examined four turtle species all from the family Geoemydidae, to understand among-species as well as sexual variation in the composition of mental gland secretions. Differential expression of a relatively small number (ca. 65) of proteins explained a large portion of the proteome variation across species, highlighting the existence of specific signals evolving even in closely related species. Lipidomic analysis revealed high inter-individual variation, but species differences could be attributed to five different lipid classes. The lipids found in mental glands could have a dietary origin or be related to individual condition, but may nonetheless be used in communication. We also examined sex-specific differences in the proteome of a single species (Mauremys leprosa) and found that males expressed a much larger array of proteins than females. Our findings establish a group of candidate proteins potentially involved in chemical signaling in freshwater geoemydid turtles. Alternatively, differently expressed proteins in mental glands could have an indirect link to chemical communication, being involved in pheromone transport and/or antioxidant protection.
Introduction
Many vertebrates possess secretory epidermal glands – either singled-celled structures or complex multicellular organs – as part of their integumentary systems (Quagliata et al. Citation2006). Epidermal glands secrete a wide variety of substances and are involved in many life processes and activities. For instance, some species of amphibians have skin glands that produce toxins to protect from predators and/or antimicrobial substances that inhibit pathogenic microorganisms (Toledo & Jared Citation1995; Jared et al. Citation2018). Another important function of cutaneous glands is the production of chemical signals that modulate intra-specific interactions, i.e., enable or facilitate communication between individuals of the same species (Mykytowycz & Goodrich Citation1974; Quay Citation1986; Woodley & Staub Citation2021). Despite a rapid increase in knowledge on this topic in recent years, the precise roles of epidermal glands and their chemical constituents are unknown for most vertebrates.
Most research concerning chemical communication in vertebrates has been traditionally carried out on fish and mammals, although recently other groups such as lizards have become the focus of interest (see Martín & López Citation2011; Baeckens Citation2019). Chelonians (tortoises and turtles) have ancient origins, but are currently highly imperiled as more than half of extant species are threatened with extinction (Stanford et al. Citation2020). Turtles and tortoises may use multiple kinds of signals for intraspecific communication (Mason & Parker Citation2010; Liu et al. Citation2013; Jorgewich-Cohen et al. Citation2022). A well-developed chemosensory ability allows them to detect chemical stimuli in their environs (Halpern Citation1992; Wachowiak et al. Citation2002). For instance, painted turtles (Chrysemys picta) may recognize chemical cues from their home pond and use them to orient and avoid suboptimal, unfamiliar water bodies (Quinn & Graves Citation1998). Many chelonians use chemosignals to communicate in sexual contexts. Several species of freshwater turtles release chemical signals that may convey information on reproductive status to conspecifics (Munoz Citation2004; Poschadel et al. Citation2006; Lewis et al. Citation2007; Ibáñez et al. Citation2012). Land tortoises such as male Hermann’s tortoises (Testudo hermanni) can discriminate the sex of other individuals as well as the reproductive state of females by using olfactory stimuli (Galeotti et al. Citation2007). Chelonians produce chemosignals from different sources, including at least two kinds of epidermal glands called Rathke glands and mental glands. Rathke (or musk) glands are present in many aquatic lineages (Waagen Citation1972). Musk glands emit malodour secretions that probably function as a repellent to deter predators, although a role in chemosignal production cannot be excluded (Ehrenfeld & Ehrenfeld Citation1973; Mason & Parker Citation2010).
Sexually dimorphic mental glands (MGs, also referred to as “chin glands”) are present in all four extant families of turtles (Emydidae; Platysternidae; Geoemydidae and Testudinidae) belonging to the Superfamily Testudinoidea (Winokur & Legler Citation1975). Mental glands are homologous structures in all turtles in which they are present but have been lost or reduced in multiple lineages (Ibáñez et al. Citation2021). The degree of development of MGs varies across species and sexes (Winokur & Legler Citation1975; Ibáñez et al. Citation2020, Citation2021). Behavioral studies on MG functionality have focused exclusively on land-dwelling tortoises of the genus Gopherus in which MG secretions are used in social and sexual activities (Rose Citation1970; Alberts et al. Citation1994). In addition, recent experiments with G. polyphemus indicate that both sexes may react to MG secretions from males, and that behavioral discrimination might be concentration-dependent in males (Kelley et al. Citation2021, Citation2022; Kelley & Mendonça Citation2021). Apart from Gopherus, research on other turtle species with well-developed glands producing holocrine secretions (see Ibáñez et al. Citation2020, Citation2021) could help to shed light on the evolution of chemical communication in this vertebrate group.
Chemically, MG secretions contain a rich mixture of lipids and proteins (Rose et al. Citation1969; Rose Citation1970; Alberts et al. Citation1994). In MG secretions of Gopherus, lipids such as cholesterol, triglycerides and fatty acids have been detected using chromatography (Rose et al. Citation1969; Rose Citation1970). More recently, chemical analysis of MG secretions from stripe-necked terrapins (Mauremys leprosa) has revealed steroids, especially cholesterol, and fatty acids as the most abundant lipids. Males and females share most of the compounds, and sexual differences in amount were apparent in only a few lipid components (Ibáñez et al. Citation2020). Studies on Gopherus have revealed sexual and interspecific differences in electrophoretic band profiles of MG secretions; however, the identities of proteins present in these secretions remain unknown (Rose Citation1970; Alberts et al. Citation1994).
In this study, we combine proteomic (the complete set of proteins expressed in a tissue or organism) and lipidomic (the entire lipid profile of a tissue or organism) methods to identify the chemical compounds present in MG secretions of turtles. We identify proteins expressed in several species and use annotated databases to explore protein function. Moreover, we characterize the lipidome for males of a subset of species. Chemosignal (or pheromone) production is intended to provide information to other members of the same species (Karlson & Lüscher Citation1959; Wyatt Citation2003), implying a high level of specificity. We therefore expected interspecific differences in protein profiles and lipidome composition as these molecules could be involved in chemical signaling in turtles. We also compared proteomic profiles in males and females of Mauremys leprosa. Since MGs are dimorphic, being reduced and probably non-functional in female turtles (Winokur & Legler Citation1975; Ibáñez et al. Citation2021), we also expected the sexes to strongly differ in their protein expression patterns, with male MGs likely to contain chemically active proteins in large numbers. Our study provides the first in-depth appraisal of the chemical complexity of turtle MG secretions and paves the way for further detailed studies assessing their function and relevance for communication.
Material and methods
Sampling of mental gland secretions
In this study, we used only captive turtles. Sampled individuals of M. leprosa were housed in outdoor, semi-natural conditions maintained in the Catalonian Reptile and Amphibian Rehabilitation Center, as part of a recovery program that aims to restore this species into natural ecosystems (see Ibáñez et al. Citation2020). These animals originated from natural Iberian populations (the provenance of individual specimens is unknown). Specimens of other sampled species were long-term captive animals maintained in zoological institutions and private collections. The pedigrees of the captive-bred turtles are either unknown or unavailable to us, however, close kinship is unlikely because they are housed in various locations with limited (or none at all) breeding opportunities between animals maintained at different facilities. All individuals used in this study were mature adults sexed according to external morphological features. Secretions were extracted from MGs following Ibáñez et al. (Citation2020), from a total of four species of turtles (Cuora amboinensis, Mauremys annamensis, Mauremys leprosa and Siebenrockiella crassicollis). All the samples originated from turtles of the family Geoemydidae in which MGs are widespread and often well-developed in males (Winokur & Legler Citation1975; Ibáñez et al. Citation2021; see for macroscopic aspect of MGs).
