ABSTRACT
Objective: Autism spectrum disorders (ASDs) is a heterogeneous neuropsychiatric disorder with widespread abnormalities of social interaction and communication, showing severely restricted interests and extreme repetitive behavior. The relationship between aggressive behavior, insensitivity to pain, and ASDs could not be explained completely. Therefore, we aimed to contribute to the etiology by examining the gene expressions of OPRL1, TACR1, and HTR1E in patients with ASDs.
Methods: This study was held in the Genome and Stem Cell Research Center of Erciyes University. In this present study, the expressions of OPRL1, TACR1, and HTR1E genes were studied in 22 ASD patients and 14 healthy controls. Quantitative Real-time Polymerase Chain Reaction (qRT-PCR) was used for gene expression studies.
Results: There was a statistically significant difference in terms of the expression of the three genes, which we examined between the ASD patient and control groups. Positive and strong correlations were obtained between the three gene expressions in the ASD group and this finding was found to be statistically significant.
Conclusions: Considering all these findings, large-scale and new researches are needed for revealing the roles of genes and their pathways which are related to aggression and insensitivity to pain in ASDs. Our results will lead to new research studies in this field.
Introduction
Autism spectrum disorders (ASDs) are manifested by common social interaction and communication abnormalities, a severe degree of limited interest, and excessive repetitive behaviors [Citation1,Citation2]. It is observed four times more commonly in boys than in girls [Citation3]. Except for these core symptoms, approximately 31% of ASD patients will also have intellectual disability (ID) and 20–25% will have seizure disorder [Citation4,Citation5]. ASD etiopathogenesis remains largely unknown, including genetic and environmental risk factors [Citation6]. Genetic studies can point out the genes and pathways that bridge the gap between behavior and biology [Citation7].
It is hypothesized that ASD is related to aggression, but very little research has been done so far [Citation8]. The available limited data report correlations of autism with aggression in intellectually disabled children [Citation9]. So, aggression can be acceptable as a risk factor for ASD cases with ID [Citation10]. Insensitivity to pain has been included as one of the diagnostic criteria in Diagnostic and Statistical Manual of Mental Disorders (DSM-5) since May 2013 [Citation11]. The prevalence of insensitivity to pain is approximately 25–40% in ASD [Citation12]. ASD patients may react to stimulations from one or more senses (taste, touch, hearing, sight, etc.) excessively or may be unresponsive [Citation11,Citation12]. The patients can be highly insensitive to pain or tolerant to pain [Citation13]. Both experimental and case studies imply that there is insensitivity to pain in ASD cases [Citation13–17]. The pain assessment of individuals with ASD is largely unexplained [Citation18,Citation19].
The three genes that are also expressed in whole blood were selected for this pilot study. To the best of our knowledge, these genes were not examined in ASD patients in the current literature. OPRL1 (opiate receptor) gene is a G protein-dependent receptor and it plays a role in a large number of brain activities, especially in the regulation of instinctive and emotional behaviors [Citation20,Citation21]. All of the opioid receptor subfamilies have revealed exciting opportunities for mechanistic insight into the molecular mechanisms underlying the biology of nociception, reward, and higher cognitive functions, as well as promises for progress in several clinical areas such as pain management and mood [Citation16]. TACR1 (tachykinin receptor) belongs to the family of tachykinin receptors. This gene encodes the receptor of tachykinin P substance, referred to as neurokinin 1 (NK1) [Citation22]. It has been shown in earlier studies that the variants of TACR1 are related to aggression and chronic pain [Citation20,Citation22,Citation23]. HTR1E (5-hydroxytryptamine [serotonin] receptor 1 E) is a protein-encoding gene connected to the G protein. HTR1E is a gene which was studied in the groups with attention-deficit hyperactivity disorder (ADHD), and was reported to be related to aggressive behavior [Citation24,Citation25].
