ABSTRACT
Objectives: Interferon (IFN) treatment has many neuropsychiatric side effects such as depression and anxiety disorder. Although untreated depression is a major contributor to dosage reduction or premature discontinuation of the IFN treatment, it is found that depressive symptoms among patients undergoing hepatitis C virus (HCV) treatment are commonly overlooked during routine clinical interviews. Besides depression, anxiety disorders are shown to affect adherence to pegylated IFN (Peg-IFN) treatment in patients with hepatitis C. Despite the occurrence of neuropsychiatric side effects of IFN treatment being widely reported in patients with hepatitis C, there are few studies studying patients infected with hepatitis B virus (HBV). The aim of this prospective study was to evaluate the incidence and risk factors of depressive and anxiety disorders that occur during Peg-IFN treatment of patients with HBV.
Methods: The sample consisted of volunteer patients who were diagnosed with HBV infection and who were decided to receive IFN treatment. During the study period, all consecutive patients with HBV infection and who would have IFN treatment were informed about the study and invited to participate. Thirty-seven chronic hepatitis B (CHB) patients were recruited for the study, but four of them were excluded due to psychiatric diagnosis at the initiation of the treatment. Therefore, the sample consisted of 33 patients with CHB, meeting the inclusion criteria. The participants had psychiatric assessment before the treatment and at 1st, 3rd, 6th, and 12th months. At each visit, the subjects were assessed with Clinical Global Impressions Scale, Hamilton Rating Scale for Depression (HRSD), and Hamilton Anxiety Rating Scale (HAM-A).
Results: Among the 33 patients with HBV, 22 (66.7%) were men. Mean age was 35.97 ± 10.73 years. While follow-up, 6 patients dropped out from the study. Also, 13 patients were excluded from the study as they developed depression and/or anxiety disorder. Mean baseline HRSD and HAM-A scores were smaller than the following visits’ scores. The difference was not statistically significant only for the 12th month assessments. Totally 14 (42.4%) patients developed depression/anxiety disorder during 1 year follow-up. Six (42.86%) of them received the diagnosis at the 1st month, 3 (21.43%) at the 3rd, 4 (28.57%), at 6th months, and 1 (7.14%) at the 12th month. When we compared the patients who developed depression/anxiety disorder with the patients who did not develop any psychiatric disorder, we found that the mean baseline HRSD score (t = 2.303, p = .028) and female percentage (p = .017) were statistically significantly higher in the depression/anxiety disorder group.
Conclusions: There is an incidence of 42.4% for depression and/or anxiety disorders during Peg-IFN treatment. Females and patients with subsyndromal depressive symptoms should be referred to a psychiatrist and closely monitored especially for the first three months of the IFN treatment.
Introduction
Hepatitis B virus (HBV) infection is a major health problem in many countries. It can lead to chronic hepatitis, liver cirrhosis, hepatocellular cancer, and death [Citation1]. The World Health Organization estimates that 240 million people are chronically infected with hepatitis B and more than 780,000 people die every year due to complications of chronic hepatitis B (CHB) [Citation2]. The primary aim of CHB treatment is to permanently suppress HBV replication. Interferon (IFN), which is an endogenous cytokine with antiviral and immunomodulatory effects, has been the mainstay of HBV treatment for a long time [Citation3,Citation4]. It enhances the host immune system to mount a defence against HBV. Pegylation of IFN-α improves its pharmacokinetics and prolongs its half-life; so pegylated interferon (Peg-IFN) had replaced the conventional IFN because of a better pharmacokinetic profile, more convenient once weekly dosing, and superior efficacy [Citation3]. Despite new treatment strategies for CHB, IFN treatment is in use either by monotherapy or by combination [Citation5–7].