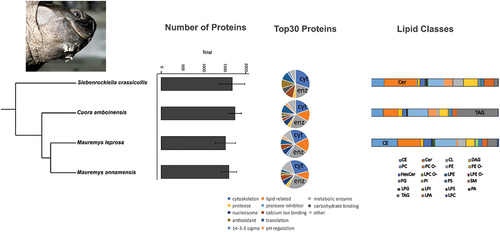
Figure 1. Results of the proteomic and lipidomic analysis for mental gland secretions of males of four turtle species. Left: number of proteins identified of each species (mean±SE). Middle: functional classification of the Top30 proteins, cytoskeleton (cyt) and metabolic enzymes (enz) are highlighted. Right: barplot with the amount (mol%) of each lipid class, the most abundant lipid class is highlighted. Phylogenetic relationships based on Pereira et al. (Citation2017), rooted with Sternotherus odoratus and Chelydra serpentina (not shown). Photo at top left shows the macroscopic aspect of mental glands in a male Siebenrockiella crassicollis. CE = cholesterol esters; Cer = ceramide; CL = cardiolipin; DAG = diacylglycerol; HexCer = hexosylceramide; LPA = lyso-phosphatidate; LPC = lyso-phosphatidylcholine; LPC O- = lyso-phosphatidylcholine-ether; LPE = lyso-phosphatidylethanolamine; LPE O- = lyso-phosphatidylethanolamine-ether; LPG = lyso-phosphatidylglycerol; LPI = lyso-phosphatidylinositol; LPS = lyso-phosphatidylserine; PA = phosphatidate; PC = phosphatidylcholine; PC O- = phosphatidylcholine-ether; PE = phosphatidylethanolamine; PE O- = phosphatidylethanolamine-ether; PG = phosphatidylglycerol; PI = phosphatidylinositol; PS = phosphatidylserine; SM = sphingomyelin; TAG = triacylglycerol.
Samples for proteomic analysis were processed as previously described (Ibáñez et al. Citation2022). We used the S-trap method, which allows for the identification of a large number of proteins (higher than other methods) of differing solubilities ranging from cytosolic to membrane proteins, thus providing a comprehensive overview of the turtle secretory proteome (Bang et al. Citation2022; Templeton et al. Citation2023). Prepared peptide samples for proteomics measurements were stored at −80°C until analysis at the Proteomics and Mass Spectrometry Core Facility of the Malopolska Centre of Biotechnology at Jagiellonian University (MCB UJ). Samples for lipidomics were dissolved in 300 or 500 µl PBS. Lipidomic samples were homogenized with pellet pestles, stored at −80°C and shipped on dry ice to Lipotype GmbH (Dresden, Germany) for laboratory analysis.
Proteomics analysis and data processing
Proteomic samples were subjected to LC-MS/MS analysis involving a Q Exactive mass spectrometer (Thermo Fisher Scientific) coupled with a nanoHPLC (UltiMate 3000 RSLCnano System, Thermo Fisher Scientific). Parameter settings conformed to those used to assay the protein composition of sand lizard femoral gland secretions (Ibáñez et al. Citation2022). The obtained mass-spectrometric data were processed using MaxQuant (version 1.6.7.0) (Tyanova et al. Citation2016) and searched using Andromeda (Cox et al. Citation2011) against Testudinoidea SwissProt + TrEMBL database (193,745 sequences, downloaded on 20 May 2022), supplemented with common protein contaminant sequences. Initially, 26 samples were used in the protein search, including two samples from the same individual (one of them was later excluded for further analyses; see supplementary file S1). iBAQ quantification (Schwanhäusser et al. Citation2011) and the LFQ algorithm were enabled. Other software settings were default and included a false discovery rate (FDR) below 1% for peptide and protein identification. The resulting table with identified protein groups was further processed using Perseus (version 2.0.7.0) (Tyanova et al. Citation2016). Protein groups from the reverse database, common protein contaminants, as well as proteins only identified by site were filtered out. Afterwards, iBAQ values were uploaded to assess protein abundance within MG secretions. Data were log transformed and median iBAQ values were calculated for protein groups that had valid iBAQ values in at least two-thirds of the samples of a given group (Cuora amboinensis, Mauremys annamensis, Mauremys leprosa males, M. leprosa females and Siebenrockiella crassicollis). Protein groups were ranked according to their median iBAQ values (from highest to lowest), and 30 proteins with the highest values (Top30) were manually grouped by function, including uncharacterized proteins present in our dataset, according to the information available in the UniProt database (UniProt Consortium Citation2021). With this first step, we intended to provide an overview of the number of proteins detected across samples and a functional categorization of the most abundant proteins in all four turtle species. In addition, a sexual comparison on MG protein profiles was conducted in M. leprosa for which samples of both sexes were analyzed.
A comparison between species was done using samples of males only and was based on log transformed LFQ intensities. Samples with low number of identified protein groups and expression pattern very different from the rest of the samples (Ml_1, Ml_7, Sc_4, Ca_7; see supplementary Figure S1) were excluded from interspecific comparison. Afterwards, an ANOVA with a permutation-based FDR threshold of 0.05 was carried out to test for differences in protein abundance in MG secretions. Moreover, Tukey’s Honest Significant Difference (HSD) test with FDR threshold of 0.05 was applied to determine pairwise differences between species. Fold changes were calculated and used to represent inter-species comparisons. Protein groups revealed as differential by the ANOVA test were analyzed using DeepLoc to predict their subcellular location (Thumuluri et al. Citation2022) by retrieving sequences for first major protein IDs from the UniProt database. A non-metric multidimensional plot (NMDS) plot was done to visualize the clustering of species considering differentially expressed proteins revealed in the ANOVA test. First, a distance matrix was created with the vegdist function (Bray–Curtis option). Then, the function metaMDS of the vegan package (Oksanen et al. Citation2019) in R was used to create the NMDS plot. Furthermore, a permutational multivariate analysis of variance (PERMANOVA), function adonis2, was carried out using intensities for differentially expressed proteins with species as a factor. Heterogeneity of variances across groups was examined with betadisper.
Lipid extraction, MS data acquisition and post-processing
Mass spectrometry-based lipid analysis was performed by Lipotype GmbH (Dresden, Germany) as described in Sampaio et al. (Citation2011). Lipids were extracted using a two-step chloroform/methanol procedure (Ejsing et al. Citation2009). Samples were spiked with internal lipid standard mixture containing cardiolipin 14:0/14:0/14:0/14:0 (CL), ceramide 18:1;2/17:0 (Cer), diacylglycerol 17:0/17:0 (DAG), hexosylceramide 18:1;2/12:0 (HexCer), lyso-phosphatidate 17:0 (LPA), lyso-phosphatidylcholine 12:0 (LPC), lyso-phosphatidylethanolamine 17:1 (LPE), lyso-phosphatidylglycerol 17:1 (LPG), lyso-phosphatidylinositol 17:1 (LPI), lyso-phosphatidylserine 17:1 (LPS), phosphatidate 17:0/17:0 (PA), phosphatidylcholine 17:0/17:0 (PC), phosphatidylethanolamine 17:0/17:0 (PE), phosphatidylglycerol 17:0/17:0 (PG), phosphatidylinositol 16:0/16:0 (PI), phosphatidylserine 17:0/17:0 (PS), cholesterol ester 20:0 (CE), sphingomyelin 18:1;2/12:0;0 (SM), and triacylglycerol 17:0/17:0/17:0 (TAG). After extraction, the organic phase was transferred to an infusion plate and dried in a speed vacuum concentrator. First step dry extract was re-suspended in 7.5 mM ammonium acetate in chloroform/methanol/propanol (1:2:4, V:V:V) and second step dry extract in 33% ethanol solution of methylamine in chloroform/methanol (0.003:5:1; V:V:V). All liquid handling steps were performed using a Hamilton Robotics STARlet robotic platform with the Anti Droplet Control feature for pipetting of organic solvents.
Samples were analyzed by direct infusion on a Q Exactive mass spectrometer (Thermo Fisher Scientific) equipped with a TriVersa NanoMate ion source (Advion Biosciences). Samples were analyzed in both positive and negative ion modes with a resolution of Rm/z = 200 = 280000 for mass spectrometry (MS) and Rm/z = 200 = 17500 for tandem mass spectrometry (MSMS) experiments, in a single acquisition. Tandem mass spectrometry was triggered by an inclusion list encompassing corresponding MS mass ranges scanned in 1 Da increments (Surma et al. Citation2015). Both MS and MSMS data were combined to monitor CE, DAG and TAG ions as ammonium adducts; PC and PC O- as acetate adducts; and CL, PA, PE, PE O-, PG, PI and PS as deprotonated anions. MS only was used to monitor LPA, LPE, LPE O-, LPI and LPS as deprotonated anions; Cer, HexCer, SM, LPC and LPC O- as acetate adducts.