These clinical findings which have not yet been fully explained are the objective of this research. In this preliminary study, within the context of clinical symptoms of aggression and insensitivity to pain, we aimed to examine the OPRL1, TACR1, and HTR1E gene expressions which were expressed in blood and also to reveal their relationships to ASDs.
Methods
Subjects
Twenty-two children were selected from the patients diagnosed with ASD after an evaluation according to the DSM-5 criteria by two different psychiatrists at the Erciyes University School of Medicine, Department of Child Psychiatry. These patients were recruited from the ones followed up with the diagnosis of ASD who first presented in the outpatient clinics of Child Psychiatry and Child Neurology, Erciyes University Medical Faculty, with complaints of speech, social, and communication delay. Taking into account the selected patients’ age and gender, 14 healthy individuals without any chronic medical, psychiatric, and genetic conditions and not taking any psychiatric or other medications, between the ages of 2 and 10 years, were enrolled as controls. The intellectual profile of the ASD patients and healthy controls was determined with the Wechsler Intelligence Scale for Children-Revised (WISC-R) scale [Citation26] and Ankara Developmental Screening Inventory (ADSI) [Citation27]. The patients and control group were selected from the same location and ethnic origin. The study was approved by the Ethics Committee of Erciyes University. Written informed consents were obtained from the parents of the patients. The study was conducted in accordance with the guidelines of the Helsinki Declaration. Information about the demographic and clinical characteristics of the patients was collected using a socio-demographic form, which was prepared by the researchers. Socio-demographic data form, Childhood Autism Rating Scale (CARS), and ADSI were used in this study.
Instruments
The socio-demographic data form was prepared in order to obtain information about the patients’ first symptoms, prenatal and developmental characteristics, any kind of treatment received (pharmacotherapy, special training, etc.), and their current complaints. In addition to the socio-demographic form, further information regarding any medical treatments or genetic disorders were asked and recorded by the researchers.
Childhood Autism Rating Scale (CARS)
This scale consists of ratings in 15 different areas of functioning significant for autism, which include relating to people; imitation; emotional response; body use; object use; adaptation to change; visual response; listening response; taste, smell, and touch response and use; fear or nervousness; verbal communication; non-verbal communication; activity level; level and consistency of intellectual response; and general impressions. The child is rated on observed behavior by a 7-point Likert-type scale ranging from 1 to 4, with intermediate values between the units. The relationships of children with other people and their body and object use, their adaptation to change, their verbal and non-verbal communication and imitation skills, as well as their sensory properties, fears, and activity levels are evaluated and scored by professionals. A score of “one point” is defined as normal behavior, whereas “four points” is described as abnormal and inappropriate behavior. In CARS, scores of 38‒60 points constitute an autistic group showing severe symptoms, scores of 30‒38 points constitute an autistic group showing mild to moderate symptoms, while scores of 15‒29 constitute a group of non-autistic symptoms.
Ankara Developmental Screening Inventory (ADSI)
ADSI is a widely used developmental screening test for children under six years of age in Turkey. It was developed by Savasir and her colleagues in 1992, consisting a total of 154 questions for the mother and the child. The questions represent the different but related areas of development. The ADSI yields four dimensional scores: cognitive language score, fine motor score, gross motor score, and social and self-help score. The total of these four scores is the general developmental score. The raw scores of general development are then converted to standard T scores, which are compared with the scores of children in the same-age normative groups.
Genetic studies
Two milliliter of blood was collected from all the participants and these samples were taken before the patients’ treatment. All of the genetic studies were performed at the Erciyes University Genome and Stem Cell Center (GENKOK). Total RNA was extracted with TRIzol reagent from whole blood samples of the groups (Roche, Germany). The quantity (absorbance at 260 nm) and quality (ratio of absorbance at 260 and 280 nm) of RNA were estimated with a BioSpec-Nano Spectrophotometer. First-strand complementary DNA (cDNA) was synthesized from total RNA with the Transcriptor High Fidelity cDNA Synthesis kit (Roche, Germany) according to the manufacturer’s instructions. Gene expressions were assessed by quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) with RotorGene (Qiagen, Germany). Each sample was studied double and beta-actine (ACTB) was used as the endogenous reference. The changes in gene expressions between the case and control groups were determined by the ΔΔCt method of relative quantification.