IFN treatment has many side effects such as fatigue, myalgia, influenza-like symptoms, alterations in libido, and neuropsychiatric symptoms [Citation4,Citation8]. Neuropsychiatric symptoms may include sadness, irritability, anger, anxiety, emotional instability, fatigue, and changes in sleep and appetite [Citation9]. Severe depression and less commonly suicidal ideation, mania, delirium, and psychosis can occur [Citation9–11]. The pathophysiology of IFN-α-induced behavioral change is not clear. It is supposed that the central role is played by the interaction between the endocrine system, the immune system, the serotoninergic system, and the opioid receptors [Citation4]. Although untreated depression is a major contributor to dosage reduction or premature discontinuation of the IFN treatment, it is found that depressive symptoms among patients undergoing hepatitis C virus (HCV) treatment are commonly overlooked by routine clinical interviews [Citation9,Citation12]. In addition to depression, anxiety disorders are shown to affect adherence to Peg-IFN treatment in patients with hepatitis C [Citation13]. Some risk factors such as female gender, younger age, past history of a psychiatric disorder, higher baseline depression scores, and lower educational level were defined for the emergence of depression in patients with hepatitis C during IFN treatment [Citation9,Citation12,Citation14–16].
Although the occurrence of neuropsychiatric side effects of IFN treatment has been widely reported in patients with hepatitis C, there are a few studies studying patients with hepatitis B. A higher incidence of psychiatric symptoms was shown in chronic hepatitis C (CHC) patients compared to CHB patients who have no treatment [Citation17]. Koskinas et al. [Citation15] found that IFN-induced depression, assessed by the Zung Self-rating Depression Scale, occurs more frequently in CHC patients than in patients with CHB [Citation15]. In the multicentre study of Marcellin et al. [Citation11], quality of life was assessed by Medical Outcomes Study Short-Form Health Survey (SF-36) at treatment weeks 12, 24, 48, and 24 after the end of the treatment in CHB and CHC patients [Citation11]. Stratification of depression-related events showed that they were less frequent in CHB patients compared with CHC patients, irrespective of ethnicity. It was found that Peg-IFN has less impact on health-related quality of life in patients with CHB compared with CHC. In the study of Huang et al. [Citation18], 73 patients with CHB and 85 patients with CHC were assessed with the Hamilton Rating Scale for Depression (HRSD) by trained nurses during 24 or 48 weeks of IFN treatment, and depression was defined as a score higher than 11 [Citation18]. In that study, median baseline HRSD score was lower in CHB patients than in CHC patients and depression was detected more in CHC patients. Pre-existing depression, treatment duration longer than 48 weeks, and hepatitis B envelope antigen (HBeAg) positivity at treatment week eight predicted the presence of more depression, whereas baseline logarithm of serum HBV DNA and HBV DNA level at week 24, and HBeAg positivity at treatment weeks 12, 16, and 20 predicted the presence of less depression.
There is a paucity of data about the neuropsychiatric side effects of IFN in CHB patients. The primary purpose of this prospective study was to determine the incidence of depressive and/or anxiety disorders that occur during Peg-IFN treatment of patients with hepatitis B, by using clinician-rated psychiatric scales and clinical interviews. We also aimed to evaluate the risk factors for developing major depressive disorders and/or anxiety disorders during Peg-IFN treatment.
Methods
This prospective study was performed at the Infectious Diseases and Clinical Microbiology Department and Psychiatry Department of Ege University School of Medicine Hospital.
Participants
The sample consisted of volunteer patients diagnosed with HBV infection and who were decided to receive IFN treatment. During the study period, all consecutive patients with CHB infection and who would have IFN treatment were informed about the study and invited to participate.
The inclusion criteria were as follows: (Citation1) able to provide informed consent, (Citation2) HBV status confirmed by the medical record (HBV viral load based on polymerase chain reaction test at the time of study enrolment), and (Citation3) age above 18 years.
The exclusion criteria were as follows: (Citation1) history of antiviral therapy or chemotherapy for any purpose, (Citation2) currently unstable medical or psychiatric condition, (Citation3) currently using any psychotropic medication, (Citation4) having a diagnosis of psychosis, dementia, or organic brain syndrome, (Citation5) patients with coinfection of two viral hepatitis infections, (Citation6) HIV infection, and (Citation7) using medications that can lead to depression or anxiety disorder.