Raw data were processed at Lipotype GmbH as described below. Data were analyzed with in-house developed lipid identification software based on LipidXplorer (Herzog et al. Citation2011, Citation2012). Data post-processing and normalization were performed using an in-house developed data management system. Only lipid identifications with a signal-to-noise ratio >5 and a signal intensity fivefold higher than in corresponding blank samples were considered for further analysis. The identified lipid molecules were quantified by normalization to a lipid class specific internal standard. The amounts in pmoles of individual lipid molecules of a given lipid class were summed to yield the total amount of the lipid class. The amounts of the lipid classes were normalized to the total lipid amount yielding mol% per total lipids. The values are thus given as mol% of the lipid class.
Afterwards, lipidomic data were analyzed with the web-interface LipotypeZoom, R and Statistica (version 13.3, StatSoft). Overall, 16 samples from three turtle species (C. amboinensis, M. leprosa and S. crassicollis) were analyzed. A total of 23 lipid classes were considered for which we calculated the mean of the relative amount (mol%) but only 12 classes (“major lipid classes”: CE, Cer, DAG, HexCer, LPE, PC, PE O-, SM, TAG, PC O-, PE, PG) appeared in all samples. Therefore, as a first step, we carried out a hierarchical clustering including all samples (three species) and focusing on 12 major lipid classes to visualize the data (see supplementary Figure S2). The relative amounts (mol%) were not re-standardized or transformed in subsequent analysis. To formally test which major lipid classes contribute to species differences, we performed a nonparametric Kruskal–Wallis test (R function kruskal.test). Post hoc comparisons were performed with pairwise.wilcox.test function from the “stats” package when the result of the Kruskal–Wallis test was significant. A NMDS plot (as described above) was used to visualize variation across individuals and species according to lipid composition using only the amounts of lipid classes previously showing differences in the Kruskal–Wallis test. A PERMANOVA was conducted to test for the effect of species on lipid composition using the same dataset. The heterogeneity of variance across groups was tested with the betadisper R function. In both NMDS and PERMANOVA analyses, we used only lipid classes differing across species (i.e., lipids showing significant differences in the Kruskal–Wallis tests).
Results
Protein characterization
Most of the samples for proteomics originated from males (N = 22) – covering four species (see ) – and only three samples were from females (see below). Considering iBAQ values, the number of identified proteins across samples for males ranged from 423 (sample from M. leprosa) to 2328 (sample from C. amboinensis; see supplementary file S1). When considering average values from each species separately, the maximum number of proteins was detected in C. amboinensis (average ± SD; 1746 ± 437, N = 8), S. crassicollis (1682 ± 599, N = 4), M. annamensis (1603 ± 326, N = 3), M. leprosa (1521 ± 643, N = 7; see ). Based on proteins with valid iBAQ values in at least two-thirds of the samples in each species, a total of 700 proteins were shared by all four turtle species. The number of uncharacterized proteins in males (considering first ID and proteins with valid iBAQ values in at least two-thirds of the samples of a given species) ranged from 223 in M. annamensis, through 176 in S. crassicollis, 152 in M. leprosa to 141 in C. amboinensis.
Proteins were sorted according to median iBAQ values, and the functions for the Top30 proteins were then examined for males of four species of turtles. In general, cytoskeleton proteins and metabolic enzymes were the most common functional categories of Top30 proteins (, ). Cytoskeleton proteins ranged from 20 to 30% across turtle species. A large portion of Top30 proteins were classified as metabolic enzymes (). Pooled metabolic enzymes and lipid-related proteins (see different categories in ) accounted for 40% of the Top30 proteins in M. leprosa to a minimum of 20% in S. crassicollis. Antioxidant proteins were present in all species but M. leprosa reaching a maximum of 10% of the Top30 proteins in S. crassicollis.
Table I. Functions for Top30 proteins according to annotations in the UniProt database (UniProt Consortium Citation2021) for males of four turtle species. The number of proteins exhibiting each function is provided (see Material and Methods for details).
Sexual differences in M. leprosa
Only three samples for proteomic characterization from a single species (Mauremys leprosa) were analyzed. When comparing males and females M. leprosa, we found out that the number of proteins varied strongly between sexes. Females had significantly lower number of proteins than males (ANOVA; F = 12.74; p = 0.007). On average (±SD) females had only 146 (±86) proteins, contrasting with the high number of proteins found in males (1521 ± 643) ().
Figure 2. a) Boxplot showing the number of protein groups identified in mental gland secretions (based on iBAQ) for each sex in Mauremys leprosa. b) Functions for Top30 proteins in males and females of M. leprosa. Most numerous categories are highlighted (i.e. cytoskeleton and protease inhibitor in females; cytoskeleton, lipid related and metabolic enzymes in males).
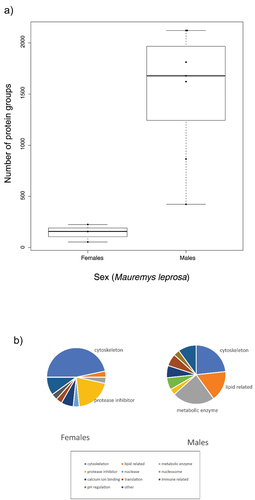
From a functional point of view, cytoskeleton proteins predominate among Top30 proteins in MG secretions from females (almost 47%; ). Protease inhibitors were also important reaching 20% of female Top30 proteins. In contrast, a large portion of Top30 proteins in males were classified either as metabolic enzymes or lipid-related proteins (23.3% and 16.7% respectively). Although less common than in females, cytoskeleton proteins appeared consistently in males ().
Species-specific protein expression in turtle mental gland secretions
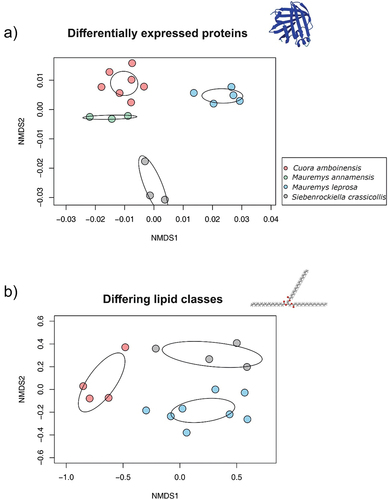
Interspecific differences in proteomic profiles were examined using only males and after applying strict filtering criteria for quality and number of samples (see Methods and supplementary Figure S1). Statistical analysis (ANOVA) revealed a total of 65 proteins differentially expressed across species (supplementary file S2). Predictions on the subcellular locations for these 65 proteins were performed with DeepLoc. Considering proteins with a single subcellular location predicted (55 proteins), we found that most of them were cytoplasmic (42), while the rest were predicted in other locations such as mitochondrion (3), endoplasmic reticulum (3), cell membrane (2), lysosome/vacuole (2), extracellular (2) and nucleus (1). A total of 10 proteins presented more than one location. Thus, species-specific proteins were overwhelmingly intracellular. A PERMANOVA taking into account 65 differentially expressed proteins showed a significant effect of species (Pseudo-F = 16, p = 0.001) accounting for a large portion of the total variance (77.4%). Differences in multivariate dispersion among species were not significant (betadisper, p = 0.43). In concordance with the PERMANOVA, samples clustered according to species in the NMDS plot ().
Figure 3. Visualization of sample distribution on non-metric multidimensional scaling (NMDS) plots. Each dot represents one sample. Ellipses represent 95% confidence intervals for centroids based on standard error (ordiellipse function; conf = 0.95, kind=“se”). Color coding denotes species. The structures were generated with Molview (Smith Citation1995) and AlphaFold (Jumper et al. Citation2021). a) For proteomics, the NMDS plot was based on 65 differentially expressed proteins found in secretions of turtle mental glands. Only males were considered in this analysis (N = 18). b) For lipidomics, NMDS plot was based on five lipid classes differing across turtle species. All samples originate from males (N = 16).