Statistical analysis
To assess data normality, histogram and q–q plots were examined; also Shapiro–Wilk’s test was performed. Levene test was used to test variance homogeneity. To compare the differences between patient groups, a two-sided independent samples t test or Mann–Whitney U test was used for continuous variables and Fisher’s exact test was used for categorical variables. Spearman’s correlation analysis was used to determine the linear association between genes in ASDs. Values were expressed as frequencies and percentages, mean and standard deviation, or median (25‒75% percentiles). Analyzes were conducted using R 3.0.3 (www.r-poject.org) software. The alpha level of 0.05 was set up to indicate significance.
Results
Twenty-two patients were diagnosed with ASD. The average age of the ASD patients was 4.79 ± 2.20 years. Eighteen of the ASD patients (81.8%) were male and four (18.2%) were female. The control group consisted of 10 (71.4%) boys and 4 (28.6%) girls. The mean age of the control group was 5.71 ± 2.33 years. Age and gender differences were not statistically significant between the two study groups (p = .244 and p = .683, respectively). According to the socio-demographic data form, 50% of the patients had aggression. Insensitivity to pain symptoms was observed in 23% of the patients (). When the patients were evaluated according to their intellectual functioning, 15 (34.1%) of the autism patients were diagnosed as intellectually disabled. All of the clinical findings of the patients are presented in .
Table 1. Clinical and socio-demographic features of ASD patients.
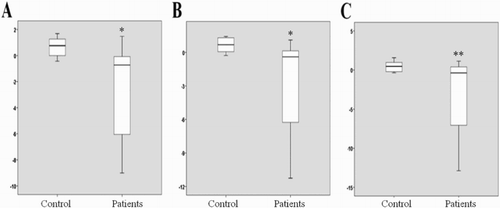
The expressions of the three genes were statistically significantly lower in ASD patients compared to that in the control group. As for the separate evaluation of groups, the OPRL1 gene expression was 0.61 (0.02–0.95) in the ASD group and 1.69 (1.00–2.41) in controls, with a p value of less than .001; the TACR1 gene expression was 0.76 (0.01–1.11) in the ASD group and 1.62 (1.05–2.49) in the control group, with a p value of less than .001. The HTR1E gene expression of the ASD group was 0.78 (0.01–1.33) and 1.39 (0.87–1.97) in the control group (p = .007) ().
Figure 1. (A) OPRL1 gene expression between the groups (p < .001), (B) TACR1 gene expression between the groups (p < .001), (C) HTR1E gene expression between the groups (p = .007).
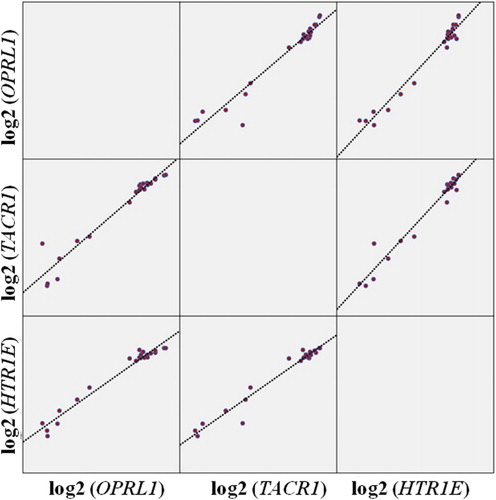
The association between the three gene expressions was found to be statistically significant, with positive and strong correlations in the ASD group (OPRL1–TACR1: rho = 0.960, p < .001; OPRL1–HTR1E: rho = 0.892, p < .001; TACR1–HTR1E: rho = 0.900, p < .001) (). When the ASD group was evaluated in terms of the parameter of insensitivity to pain, the HTR1E gene expression of the positive group was found to be statistically significant and lower compared to the negative group (p = .038), while the OPRL1 and TACR1 gene expressions observed were lower in this group but not statistically significant.