Thirty-seven CHB patients were enrolled for the study, but four of them were excluded due to current psychiatric diagnosis at the initiation of the treatment. Thus, the study sample consisted of 33 patients with CHB, meeting the inclusion criteria.
Procedure
This study was approved by the local Ethics Committee and by the Ethics Committee of the Turkish Health Ministry. Procedures were in accordance with the Helsinki Declaration of 1975. All subjects were recruited after obtaining proper written informed consent.
The participants had psychiatric assessment by a psychiatrist before the IFN treatment and at the 1st, 3rd, 6th, and 12th months. At the first visit (before the treatment), clinical interviews were conducted using a structured clinical case report form, developed specifically for this study, including prompts to screen patients based on each inclusion criterion, gather relevant demographic data, and assess for a full range of current and past axis I psychiatric disorders using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Besides these, data about liver disease such as HBV DNA and alanine aminotransferase (ALT) level were collected. At each psychiatric visit, the subjects underwent clinician-rated evaluations for depression level using the HRSD [Citation19], for anxiety level using the Hamilton Anxiety Rating Scale (HAM-A) [Citation20], and for severity of psychopathology with the Clinical Global Impressions (CGI) Scale [Citation21]. The adaptation and reliability study of the HRSD and HAM-A for Turkish patients were performed previously [Citation22,Citation23]. Cutoff scores for the Turkish versions of the HRSD and HAM-A were not reported in the Turkish adaptation studies. In the literature, there is variety of cutoff scores for bands of severity on the HRSD. For the 17-item HRSD scale, a cutoff of 7 is generally regarded as appropriate for the diagnosis of depression [Citation24]. During this one year follow-up, patients who had developed clinically significant depression and/or anxiety disorder were referred and initiated proper psychiatric treatment and excluded from the study.
The CHB patients received once weekly injections of 180 μg of Peg-IFN α-2a or 1.5 μg/kg of Peg-IFN α-2b for one year.
Statistics
Data analyzes were performed by Statistical Package for Social Sciences (SPSS), version 16.0 for Windows. The Kolmogorov–Smirnov test was used to analyse the distribution of continuous variables. Quantitative data were presented as mean and standard deviations (SD) if there is normal distribution, and as median and percentiles if there is skewed distribution. Minimum and maximum values were also provided. Fisher’s exact test was used for the comparison of relative frequencies. As it was assumed that the group which developed depression and/or anxiety disorder would have more psychopathology and female predominance, one-sided Fisher’s exact test was used for the comparison of the groups. For the analysis of paired data, paired t test and the Wilcoxon signed-rank test were used, and differences between groups of unpaired observations were analysed by Student’s t-test or the Mann–Whitney U test. The alpha level of 0.05 was set up to indicate statistical significance.
Results
Among the 33 patients with CHB, 22 (66.7%) were male and 11 (33.3%) were female, with a mean age 35.97 ± 10.73 years (between 22 and 60 years). Socio-demographic variables of the sample are shown in . The mean year of education was 8.42 ± 3.79.
Table 1. Socio-demographic and clinical data of the sample.
During follow-up, totally six patients dropped out from the study. Reasons for drop out were treatment failure for two (6.1%) patients, financial difficulties for one (3.0%) patient (he moved to another city and because of financial difficulties he could not come to our university; instead he continued his treatment in another hospital), and unwillingness to be in psychiatric follow-up for three (9.1%) patients. Also, 13 patients were excluded from the study as they were started on antidepressant treatment.
Results of the psychiatric assessment are shown in and and and . Mean baseline HRSD and HAM-A scores were smaller than the following visits’ scores. The difference was not statistically significant only for the 12th month assessments (). In total, 14 (42.4%) patients developed depression/anxiety disorder and were started on antidepressant treatment, during 1 year follow-up. Six (42.86%) of them received the diagnosis at the 1st month, 3 (21.43%) at the 3rd, 4 (28.57%) at the 6th, and 1 (7.14%) at the 12th month. Comparison of the patients who developed depression/anxiety disorder and who did not during the IFN treatment is shown in . Mean of the baseline HRSD score and female percentage were statistically significantly higher in the depression/anxiety disorder group. There were no statistically significant differences for baseline CGI between the groups, but 80% of the patients who were borderline mentally ill before the IFN treatment developed depression/anxiety disorder during the treatment.