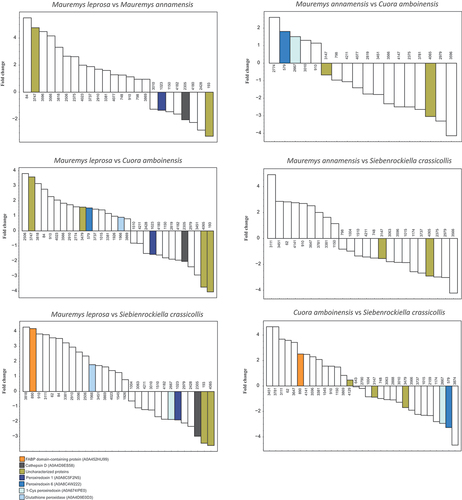
Further pairwise post-hoc comparisons of proteins differing in the ANOVA (see above) were carried out. Proteins related to lipid metabolism, antioxidant activity, and other functions were upregulated or downregulated in one or more species (see and supplementary file S2). For instance, a fatty acid-binding protein (FABP) was found among differentially expressed proteins. FABP domain-containing protein (A0A452HU99) was upregulated in M. leprosa and C. amboinensis when compared to S. crassicollis (). In addition, several antioxidant proteins were differentially expressed including three peroxiredoxins and glutathione peroxidase (A0A4D9E0D3). Cathepsin D (A0A4D9ES58), a protease, was expressed at high levels in all species, but in M. leprosa it was downregulated in comparison to other species. Interestingly, the list of differentially abundant proteins revealed by ANOVA contained several uncharacterized proteins (see ). An exhaustive list of pairwise comparisons with all upregulated and downregulated proteins can be found in supplementary file S2.
Figure 4. Pairwise comparisons of protein abundances in turtle mental gland secretions for the chelonian species Mauremys leprosa, M. annamensis, Cuora amboinensis and Siebenrockiella crassicollis. Positive and negative fold change values (in logarithmic scale) represent upregulated and downregulated proteins, respectively. Numbers at the bases of the bars are protein identifiers as shown in supplementary file S2 (see column id). Some selected proteins are colored, for details see Results.
Lipidomics of mental gland secretions
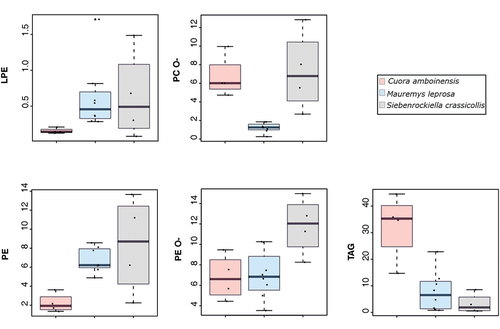
Lipid classes were detected in MGs from three turtle species analyzed (N = 16). Remarkable variation in lipid composition was observed across as well as within these species ( and supplementary file S3). For instance, the two main classes in S. crassicollis were Cer (average ± SD: 25.7 mol% ± 15.3, N = 4) and PC (12.9 mol% ± 12.7, N = 4), while in C. amboinensis the most abundant lipids were TAG (32.4 mol% ± 12.6, N = 4) and PC (15.8 mol% ± 4.8, N = 4). In the case of M. leprosa, MG secretions were dominated by cholesterol esters (CE, 22.4 mol% ± 7.6, N = 8) followed by Cer (17.9 ± 20.4, N = 8). Typically, the lipid classes analyzed here were present in at least one sample of each species; however, only 12 classes appeared consistently in all samples (see supplementary file S3). Therefore, we focused on these 12 major lipid classes for further analyses.
To formally test which major lipid classes could contribute to differences among species we used Kruskal–Wallis tests. Statistically significant differences were found in 5 out of 12 major classes of lipids (). The PERMANOVA test showed that lipid composition (based on five differing lipid classes) was affected by species (pseudo-F = 8.66, p = 0.001) and 57.1% of the total variation was explained by this factor. Differences in multivariate dispersion among species were not significant (betadisper, p = 0.52). The NMDS plot (based on five lipid classes) showed that samples grouped according to species (see ). Overall, the results indicate that despite a considerable level of overlap in MG lipidome among species is present, species differences on lipid composition are mainly driven by five lipid classes.
Table II. Results of the Kruskal–Wallis test for the 12 major lipid classes found in turtle mental gland secretions. Statistical Kruskal–Wallis chi-squared (K-W) and P are shown along with median and minimum-maximum range values for each species. Post hoc comparisons that are significant are highlighted (in bold) and specified in the last column.
Further post-hoc tests were carried out for the five major lipid classes previously detected in Kruskal–Wallis tests. Significant differences in some species pairwise comparisons were revealed in the post-hoc tests (). For instance, C. amboinensis had larger amounts of TAG than both S. crassicollis and M. leprosa (both post-hoc tests p < 0.05; see and ). Also, PC-O was present in significantly lower amounts in M. leprosa compared to both S. crassicollis and C. amboinesis (p < 0.05; ; ).
Discussion
Proteins and lipids are important compounds found in cutaneous glands of many vertebrates. Here, we used modern analytical techniques for large-scale identification of molecules in turtle mental gland (MG) secretions, providing a foundation for more detailed studies on their biological functions and potential role in chemical communication. Proteins have been neglected in the study of reptilian chemical communication, particularly in chelonians. To fill this research gap, we analyzed the molecular profiles in multiple species of turtles: (i) C. amboinensis, M. annamensis, M. leprosa and S. crassicollis for proteomics and (ii) C. amboinensis, M. leprosa and S. crassicollis for lipidomics. The relatively few proteins that demonstrate a species-specific component may be directly or indirectly related to chemical signaling in these species. We also show that protein profiles highly differ between sexes of M. leprosa. Far more proteins are expressed in male MGs compared to female MGs of this species. In males, highly expressed proteins are related to metabolic enzymes, lipid metabolism as well as cytoskeleton among other functions. We detected 12 major lipid classes in MGs of three chelonians. However, inter-specific signatures on MG lipidome were mainly driven by five lipid classes.
Species-specific component
We expected enhanced expression of proteins involved in chemical signaling (Van Bocxlaer et al. Citation2015; Maex et al. Citation2018) and a species-specific component in protein profiles (Mangiacotti et al. Citation2021). We found that relatively few proteins were expressed differentially among turtle species. This suggests that most proteins expressed in turtle MGs may not be linked to chemical signaling. Alternatively, the same protein(s) could play a role in chemical signaling in many related species. For instance, Sodefrin Precursor-Like Factors are pheromone proteins expressed in breeding glands of different lineages of amphibians (Bossuyt et al. Citation2019). Several previous studies dealing with gland-protein variation in lizards relied on electrophoretic banding patterns and did not identify the proteins contributing to differences between species (Alberts Citation1991; Mangiacotti et al. Citation2021). However, recent studies have been able to characterize proteins expressed in lizard epidermal glands (Tellkamp et al. Citation2020; Ibáñez et al. Citation2022; Mangiacotti et al. Citation2023). Here, we were able to identify the proteins in turtle MGs by mass spectrometry proteomics using an annotated protein database.
Several proteins differing in expression patterns among turtle species are related to lipids or metabolic routes. Noteworthy is the presence of a fatty acid-binding protein (FABP) that was upregulated in M. leprosa and C. amboinensis compared to S. crassicollis (see ; supplementary file S2). In mammalian pheromones, proteins may facilitate the transport and/or persistence of chemosignals by reversible binding. For instance, in rodents, major urinary proteins (MUPs) bind small volatile odorant molecules that are slowly released from scent marks, and thus increase the persistence of chemical signals (reviewed in Wyatt Citation2014). A similar role has been observed for serum albumin in Asian elephants (Elephas maximus). Serum albumin may bind and facilitate the dispersion and chemoreception of (Z)-7-dodecenyl acetate - a sex pheromone in Asian elephants (Lazar et al. Citation2004). We hypothesize that lipid metabolic proteins such as FABPs found in turtle MG secretions could bind some of the lipophilic compounds (Rose Citation1970; Ibáñez et al. Citation2020) that may be transferred to the chemosensory organs of other conspecifics. For instance, fatty acid blends produced by MGs of male Texas tortoises (Gopherus berlandieri) may mediate courtship and aggressive behavioral interactions (Rose Citation1970). Therefore, we hypothesize that FABP specifically upregulated in turtles could be involved in the pathways for the transport and detection of volatile or semi-volatile lipids potentially used in chemical signaling, similar to the chemosensory pathways described for other vertebrates.