Figure 2. Displaying the scatterplot of the relationship between the OPRL1, TACR1, and HTR1E genes in the ASD group.
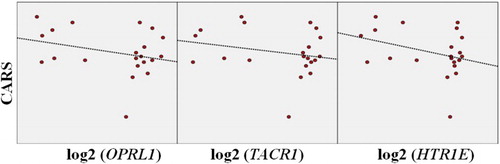
A negative correlation was observed when the CARS score and the gene expressions were compared, but these findings were not statistically significant (r = −0.292, r = −0.231, r = −0.333, respectively; p > .05) ().
Discussion
ASD is primarily a complex genetic disorder, but it has been also accepted that it shows genetic heterogeneity [Citation1]. In this preliminary study, the whole blood mRNA expressions of OPRL1, TACR1, and HTR1E related to aggression and pain have been examined in ASD patients. Our findings, namely, the comparison of clinical features of aggression and insensitivity to pain and OPRL1, TACR1, and HTR1E gene expressions could provide a new contribution in understanding the neurobiology of ASDs.
It has been known that children with autism have unusual reactions to sensory stimuli. This characteristic of children with autism, independent from age and IQ development, is one of the significant characteristics differentiating them from normal developing peers [Citation28]. Pain assessment of individuals with ASD is largely unexplained [Citation18]. Studying insensitivity to pain, which is a part of unusual response, is the objective of our research, but studies regarding the perception of pain and its form of expression in individuals with ASD are limited. Some of these studies are case studies, whereas others are in the form of empirical studies. The pressure pain threshold was measured in a study, which indexes sensitization of peripheral nociceptors, and assessed subjective ratings of unpleasantness and pain intensity in response to empathy-eliciting stimuli depicting physical bodily injuries in three age- and sex-matched participant groups; namely, ASD, conduct disorder symptoms (CDS), and typically developing controls (TDC) [Citation29]. Their findings indicated that the pain threshold was lowest in the ASD group and highest in the CDS group. The genes of OPRL1 and TACR1 were associated with insensitivity to pain. In our study, it has been observed that the expression of OPRL1 and TACR1 genes is statistically significant (p < .001); thus, it supports the relationship between insensitivity to pain and autism. Although there are no available data in the literature associated with autism and TACR1 gene expression, these data may be very important. It is clear that there is a need for more genetic studies about pain-related genes in individuals with ASD with larger sample sizes.
Our knowledge about aggression in children with ASD is also insufficient. However, the available data indicated that aggression is common in patients with ASD. In a study conducted interviews with families was selected and method of aggression in patients with ASD was found to be 65% independently from ID [Citation30]. Again interviewing with the families of patients with ASD was selected in another study in which the parents of 67 children were interviewed. It was reported that two-thirds of these children had been having severe temper tantrums and one-third of these children had manifested aggression in the past [Citation31]. A broader study shows that in more than 50% of ASDs, physical aggression is seen as a snapshot of clinical finding, while 70% stated that aggression against caregivers was observed in their life span [Citation32,Citation33]. Many studies in ASDs have emphasized that there is no change in the level of aggression with age [Citation31,Citation32–Citation34]. Aggression has a clear relationship with HTR1E. Low serotonergic activity correlates with increased impulsive-aggressive behavior. In 2008, HTR1E (rs1406946) gene polymorphism was evaluated with other serotonergic system gene polymorphisms in the ADHD group. This study revealed that HTR1E polymorphism was under-expressed in aggressive behavioral impulsivity [Citation25]. Similar to this result, the expression of HTR1E gene was lower (p = .007) in our ASD group. Our study is different from Oades et al.’s study by examining the gene expression of HTR1E. Therefore, we thought that this is an important finding supporting the characteristic of aggression in children with ASD. As a limitation, in this preliminary study, we selected only the three genes to evaluate the symptoms of aggression and pain in a small-sized ASD group. Further studies with larger samples are required for future genetic studies.