Figure 1. Error-bar graph showing mean HRSD score and 95% confidence intervals by the duration of the IFN treatment.
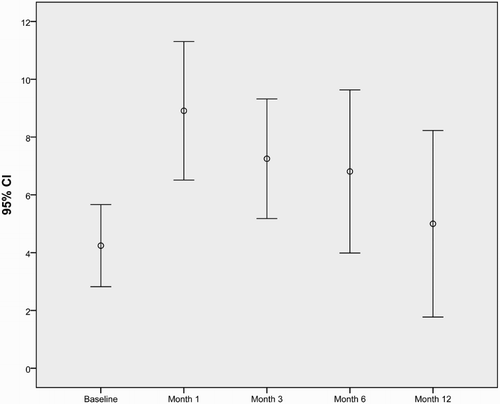
Figure 2. Error-bar graph showing mean HAM-A score and 95% confidence intervals by the duration of the IFN treatment.
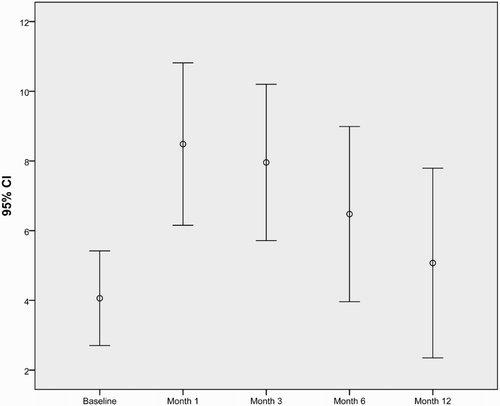
Table 2. Results of the psychiatric scales.
Table 3. CGI results of the study sample.
Table 4. Comparison of patients who developed depression/anxiety disorder during IFN treatment with the other patients.
For the HRSD scale, 22 (66.7%) patients received a score higher than seven at least once during the follow-up. There were no statistically significant differences for gender, marital status, alcohol use, substance use, having past psychiatric diagnosis, past psychiatric treatment, or suicide attempt between the group having HRSD score higher than seven and the remaining group.
Six IFN-treated patients dropped out because of IFN treatment failure, financial difficulties, and unwillingness to be in psychiatric follow-up. These patients have been analysed in depression and/or anxiety disorder-free group, although we do not know their full psychiatric course, as we could not follow them up for a year. Therefore, we made an analysis after the exclusion of this group. In this case, again female percentage was statistically significantly higher in the depression/anxiety disorder group (p = .009). But there were no statistically significant differences for being single, having any lifetime psychiatric diagnosis, any lifetime psychiatric treatment, suicide attempt, being borderline ill in baseline CGI, year of education, baseline HRSD score, baseline HAM-A score, baseline HBV DNA, and baseline ALT between the groups (p > .05).
Discussion
During one year follow-up of the Peg-IFN treatment of patients with CHB, both depression and anxiety scores increased. This is consistent with the literature of IFN treatment in patients with CHC [Citation13]. When we compared the baseline scores with the following visit scores, only the differences between the baseline and the 12 month assessment were not statistically significant for depression and anxiety. This may be because of the exclusion of patients who suffered depression and/or anxiety disorder till then. Only one patient received diagnosis in the last visit. So we can say that in the 12th month, mostly the resilient patients were still in the study.