Several peroxiredoxins and glutathione peroxidase were differentially expressed across species. These molecules are antioxidant enzymes playing a crucial role in the protection against reactive oxygen species (ROS) involved in oxidative stress (Molavian et al. Citation2015; Cao & Lindsay Citation2017). Antioxidants were found using similar proteomic methods in sand lizard femoral gland secretions (Ibáñez et al. Citation2022). It has been hypothesized that antioxidants could protect the lipid fraction from oxidation in humid conditions in the terrestrial habitats inhabited by lizards. In turtles, antioxidant proteins may carry out an analogous function and protect other molecules secreted by MGs from oxidation. Most of the turtle species studied herein live in tropical and subtropical habitats with high humidity levels in which lipid peroxidation seems likely to occur, e.g., S. crassicollis occurs in wetlands of southeastern Asia. Indeed, antioxidant proteins were among the highly expressed proteins (i.e., Top30; see ) found in this species, suggesting their involvement in safeguarding against the oxidation of other chemosignaling molecules. Alternatively, these proteins could be released by MGs to act as scavengers of ROS produced in the skin due to the action of aggressive agents such as UV-radiation.
Cathepsin D was among the differentially expressed proteins found in turtle MGs. This aspartic protease is involved in multiple biological processes such as protein degradation, activation of enzymatic precursors, antigen presentation and regulation of apoptosis, among others (Benes et al. Citation2008). Cathepsin D is one of the most abundant proteins in femoral gland secretions of sand lizards (Lacerta agilis) and marine iguanas (Amblyrhynchus cristatus) (Tellkamp et al. Citation2020; Ibáñez et al. Citation2022). Here, cathepsin D was also abundant (Top30) in S. crassicollis, C. amboinensis and M. annamensis but was downregulated in M. leprosa MG secretions (see ). Several uncharacterized proteins were differently expressed across species, and thus further work should be aimed at characterizing these proteins – e.g., using blast-like methods to annotate turtle proteins in the UniProt database. Further studies should include transcriptome sequencing of entire mental glands to improve protein identification (Bossuyt et al. Citation2019). In addition, experimental work focusing on species-specific proteins identified in MG secretions could demonstrate whether they have any direct pheromonal activity. For instance, a similar framework as previously used in lacertid lizards for behavioral tests of the bioactivity of proteins (Mangiacotti et al. Citation2019) could be adapted to turtles, although we acknowledge that this could be methodologically challenging.
A qualitative comparison of the most highly expressed proteins in turtle MGs and lizard femoral glands reveals some similarities. For example, cathepsin D and carbonic anhydrase were among the most abundant proteins in femoral glands (Tellkamp et al. Citation2020; Ibáñez et al. Citation2022). Cathepsin D and carbonic anhydrase were also among the Top30 proteins found in MG secretions from some species of turtles studied here. In addition, keratins and cytoskeletal proteins, lipid-related proteins, antioxidants and histones are present in the Top30 proteins in femoral glands of sand lizards (Ibáñez et al. Citation2022). This result indicates that some types of proteins are expressed at high levels in epidermal glands both in squamates and in the phylogenetically distant chelonians. This might be explained by the lipogenic nature of femoral and mental glands since both are considered “sebaceous-like glands” exclusive to amniotes (Quagliata et al. Citation2006). It is likely that some of these proteins are housekeeping, necessary for the maintenance of epidermal glands, and are not involved in chemical communications.
Proteins of varied abundance in different species expressed in MGs could be used directly for intraspecific communication, although an indirect effect on chemical signaling is also possible. A recent study by Mangiacotti et al. (Citation2023) found an association between some enzymatic proteins (carbonic anhydrase and protein disulfide isomerases) and provitamin D3, a lipophilic compound known to be involved in lizard chemical signaling (Martín & López Citation2006; López et al. Citation2009). An analogous situation in which proteins respond to a dynamic microenvironment and facilitate the functioning of other molecules in signaling processes in turtle MGs seems plausible. Indeed, some of the species-specific proteins identified here are involved in multiple molecular functions that are not directly related to chemical communication but that could be linked to persistence, protection, and chemosensory reception of chemosignals (see above).
Lipidomic analysis revealed among-individual and species-specific differences in the amounts of several major lipid classes including phospholipids such as phosphatidylethanolamine (PE), ether-linked phosphatidylethanolamine (PE O-), ether-linked phosphatidylcholine (PC O-) and lyso-phosphatidylethanolamine (LPE). Genomic analysis has revealed many olfactory gene receptors in turtles, especially class I type that has a high affinity for hydrophilic or polar compounds (Wang et al. Citation2013; Bentley et al. Citation2023). Phospholipids may be suitable for signaling in freshwater environments due to their amphipathic character, as polar heads could interact with water molecules. This hypothesis could explain differences in the abundance of these compounds in aquatic turtles. Lipidomic analysis also revealed differences in triacylglycerols (TAGs), a class of lipids abundant in MG secretions of C. amboinensis. These molecules are fats used for energy storage and are highly insoluble in water. TAG lipids are degraded by hydrolysis producing glycerol and fatty acids that can be used in other tissues depending on energetic demands. Therefore, the high variability of TAGs among individuals and species () could be related to differences in individual condition, e.g., an increased level of fat storage for C. amboinensis individuals compared to S. crassicollis and M. leprosa. Multivariate analysis showed that species-specific lipids are restricted to just a subset of the MG lipidome. Hence, the potential use of some lipids (e.g., some phospholipids and TAGs) in turtle communicative processes requires further investigation, and moreover, lipidomic analysis of further species with larger sample sizes. Alternatively, differences in these lipid classes could be a product of distinct diets taken by turtles or other uncontrolled factors influencing our results (see below).
Shotgun lipidomics is applied in large-scale lipid characterization, including neutral and polar lipids (Wu et al. Citation2014). Previous chemical analysis of turtle MGs was carried out using gas chromatography coupled to mass spectrometry (GC/MS), a method suitable for the detection of volatile and semivolatile compounds. GC/MS analysis showed that steroids and carboxylic acids are prominent components in MG secretions of M. leprosa. Many of the compounds detected through GC/MS were present in similar amounts in both males and females, and sexual differences occurred in only a few compounds which could potentially be used in intraspecific signaling (Ibáñez et al. Citation2020). Cholesterol was the most abundant compound constituting the bulk of MG secretions (Ibáñez et al. Citation2020). Lipidomics analysis performed herein revealed that cholesterol esters (CE) were the most abundant lipid class in M. leprosa. Indeed, CEs are intermediary forms of cholesterol used for transport in the blood or other tissues and this could explain their presence in turtle MGs. The lack of inter-specific differences suggests that CEs may not be relevant for chemical communication, but we cannot rule out that other volatile or semivolatile compounds could still be related to chemical signaling.
Our results show that species signatures in MG composition are more evident in proteins than in lipids. Lipids may be related to individual condition and thus be used as honest signals. Indeed, many lizards secrete a wide variety of volatile lipophilic compounds that play a pivotal role in intrasexual interactions and mate choice (Khannoon et al. Citation2011; Martín & López Citation2011). Peptide or proteinaceous pheromones are used in other aquatic or terrestrial vertebrates (Wyatt Citation2014). In amphibians, a group of vertebrates strongly linked to freshwater environments, peptides and proteins are released into water and used during courtship and reproduction (Kikuyama et al. Citation1995; Maex et al. Citation2016). While lipidome composition might be influenced by condition, health state or individual dietary intake of lipids, the amino acid composition of proteins is expected to rapidly evolve through natural selection and produce unique signals for a given species (Wyatt Citation2014; Treer et al. Citation2018). Sex pheromones should provide reliable information to the opposite sex in a given species, and therefore, species-specificity is expected as a general rule. Besides interspecific variation, prominent sexual differences (see below) were also observed in MG proteins of M. leprosa, supporting the hypothesis that these molecules are connected to chemical communication in turtles.