In conclusion, the expressions of genes are low in ASD cases compared to that in the control group; in other words, all of the gene expressions are decreased in ASD cases. This situation also supports that these three genes can be associated with ASD. However, whether these symptoms are related to etiology or are a result of the disorder needs to be clarified in further studies. Considering all these findings, there is a need to examine other genes associated with aggression and insensitivity to pain in future studies in ASD patients. Multidisciplinary studies using animal models to reveal this relationship using both tissue samples and blood are needed. We conclude that larger sample sizes may lead to more significant results, which may reveal the negative correlation observed between the expression levels of these three genes and CARS scores in the ASD group.
Acknowledgements
We would like to thank Erciyes University Genome and Stem Cell Center.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sener EF. Association of copy number variations in autism spectrum disorders: a systematic review. Chin J Biol. 2014;2014:9. doi: 10.1155/2014/713109
- Helsmoortel C, Swagemakers SM, Vandeweyer G, et al. Whole genome sequencing of a dizygotic twin suggests a role for the serotonin receptor HTR7 in autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet. 2016;171(8):1049–1056. doi: 10.1002/ajmg.b.32473
- AlSagob M, Colak D, Kaya N. Genetics of autism spectrum disorder: an update on copy number variations leading to autism in the next generation sequencing era. Discov Med. 2015;19(106):367–379.
- Sener EF, Canatan H, Ozkul Y. Recent advances in autism spectrum disorders: applications of whole exome sequencing technology. Psychiatry Investig. 2016;13(3):255–264. doi: 10.4306/pi.2016.13.3.255
- Bourgeron T. Current knowledge on the genetics of autism and propositions for future research. C R Biol. 2016;339(7–8):300–307. doi: 10.1016/j.crvi.2016.05.004
- Sener EF, Cikili UM, Korkmaz-Bayramov K, et al. The roles of CC2D1A and HTR1A gene expressions in autism spectrum disorders. Metab Brain Dis. 2016;31(3):613–619. doi: 10.1007/s11011-016-9795-0
- Robinson EB, Neale BM, Hyman SE. Genetic research in autism spectrum disorders. Curr Opin Pediatr. 2015;27(6):685–691. doi: 10.1097/MOP.0000000000000278
- Farmer CA, Aman MG. Psychometric properties of the children’s scale of hostility and aggression: reactive/proactive (C-SHARP). Res Dev Disabil. 2010;31(1):270–280. doi: 10.1016/j.ridd.2009.09.014
- Benson BA, Brooks WT. Aggressive challenging behaviour and intellectual disability. Curr Opin Psychiatry. 2008;21(5):454–458. doi: 10.1097/YCO.0b013e328306a090
- Holden B, Gitlesen JP. A total population study of challenging behaviour in the county of Hedmark, Norway: prevalence, and risk markers. Res Dev Disabil. 2006;27(4):456–465. doi: 10.1016/j.ridd.2005.06.001
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013.