We found an incidence of 42.4% for depression and/or anxiety disorders during the Peg-IFN treatment of patients with HBV. This incidence is much higher than that found for depression in the study of Huang et al. [Citation18], conducted in Taiwan. In that study among 73 patients with HBV, only 3 of them developed depression [Citation18]. This may be explained by the differences in ethnicity. Marcellin et al. [Citation11] reported that Asians have significantly lower frequency for depression-related events than Caucasians in both HCV and HBV studies [Citation11]. Also, screening instruments used for detection and being assessed by a psychiatrist may be the cause of getting a higher incidence. Similar to our results, Martin-Santos et al. [Citation13] reported an incidence of 36% for depression and anxiety syndromes during 24 weeks’ follow-up of Peg-IFN and ribavirin treatment in patients with hepatitis C [Citation13]. Udina et al. [Citation16] reported an overall cumulative incidence of depression of 25% for 24 weeks of treatment and 28% for 48 weeks of treatment, in their meta-analysis evaluating 26 prospective observational studies about IFN treatment of patients with HCV [Citation16]. Our higher incidence is probably due to the contribution of the incidence of anxiety disorders which are not well-studied in the literature of IFN treatment.
In our study, nearly 45% of the depression/anxiety disorders appeared in the first month and nearly 65% appeared in the first three months of the Peg-IFN treatment. Likewise in the study of Martin-Santos et al. [Citation13], half of the depression and anxiety syndromes appeared in the first month and more than 90% in the first 4 months, and in the study of Castera et al. [Citation12], mood disorders associated with Peg-IFN treatment emerged mostly (87%) in the first 12 weeks of 24 weeks of follow-up of patients with hepatitis C [Citation12,Citation13]. This shows that it is important to be alert for depressive symptoms especially in the first 3‒4 months of the Peg-IFN treatment. On the other hand, it should be kept in mind that neuropsychiatric side effects of IFN may emerge any time, even after the cessation of IFN treatment and it can be persistent. Suicidal ideation following withdrawal of IFN has been reported [Citation25].
We aimed to analyse the risk factors associated with the emergence of depression/anxiety disorder during the IFN treatment of patients with HBV and we found a statistically significant difference for female gender and baseline HRSD scores. Similar to our results, Martin-Santos et al. [Citation13] found baseline depression score but not anxiety score as a risk factor for depression/anxiety syndrome in a cohort of hepatitis C patients [Citation13]. Udina et al. [Citation16], in their meta-analysis, defined female gender, history of psychiatric disorder, subthreshold depressive symptoms, and low educational level as predictive variables of depression during IFN treatment of patients with HCV [Citation16]. After the exclusion of patients who were dropped out, the significance of the baseline HRSD score disappeared. It is noteworthy that the small sample size might be the reason for our non-significant results for the other variables. Consistent with our results, there are some other studies that showed no significant associations between age and history of depression [Citation9,Citation26].
There are many opinions about the mechanism of IFN-induced depression. It was found that having been exposed to mood disorders in the past and carrying G allele variation of 5-hydroxytryptamine receptor 1-A (HTR1-A) significantly influenced the increase in IFN-induced depression [Citation27]. Besides, reduced glucocorticoid negative feedback sensitivity leading to flattening of the diurnal cortisol slope [Citation28], direct effect on central serotonin neurotransmission [Citation29–32], activation of indoleamine 2,3-dioxygenase which indirectly converts tryptophan to kynurenine [Citation33], and a decrease in the levels of brain-derived neurotrophic factor after IFN treatment might have played a role in the etiology of IFN-induced depression [Citation34]. These associations are outside the scope of this study.
Pretreatment with an antidepressant is suggested to be a reasonable strategy for patients at high risk of developing depression with IFN treatment. In patients who are at risk for depression or in whom IFN-induced depression has already developed, drug–drug interaction and adverse effects of the antidepressants should be considered [Citation30]. As decreased serotoninergic neurotransmission is a pathophysiological mechanism of IFN-induced depression, selective serotonin reuptake inhibitor is a good choice for treatment and prevention of it. Mirtazapine can be a better choice of treatment for patients who have insomnia and anorexia. There is no sufficient evidence for the safe and recommended use of tricyclic antidepressants. Also for patients with IFN-induced cognitive impairments, anticholinergic effects of tricyclic antidepressants may cause further disturbances. Mostly pharmacotherapy is effective and response occurs at relatively small doses with a rapid onset [Citation35].
In the absence of a psychiatrist, easily administered self-report scales such as the Beck Depression Inventory and the Beck Anxiety Inventory may be used to screen depression and anxiety disorders in the assessment of IFN-treated patients [Citation36,Citation37].