Sexual differences in Mauremys leprosa
Previous studies have shown that MGs are sexually dimorphic organs for many turtle species (Winokur & Legler Citation1975; Ibáñez et al. Citation2020, Citation2021). In Mauremys leprosa, females possess MGs that resemble small and highly keratinized invaginations, while males have well-developed and prominent MGs that produce holocrine secretions (Ibáñez et al. Citation2020). In line with previous findings, we found stark differences in the number of proteins in MG secretions between sexes. Substantially lower numbers of proteins were expressed in MGs from females compared to males (146 ± 86 vs. 1521 ± 643, respectively). This finding further indicates that MGs in female M. leprosa are likely non-functional vestigial traits and reinforce the idea that male MGs are compound organs likely involved in pheromone production. It is worth noting that among the most abundant Top30 proteins expressed in MGs of M. leprosa males (as opposed to females), we found some metabolic enzymes and lipid-related proteins, providing further evidence that male MGs in this species are functional organs, secreting a rich mixture of chemically active molecules.
Study caveats
An important limitation here concerns the poor annotation of the existing proteomic databases for chelonian taxa, as evidenced by the relatively large number of uncharacterized proteins in our analyses. Moreover, some of our annotations should be considered as preliminary. This imposes limits on the accuracy of inferring the molecular activities of proteins and hence the biological functions of MG secretions. Here, we do not attempt to explore the physiological or molecular functions of the proteins but instead we search for interspecific signatures that can establish a baseline for the further characterization of protein-based pheromones in turtles. Experimental research is needed to fully understand the roles of MG proteins.
Several uncontrolled factors and limitations could have affected our results. First, different turtle species might have distinct diets that could affect MG lipidome composition. For example, S. crassicollis has been considered as more carnivorous than C. amboinensis (Kimmel Citation1980). Many freshwater turtles are good scavengers, opportunistically feeding on a variety of food items, playing a pivotal role in natural ecosystems (Lovich et al. Citation2018). Three species (M. leprosa, S. crassicollis and C. amboineisis) considered here are to some extent omnivorous (Kimmel Citation1980; Bertolero & Busack Citation2017). Nonetheless, individual diets certainly vary, and considering that captive individuals were used in this study, diet-related differences or individual condition status could have influenced lipid composition. In the same way, we could not control the genetic relatedness of the sampled turtles, although we tried to ameliorate the effects of potentially close kinship by sampling animals from different zoological collections. Although beyond the scope of this study, testing the effects of diet and genetic relatedness on MG lipidome and proteome profiles would definitely improve our understanding of the sources of variation on reptilian glandular chemistry.
Conclusions
This study revealed that interspecific variation in proteome composition is due to a relatively small number of proteins that could be involved, either directly or indirectly, in communication in freshwater geoemydid turtles. Sexual dimorphism in gland-expressed proteins was evident in M. leprosa, and supports the hypothesis that MGs are functional organs in males, but not females, of this species. Inter-individual variation in lipid composition was high and differences among species were observed in some lipid classes, although their exact roles in turtle communication need further research. This study lays the foundation for future studies using proteomic and lipidomic tools applied to non-model taxa as chelonians.
Supplementary figures_and_captions_EZJ_rev.docx
Download MS Word (349.8 KB)SuppFileS3_Lipid_class_rev.xls
Download MS Excel (32.5 KB)SuppFileS1_samples_proteomics_rev.xlsx
Download MS Excel (11.2 KB)SuppFileS2_ANOVAsig_65_posthoc.xlsx
Download MS Excel (60.4 KB)Acknowledgments
We thank zoological institution personnel for their help during sampling. Animals used in this study were already in captivity when sampled. Turtles were sampled in accordance with European Union directive 2010/63/UE on the protection of animals used for scientific purposes. Following directive 2010/63/UE, the extraction of mental gland exudates does not qualify as an experimental procedure because it does not puncture the tissue or harm the turtle.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Proteomic mass spectrometry data were deposited to the ProteomeXchange via the MassIVE repository with the dataset identifier PXD049363. Supplementary file S1 shows the names for proteomic samples together with the number of identified proteins. A table with the amounts of lipid classes analyzed in this study is available at the supplementary material (supplementary file S3).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2024.2376589
Additional information
Funding
References
- Alberts AC. 1991. Phylogenetic and adaptive variation in lizard femoral gland secretions. Copeia 1991(1):69–79. DOI: 10.2307/1446249.
- Alberts AC, Rostal DC, Lance VA. 1994. Studies on the chemistry and social significance of chin gland secretions in the desert tortoise, Gopherus agassizii. Herpetological Monographs 8:116–124. DOI: 10.2307/1467075.
- Baeckens S. 2019. Evolution of animal chemical communication: Insights from non-model species and phylogenetic comparative methods. Belgian Journal of Zoology 149:63–93. DOI: 10.26496/bjz.2019.31.
- Bang G, Lee H, Kim H, Han EH, Park YH, Kim JY. 2022. Comparison of protein characterization using in solution and S-Trap digestion methods for proteomics. Biochemical & Biophysical Research Communications 589:197–203. DOI: 10.1016/j.bbrc.2021.12.026.
- Benes P, Vetvicka V, Fusek M. 2008. Cathepsin D—many functions of one aspartic protease. Critical Reviews in Oncology/Hematology 68(1):12–28. DOI: 10.1016/j.critrevonc.2008.02.008.
- Bentley BP, Carrasco-Valenzuela T, Ramos EK, Pawar H, Souza Arantes L, Alexander A, Banerjee SM, Masterson P, Kuhlwilm M, Pippel M. 2023. Divergent sensory and immune gene evolution in sea turtles with contrasting demographic and life histories. Proceedings of the National Academy of Sciences of the United States of America. 120:e2201076120. DOI: 10.1073/pnas.2201076120.
- Bertolero A, Busack S. 2017. Mauremys leprosa (Schoepff in Schweigger 1812)–Mediterranean pond turtle, Spanish terrapin, Mediterranean stripe‐necked terrapin. Conservation Biology of Freshwater Turtles & Tortoises. Chelonian Research Monographs 5:102.101–102.119.
- Bossuyt F, Schulte LM, Maex M, Janssenswillen S, Novikova PY, Biju S, Van de Peer Y, Matthijs S, Roelants K, Martel A, Van Bocxlaer I. 2019. Multiple independent recruitment of sodefrin precursor-like factors in anuran sexually dimorphic glands. Molecular Biology and Evolution 36(9):1921–1930. DOI: 10.1093/molbev/msz115.
- Cao Z, Lindsay JG. 2017. The peroxiredoxin family: An unfolding story. Macromolecular Protein Complexes: Structure and Function 83:127–147.
- Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. 2011. Andromeda: A peptide search engine integrated into the MaxQuant environment. Journal of Proteome Research 10(4):1794–1805. DOI: 10.1021/pr101065j.
- Ehrenfeld JG, Ehrenfeld DW. 1973. Externally secreting glands of freshwater and sea turtles. Copeia 1973(2):305–314. DOI: 10.2307/1442969.
- Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, Ekroos K, Klemm RW, Simons K, Shevchenko A. 2009. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America 106:2136–2141. DOI: 10.1073/pnas.0811700106.
- Galeotti P, Sacchi R, Rosa DP, Fasola M. 2007. Olfactory discrimination of species, sex, and sexual maturity by the Hermann’s Tortoise Testudo hermanni. Copeia 2007(4):980–985. DOI: 10.1643/0045-8511(2007)7[980:ODOSSA]2.0.CO;2.
- Halpern M. 1992. Nasal chemical senses in reptiles: Structure and function. In: Gans C, Crews D, editors Biology of the Reptilia: Hormones, brain, and behavior. Chicago: University of Chicago Press. pp. 423–523.
- Herzog R, Schuhmann K, Schwudke D, Sampaio JL, Bornstein SR, Schroeder M, Shevchenko A, Brennan L. 2012. LipidXplorer: a software for consensual cross-platform lipidomics. PLOS ONE 7(1):e29851. DOI: 10.1371/journal.pone.0029851.
- Herzog R, Schwudke D, Schuhmann K, Sampaio JL, Bornstein SR, Schroeder M, Shevchenko A. 2011. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biology 12(1):R8. DOI: 10.1186/gb-2011-12-1-r8.