- Moore DJ. Acute pain experience in individuals with autism spectrum disorders: a review. Autism. 2015;19(4):387–399. doi: 10.1177/1362361314527839
- Allely CS. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. Sci World J. 2013;2013. Article id: 916178. doi: 10.1155/2013/916178
- Elwin M, Ek L, Schroder A, et al. Autobiographical accounts of sensing in Asperger syndrome and high-functioning autism. Arch Psychiatr Nurs. 2012;26(5):420–429. doi: 10.1016/j.apnu.2011.10.003
- Mieres AC, Smallwood V, Nicholson SK. Retrospective case report: evaluation of pain in a child with pervasive developmental disorder. Pediatr Phys Ther. 2011;23(2):194–200. doi: 10.1097/PEP.0b013e318218f35f
- Provasi D. Computational structural biology of opioid receptors. Methods Mol Biol. 2015;1230:13–38. doi: 10.1007/978-1-4939-1708-2_2
- Ross-Russell M, Sloan P. Autoextraction in a child with autistic spectrum disorder. Br Dent J. 2005;198(8):473–474. doi: 10.1038/sj.bdj.4812250
- Ely E, Chen-Lim ML, Carpenter KM, et al. Pain assessment of children with autism spectrum disorders. J Dev Behav Pediatr. 2016;37(1):53–61. doi: 10.1097/DBP.0000000000000240
- MacLean WE Jr, Tervo RC, Hoch J, et al. Self-injury among a community cohort of young children at risk for intellectual and developmental disabilities. J Pediatr. 2010;157(6):979–983. doi: 10.1016/j.jpeds.2010.05.052
- Giegling I, Rujescu D, Mandelli L, et al. Tachykinin receptor 1 variants associated with aggression in suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(6):757–761. doi: 10.1002/ajmg.b.30506
- Henderson G, McKnight AT. The orphan opioid receptor and its endogenous ligand- nociceptin/orphanin FQ. Trends Pharmacol Sci. 1997;18(8):293–300. doi: 10.1016/S0165-6147(97)90645-3
- Steinhoff MS, von Mentzer B, Geppetti P, et al. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265–301. doi: 10.1152/physrev.00031.2013
- Yan TC, McQuillin A, Thapar A, et al. NK1 (TACR1) receptor gene ‘knockout’ mouse phenotype predicts genetic association with ADHD. J Psychopharmacol. 2010;24(1):27–38. doi: 10.1177/0269881108100255
- Turecki G, Sequeira A, Gingras Y, et al. Suicide and serotonin: study of variation at seven serotonin receptor genes in suicide completers. Am J Med Genet B Neuropsychiatr Genet. 2003;118B(1):36–40. doi: 10.1002/ajmg.b.10006
- Oades RD, Lasky-Su J, Christiansen H, et al. The influence of serotonin- and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): findings from a family-based association test (FBAT) analysis. Behav Brain Funct. 2008;4:48. doi: 10.1186/1744-9081-4-48
- Wechsler D. Wechsler intelligence scale for children-revised. New York: Psychological Corporation; 1974.
- Savaşır I, Sezgin N, Erol N. 0-6 Yaş Çocukları için Gelişim Tarama Envanteri Geliştirilmesi: Ön Çalışmalar. Türk Psikiyatri Dergisi. 1992;3(2):33–38.
- Wing L, Gould J, Gillberg C. Autism spectrum disorders in the DSM-V: better or worse than the DSM-IV? Res Dev Disabil. 2011;32(2):768–773. doi: 10.1016/j.ridd.2010.11.003
- Chen C, Hung AY, Fan YT, et al. Linkage between pain sensitivity and empathic response in adolescents with autism spectrum conditions and conduct disorder symptoms. Autism Res. 2016. DOI:10.1002/aur.1653
- Totsika V, Hastings RP, Emerson E. A population-based investigation of behavioural and emotional problems and maternal mental health: associations with autism spectrum disorder and intellectual disability. J Child Psychol Psychiatry. 2011;52(1):91–99. doi: 10.1111/j.1469-7610.2010.02295.x
- Dominick KC, Davis NO, Lainhart J, et al. Atypical behaviors in children with autism and children with a history of language impairment. Res Dev Disabil. 2007;28(2):145–162. doi: 10.1016/j.ridd.2006.02.003
- Kanne SM, Mazurek MO. Aggression in children and adolescents with ASD: prevalence and risk factors. J Autism Dev Disord. 2011;41(7):926–937. doi: 10.1007/s10803-010-1118-4
- Mazurek MO, Kanne SM, Wodka EL. Physical aggression in children and adolescents with autism spectrum disorders. Res Autism Spectr Disord. 2013;7(3):455–465. doi: 10.1016/j.rasd.2012.11.004
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disord. 2006;36(8):1101–1114. doi: 10.1007/s10803-006-0147-5