Hospital Anxiety and Depression Scale may also be a good option as it is found to be a reliable instrument for detecting depression and anxiety in the setting of a hospital medical outpatient clinic [Citation38].
This study has certain limitations. Firstly, our sample size was small and exclusion of patients who developed psychopathology made it further smaller. Secondly, six patients who were excluded for reasons other than psychiatric disorder emergence were analysed in the group which had no depression and/or anxiety disorder, although we did not know whether they had psychiatric disorder after exclusion. Another limitation is the absence of a control group of CHB patients without treatment; but it would not be possible for widely accepted ethical reasons. Although we have a prospective design, we cannot rule out other factors that may be involved in the emergence of the depression/anxiety disorders. We did not have data about thyroid function tests or hemoglobin changes during treatment. Lastly, like most of the other studies, our research was conducted in a tertiary center, so it is difficult to generalize our results to the general population of patients with HBV.
In conclusion, females and patients with subsyndromal depressive symptoms should be referred to a psychiatrist and closely monitored, especially for the first three months. This cooperation will improve the quality of life and reduce the risk of discontinuation of treatment. Although IFN-induced depression in patients with HCV is well-studied, there is a research gap for patients with HBV. To date, only a few studies have prospectively assessed the frequency and time course of depressive/anxiety syndromes during treatment of HBV infection with Peg-IFN, using validated psychiatric tools. Hence, our data obtained using clinician-rated scales and via face-to-face interviews by psychiatrists are important contributions to the literature. Further prospective studies with larger samples are needed.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ozlem Kuman Tuncel http://orcid.org/0000-0002-9632-6173
References
- Niederau C. Chronic hepatitis B in 2014: great therapeutic progress, large diagnostic deficit. World J Gastroenterol. 2014;20(33):11595–11617. doi: 10.3748/wjg.v20.i33.11595
- World Health Organization. Hepatitis B. 2015. [Internet] [cited 2016 April]. Available from: http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng.pdf
- Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6(3):531–561. doi: 10.1007/s12072-012-9365-4
- Zahiu CD, Rimbas M. Neuropsychiatric side-effects of interferon-alpha treatment: pathophysiology and therapeutic options. Maedica (Buchar). 2014;9(2):121–126.
- Zhang Y, Chen B, Wang L, et al. HBsAg seroclearance or seroconversion induced by peg-interferon alpha and lamivudine or adefovir combination therapy in chronic hepatitis B treatment: a meta-analysis and systematic review. Rev Esp Enferm Dig. 2016;108(5):263–270.
- Hagiwara S, Nishida N, Kudo M. Antiviral therapy for chronic hepatitis B: combination of nucleoside analogs and interferon. World J Hepatol. 2015;7(23):2427–2431. doi: 10.4254/wjh.v7.i23.2427
- Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–144.e10. doi: 10.1053/j.gastro.2015.09.043
- Bernstein D, Kleinman L, Barker CM, et al. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35(3):704–708. doi: 10.1053/jhep.2002.31311
- Leutscher PD, Lagging M, Buhl MR, et al. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C. Hepatology. 2010;52(2):430–435. doi: 10.1002/hep.23699
- Lotrich FE. Psychiatric clearance for patients started on interferon-alpha-based therapies. Am J Psychiatry. 2013;170(6):592–597. doi: 10.1176/appi.ajp.2013.12121572
- Marcellin P, Lau GK, Zeuzem S, et al. Comparing the safety, tolerability and quality of life in patients with chronic hepatitis B vs chronic hepatitis C treated with peginterferon alpha-2a. Liver Int. 2008;28(4):477–485. doi: 10.1111/j.1478-3231.2008.01696.x
- Castera L, Constant A, Henry C, et al. Impact on adherence and sustained virological response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2006;24(8):1223–1230. doi: 10.1111/j.1365-2036.2006.03107.x