- Ibáñez A, Fritz U, Auer M, Martínez-Silvestre A, Praschag P, Załugowicz E, Podkowa D, Pabijan M. 2021. Evolutionary history of mental glands in turtles reveals a single origin in an aquatic ancestor and recurrent losses independent of macrohabitat. Scientific Reports 11(1):10396. DOI: 10.1038/s41598-021-89520-w.
- Ibáñez A, López P, Martín J. 2012. Discrimination of conspecifics’ chemicals may allow Spanish terrapins to find better partners and avoid competitors. Animal Behaviour 83(4):1107–1113. DOI: 10.1016/j.anbehav.2012.02.001.
- Ibáñez A, Martínez-Silvestre A, Podkowa D, Woźniakiewicz A, Woźniakiewicz M, Pabijan M. 2020. The chemistry and histology of sexually dimorphic mental glands in the freshwater turtle, Mauremys leprosa. PeerJ 8:e9047. DOI: 10.7717/peerj.9047.
- Ibáñez A, Skupien-Rabian B, Jankowska U, Kędracka-Krok S, Zając B, Pabijan M. 2022. Functional protein composition in femoral glands of sand lizards (Lacerta agilis). Molecules 27(7):2371. DOI: 10.3390/molecules27072371.
- Jared C, Mailho-Fontana PL, Marques-Porto R, Sciani JM, Pimenta DC, Brodie Jr ED, Antoniazzi MM. 2018. Skin gland concentrations adapted to different evolutionary pressures in the head and posterior regions of the caecilian Siphonops annulatus. Scientific Reports 8(1):3576. DOI: 10.1038/s41598-018-22005-5.
- Jorgewich-Cohen G, Townsend SW, Padovese LR, Klein N, Praschag P, Ferrara CR, Ettmar S, Menezes S, Varani AP, Serano J, Sánchez-Villagra MR. 2022. Common evolutionary origin of acoustic communication in choanate vertebrates. Nature Communications 13(1):6089. DOI: 10.1038/s41467-022-33741-8.
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596(7873):583–589. DOI: 10.1038/s41586-021-03819-2.
- Karlson P, Lüscher M. 1959. ‘Pheromones’: A new term for a class of biologically active substances. Nature 183(4653):55–56. DOI: 10.1038/183055a0.
- Kelley MD, Finger Jr JW, Mendonça MT. 2022. Male gopher tortoise (Gopherus polyphemus) concentration-dependent social responses to diluted mental gland pheromones. Behavioural Processes 201:104729. DOI: 10.1016/j.beproc.2022.104729.
- Kelley MD, Ka C, Finger Jr JW, Mendonça MT. 2021. Behavioural discrimination of male mental gland secretions of the gopher tortoise (Gopherus polyphemus) by both sexes. Behavioural Processes 183:104314. DOI: 10.1016/j.beproc.2021.104314.
- Kelley MD, Mendonça MT. 2021. Mental gland secretions as a social cue in gopher tortoises (Gopherus polyphemus): Tortoise presence stimulates and maintains social behaviour with chemical cues. Acta Ethologica 24(1):1–8. DOI: 10.1007/s10211-020-00353-8.
- Khannoon ER, El-Gendy A, Hardege JD. 2011. Scent marking pheromones in lizards: Cholesterol and long chain alcohols elicit avoidance and aggression in male Acanthodactylus boskianus (Squamata: Lacertidae). Chemoecology 21(3):143–149. DOI: 10.1007/s00049-011-0076-4.
- Kikuyama S, Toyoda F, Ohmiya Y, Matsuda K, Tanaka S, Hayashi H. 1995. Sodefrin: A female-attracting peptide pheromone in newt cloacal glands. Science 267(5204):1643–1645. DOI: 10.1126/science.7886452.
- Kimmel CE. 1980. A diet and reproductive study for selected species of Malaysian turtles. Masters Thesis. Charleston, IL: Eastern Illinois University.
- Lazar J, Rasmussen L, Greenwood DR, Bang I-S, Prestwich GD. 2004. Elephant albumin: A multipurpose pheromone shuttle. Chemistry & Biology 11(8):1093–1100. DOI: 10.1016/j.chembiol.2004.05.018.
- Lewis CH, Molloy SF, Chambers RM, Davenport J. 2007. Response of common musk turtles (Sternotherus odoratus) to intraspecific chemical cues. Journal of Herpetology 41(3):349–353. DOI: 10.1670/0022-1511(2007)41[349:ROCMTS]2.0.CO;2.
- Liu Y-X, Davy CM, Shi H-T, Murphy RW. 2013. Sex in the half-shell: A review of the functions and evolution of courtship behavior in freshwater turtles. Chelonian Conservation and Biology 12(1):84–100. DOI: 10.2744/CCB-1037.1.
- López P, Gabirot M, Martín J. 2009. Immune activation affects chemical sexual ornaments of male Iberian wall lizards. Naturwissenschaften 96(1):65–69. DOI: 10.1007/s00114-008-0451-3.
- Lovich JE, Ennen JR, Agha M, Gibbons JW. 2018. Where have all the turtles gone, and why does it matter? BioScience 68(10):771–781. DOI: 10.1093/biosci/biy095.
- Maex M, Treer D, De Greve H, Proost P, Van Bocxlaer I, Bossuyt F. 2018. Exaptation as a mechanism for functional reinforcement of an animal pheromone system. Current Biology 28(18):2955–2960. e2955. DOI: 10.1016/j.cub.2018.06.074.
- Maex M, Van Bocxlaer I, Mortier A, Proost P, Bossuyt F. 2016. Courtship pheromone use in a model urodele, the Mexican axolotl (Ambystoma mexicanum). Scientific Reports 6(1):1–9. DOI: 10.1038/srep20184.
- Mangiacotti M, Baeckens S, Fumagalli M, Martín J, Scali S, Sacchi R. 2023. Protein–lipid association in lizard chemical signals. Integrative Organismal Biology 5(1):obad016. DOI: 10.1093/iob/obad016.
- Mangiacotti M, Baeckens S, Scali S, Martín J, Van Damme R, Sacchi R. 2021. Evolutionary and biogeographical support for species-specific proteins in lizard chemical signals. Biological Journal of the Linnean Society 134(4):912–928. DOI: 10.1093/biolinnean/blab131.
- Mangiacotti M, Gaggiani S, Coladonato AJ, Scali S, Zuffi MAL, Sacchi R. 2019. First experimental evidence that proteins from femoral glands convey identity-related information in a lizard. Acta Ethologica 22(1):57–65. DOI: 10.1007/s10211-018-00307-1.
- Martín J, López P. 2006. Links between male quality, male chemical signals, and female mate choice in Iberian rock lizards. Functional Ecology 20(6):1087–1096. DOI: 10.1111/j.1365-2435.2006.01183.x.
- Martín J, López P. 2011. Pheromones and reproduction in reptiles. In: Norris D, Lopez K, editors Hormones and reproduction of vertebrates, reptiles. Vol. 3. San Diego, California: Academic Press. pp. 141–167.
- Mason RT, Parker MR. 2010. Social behavior and pheromonal communication in reptiles. Journal of Comparative Physiology A 196(10):729–749. DOI: 10.1007/s00359-010-0551-3.
- Molavian H, Madani Tonekaboni A, Kohandel M, Sivaloganathan S. 2015. The synergetic coupling among the cellular antioxidants glutathione peroxidase/peroxiredoxin and other antioxidants and its effect on the concentration of H2O2. Scientific Reports 5(1):136201. DOI: 10.1038/srep13620.
- Munoz A. 2004. Chemo-orientation using conspecific chemical cues in the stripe-necked terrapin (Mauremys leprosa). Journal of Chemical Ecology 30(3):519–530. DOI: 10.1023/B:JOEC.0000018626.55609.31.
- Mykytowycz R, Goodrich B. 1974. Skin glands as organs of communication in mammals. The Journal of Investigative Dermatology 62(3):124–131. DOI: 10.1111/1523-1747.ep12676776.
- Oksanen J, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D. 2019. Vegan: Community ecology package. R package version 2.5–4.