- Martin-Santos R, Diez-Quevedo C, Castellvi P, et al. De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. Aliment Pharmacol Ther. 2008;27(3):257–265. doi: 10.1111/j.1365-2036.2007.03568.x
- Sarkar S, Sarkar R, Berg T, et al. Sadness and mild cognitive impairment as predictors for interferon-alpha-induced depression in patients with hepatitis C. Br J Psychiatry. 2015;206(1):45–51. doi: 10.1192/bjp.bp.113.141770
- Koskinas J, Merkouraki P, Manesis E, et al. Assessment of depression in patients with chronic hepatitis: effect of interferon treatment. Dig Dis. 2002;20(3–4):284–288. doi: 10.1159/000067682
- Udina M, Castellvi P, Moreno-Espana J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128–1138. doi: 10.4088/JCP.12r07694
- AlHuthail YR. Comparison of the prevalence of psychiatric co-morbidities in hepatitis C patients and hepatitis B patients in Saudi Arabia. Saudi J Gastroenterol. 2013;19(4):165–171. doi: 10.4103/1319-3767.114514
- Huang YW, Hu JT, Hu FC, et al. Biphasic pattern of depression and its predictors during pegylated interferon-based therapy in chronic hepatitis B and C patients. Antivir Ther. 2013;18(4):567–573. doi: 10.3851/IMP2441
- Williams JB. A structured interview guide for the Hamilton depression rating scale. Arch Gen Psychiatry. 1988;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37.
- Akdemir A, Turkcapar MH, Orsel SD, et al. Reliability and validity of the turkish version of the Hamilton depression rating scale. Compr Psychiatry. 2001;42(2):161–165. doi: 10.1053/comp.2001.19756
- Yazici MK, Demir B, Tanriverdi N, et al. [Hamilton anxiety rating scale: interrater reliability and validity study]. Turk Psikiyatri Derg. 1998;9(2):114–117. Turkish.
- Zimmerman M, Martinez JH, Young D, et al. Severity classification on the Hamilton depression rating scale. J Affect Disord. 2013;150(2):384–388. doi: 10.1016/j.jad.2013.04.028
- Rifflet H, Vuillemin E, Oberti F, et al. [Suicidal impulses in patients with chronic viral hepatitis C during or after therapy with interferon alpha]. Gastroenterol Clin Biol. 1998;22(3):353–357. French.
- Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7(9):942–947. doi: 10.1038/sj.mp.4001119
- Cozzolongo R, Porcelli P, Cariola F, et al. Serotonin gene polymorphisms and lifetime mood disorders in predicting interferon-induced depression in chronic hepatitis C. J Affect Disord. 2015;183:90–97. doi: 10.1016/j.jad.2015.04.056
- Felger JC, Haroon E, Woolwine BJ, et al. Interferon-alpha-induced inflammation is associated with reduced glucocorticoid negative feedback sensitivity and depression in patients with hepatitis C virus. Physiol Behav. 2016;166:14–21. doi: 10.1016/j.physbeh.2015.12.013
- Malek-Ahmadi P, Hilsabeck RC. Neuropsychiatric complications of interferons: classification, neurochemical bases, and management. Ann Clin Psychiatry. 2007;19(2):113–123. doi: 10.1080/10401230701333038
- Raison CL, Demetrashvili M, Capuron L, et al. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. doi: 10.2165/00023210-200519020-00002
- Asnis GM, De La Garza R. Interferon-induced depression: strategies in treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):808–818. doi: 10.1016/j.pnpbp.2005.03.006
- Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82(2):175–190. doi: 10.1016/j.jad.2004.04.002
- Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci. 2004;29(1):11–17.
- Cicek IE, Cicek E, Kayhan F, et al. The roles of BDNF, S100B, and oxidative stress in interferon-induced depression and the effect of antidepressant treatment in patients with chronic viral hepatitis: a prospective study. J Psychosom Res. 2014;76(3):227–232. doi: 10.1016/j.jpsychores.2014.01.003
- Baraldi S, Hepgul N, Mondelli V, et al. Symptomatic treatment of interferon-alpha-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531–543. doi: 10.1097/JCP.0b013e31825d9982
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004
- Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037/0022-006X.56.6.893
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x