- Pereira AG, Sterli J, Moreira FR, Schrago CG. 2017. Multilocus phylogeny and statistical biogeography clarify the evolutionary history of major lineages of turtles. Molecular Phylogenetics and Evolution 113:59–66. DOI: 10.1016/j.ympev.2017.05.008.
- Poschadel JR, Meyer-Lucht Y, Plath M. 2006. Response to chemical cues from conspecifics reflects male mating preference for large females and avoidance of large competitors in the European pond turtle, Emys orbicularis. Behaviour 143(5):569–587. DOI: 10.1163/156853906776759510.
- Quagliata S, Malentacchi C, Delfino C, Brunasso AM, Delfino G. 2006. Adaptive evolution of secretory cell lines in vertebrate skin. Caryologia 59(2):187–206. DOI: 10.1080/00087114.2006.10797915.
- Quay WB. 1986. Scent glands. In: Bereiter-Hahn J, Matoltsy A, Richards K, editors Biology of the integument, vol 2. Vertebrates. Berlin Heidelberg New York Tokyo: Springer. pp. 357–373.
- Quinn VS, Graves BM. 1998. Home pond discrimination using chemical cues in Chrysemys picta. Journal of Herpetology 32(3):457–461. DOI: 10.2307/1565467.
- Rose FL. 1970. Tortoise chin gland fatty acid composition: Behavioral significance. Comparative Biochemistry and Physiology 32(3):577–580. DOI: 10.1016/0010-406X(70)90475-5.
- Rose FL, Drotman R, Weaver WG. 1969. Electrophoresis of chin gland extracts of Gopherus (tortoises). Comparative Biochemistry and Physiology 29(2):847–851. DOI: 10.1016/0010-406X(69)91637-5.
- Sampaio JL, Gerl MJ, Klose C, Ejsing CS, Beug H, Simons K, Shevchenko A. 2011. Membrane lipidome of an epithelial cell line. Proceedings of the National Academy of Sciences of the United States of America 108:1903–1907. DOI: 10.1073/pnas.1019267108.
- Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. 2011. Global quantification of mammalian gene expression control. Nature 473(7347):337–342. DOI: 10.1038/nature10098.
- Smith TJ. 1995. MolView: A program for analyzing and displaying atomic structures on the Macintosh personal computer. Journal of Molecular Graphics 13(2):122–125. DOI: 10.1016/0263-7855(94)00019-O.
- Stanford CB, Iverson JB, Rhodin AG, van Dijk PP, Mittermeier RA, Kuchling G, Berry KH, Bertolero A, Bjorndal KA, Blanck TE, Buhlmann KA, Burke RL, Congdon JD, Diagne T, Edwards T, Eisemberg CC, Ennen JR, Forero-Medina G, Frankel M, Fritz U, Gallego-García N, Georges A, Gibbons JW, Gong S, Goode EV, Shi HT, Hoang H, Hofmeyr MD, Horne BD, Hudson R, Juvik JO, Kiester RA, Koval P, Le M, Lindeman PV, Lovich JE, Luiselli L, McCormack TEM, Meyer GA, Páez VP, Platt K, Platt SG, Pritchard PCH, Quinn HR, Roosenburg WM, Seminoff JA, Shaffer HB, Spencer R, Van Dyke JU, Vogt RC, Walde AD. 2020. Turtles and tortoises are in trouble. Current Biology 30(12):R721–R735. DOI: 10.1016/j.cub.2020.04.088.
- Surma MA, Herzog R, Vasilj A, Klose C, Christinat N, Morin‐Rivron D, Simons K, Masoodi M, Sampaio JL. 2015. An automated shotgun lipidomics platform for high throughput, comprehensive, and quantitative analysis of blood plasma intact lipids. European Journal of Lipid Science & Technology 117(10):1540–1549. DOI: 10.1002/ejlt.201500145.
- Tellkamp F, Lang F, Ibáñez A, Abraham L, Quezada G, Günther S, Looso M, Tann FJ, Müller D, Cemic F, Hemberger J, Steinfartz S, Krüger M. 2020. Proteomics of Galápagos marine iguanas links function of femoral gland proteins to the immune system. Molecular & Cellular Proteomics 19(9):1523–1532. DOI: 10.1074/mcp.RA120.001947.
- Templeton EM, Pilbrow AP, Kleffmann T, Pickering JW, Rademaker MT, Scott NJ, Ellmers LJ, Charles CJ, Endre ZH, Richards AM, Cameron VA, Lassé M. 2023. Comparison of SPEED, S-Trap, and In-solution-based sample preparation methods for mass spectrometry in kidney tissue and plasma. International Journal of Molecular Sciences 24(7):6290. DOI: 10.3390/ijms24076290.
- Thumuluri V, Almagro Armenteros JJ, Johansen AR, Nielsen H, Winther O. 2022. DeepLoc 2.0: Multi-label subcellular localization prediction using protein language models. Nucleic Acids Research 50(W1):W228–W234. DOI: 10.1093/nar/gkac278.
- Toledo RD, Jared C. 1995. Cutaneous granular glands and amphibian venoms. Comparative Biochemistry and Physiology Part A, Physiology 111(1):1–29. DOI: 10.1016/0300-9629(95)98515-I.
- Treer D, Maex M, Van Bocxlaer I, Proost P, Bossuyt F. 2018. Divergence of species‐specific protein sex pheromone blends in two related, nonhybridizing newts (Salamandridae). Molecular Ecology 27(2):508–519. DOI: 10.1111/mec.14398.
- Tyanova S, Temu T, Cox J. 2016. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nature Protocols 11(12):2301–2319. DOI: 10.1038/nprot.2016.136.
- Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J. 2016. The Perseus computational platform for comprehensive analysis of (prote) omics data. Nature Methods 13(9):731–740. DOI: 10.1038/nmeth.3901.
- UniProt Consortium. 2021. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Research 49(D1):D480–D489. DOI: 10.1093/nar/gkaa1100.
- Van Bocxlaer I, Treer D, Maex M, Vandebergh W, Janssenswillen S, Stegen G, Kok P, Willaert B, Matthijs S, Martens E. 2015. Side-by-side secretion of Late Palaeozoic diverged courtship pheromones in an aquatic salamander. Proceedings of the Royal Society B: Biological Sciences 282:20142960. DOI: 10.1098/rspb.2014.2960.
- Waagen GN. 1972. Musk glands in recent turtles. Unpublished Master of Science thesis, University of Utah.
- Wachowiak M, Cohen LB, Zochowski MR. 2002. Distributed and concentration-invariant spatial representations of odorants by receptor neuron input to the turtle olfactory bulb. Journal of Neurophysiology 87(2):1035–1045. DOI: 10.1152/jn.00522.2001.
- Wang Z, Pascual-Anaya J, Zadissa A, Li W, Niimura Y, Huang Z, Li C, White S, Xiong Z, Fang D, Wang B, Ming Y, Chen Y, Zheng Y, Kuraku S, Pignatelli M, Herrero J, Beal K, Nozawa M, Li Q, Wang J, Zhang H, Yu L, Shigenobu S, Wang J, Liu J, Flicek P, Searle S, Wang J, Kuratani S, Yin Y, Aken B, Zhang G, Irie N. 2013. The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nature Genetics 45(6):701–706. DOI: 10.1038/ng.2615.
- Winokur RM, Legler JM. 1975. Chelonian mental glands. Journal of Morphology 147(3):275–291. DOI: 10.1002/jmor.1051470303.
- Woodley SK, Staub NL. 2021. Pheromonal communication in urodelan amphibians. Cell and Tissue Research 383(1):327–345. DOI: 10.1007/s00441-020-03408-1.
- Wu Z, Shon JC, Liu K-H. 2014. Mass spectrometry-based lipidomics and its application to biomedical research. Journal of Lifestyle Medicine 4(1):17–33. DOI: 10.15280/jlm.2014.4.1.17.
- Wyatt TD. 2003. Pheromones and animal behaviour: Communication by smell and taste. Cambridge, UK: Cambridge University Press.
- Wyatt TD. 2014. Proteins and peptides as pheromone signals and chemical signatures. Animal Behaviour 97:273–280. DOI: 10.1016/j.anbehav.2014.07.025.