ABSTRACT
OBJECTIVE: The main objective of this study is to compare the rate of smoking cessation in the first month, third month, sixth month, first-year, and second year among those who quit smoking following different pharmacological and behavioural therapies administered at the Selcuk University’s Smoking Cessation Clinic in Turkey.
METHODS: In this study, 3322 people who presented to the clinic in order to quit smoking were advised one of the most suitable medical treatments (varenicline, bupropion, NRT) accompanied by behaviour therapy after their health queries and examinations were made and Fagerström scores were evaluated. Smoking cessation patients were followed up clinically and by making calls after smoking cessation.
RESULTS: The smoking cessation success rate in the cases using varenicline in the first month was 63.5% (766/1206), in the third month 46.8% (548/1170), in the sixth month 32.1% (386/1201), first year 25.6% (298/1163), and 19.9% (211/1059) in the second year. The success rate in the cases using bupropion in the first month was 49.9% (559/1120), in the third month 35.6% (405/1138), in the sixth month 26.4% (319/1210), first year 21.9% (261/1192), and 16.0% (133/832) in the second year. The success rate in the cases using NRT was 53.2% (25/47) in the first-month, 24.3% (9/37) in the third-month, and 27.3% (6/22) in the sixth-month assessments. The rates of smoking cessation in the cases using varenicline and behavioural therapy in the 1st, 3rd, 6th, 12th, and 24th month were significantly higher compared to the cases using bupropion and behavioural therapy (p = .000, p = .000, p = .008, p = .034, and p = .028; respectively).
CONCLUSIONS: It has been observed in this study that varenicline as a smoking cessation drug is better tolerated than other medications and it seems to be more effective.
KEYWORDS:
Introduction
Tobacco use is the number one cause of preventable deaths in the world. The World Health Organization (WHO) data report that there are 1.3 billion smokers in the world, and 6 million people die each year from the smoking-related causes [Citation1,Citation2]. In 2030 this number is expected to reach eight million. And at least half of the present smokers are reported to end up with losing their lives due to smoking [Citation1,Citation2].
The population of Turkey is 78 million and 965 thousand and 645 (per Turkish Statistical Institute database), at middle-income level [Citation3]. According to the Global Adult Tobacco Survey Turkey 2012, 27% of adults who are above 15 years of age were tobacco smokers (41.4% in males, 13.1% females), and the daily use of cigarettes were in 23.7% (37.3% for males – 10.7% for females). In the 13‒15 age group, the tobacco and cigarettes use rates were 11.9% and 8.4%, respectively [Citation3,Citation4]. Smoking is more frequent among 35‒44 years age group (36.2%), and 25‒34 years age group (34.9%) [Citation2,Citation4]. Prevalence of smoking among the elderly is lower compared with the younger group [Citation2]. The rate of smoking in the elderly varies between 13.2% and 25% according to some studies conducted in Turkey [Citation2,Citation5,Citation6]. More than 15 million individuals over the age of 15 years old are tobacco and cigarette smokers, and they are candidates to receive tobacco smoking cessation services [Citation3].
Smoking-related diseases such as chronic obstructive pulmonary disease (COPD), lung cancer, oesophageal cancer and cancers of the oral cavity, asthma, cardiovascular disease, diabetes mellitus and arterial hypertension, osteoporosis, cataracts, and pregnancy complications are very common, particularly among the aging population. Smoking cessation reduces mortality rate in patients with COPD and reduces the progression of asthma, cardiovascular disease, arterial stiffness, and arterial hypertension [Citation7–10].
Evaluation of smoking cessation, support, and treatment of smokers are among the interests and duties of primary health care physicians. Quitting tobacco is not easy as tobacco dependence is a cluster of behavioural, cognitive, and physiological phenomena. Very few tobacco users can successfully quit the habit in their first attempt. Average smokers attempted five times to quit before sustainable success [Citation11]. The first step in smoking cessation treatment is to motivate patients to quit smoking, to inform, to initiate the necessary support and treatment. Physicians should address all patients who use tobacco [Citation11,Citation12]. Although intensive group and individual psychological counselling are effective in helping smokers to achieve abstinence, most smokers are not interested in participating in these types of interventions [Citation11,Citation13,Citation14]. The five A’s frame work (Ask, Advise, Assess, Assist, and Arrange) has been developed to allow physicians to incorporate smoking cessation counselling into their busy clinical practices [Citation11,Citation15].
In addition to the fundamental changes in individual behaviour and psychotherapy, FDA approved medical drugs for the smoking cessation treatment. These drugs include first line; Nicotine Replacement Therapy (NRT) (e.g. nicotine gum, inhalers, lozenges, nasal spray, or patches), Bupropion Hydrochloride Sustained-Release, α4β2 nicotinic acetylcholine receptor partial agonist Varenicline. Second line; Clonidine and Nortriptyline are shown to be effective in quitting smoking in clinical trials, but their use is not common [Citation7,Citation11,Citation15].
The aim of this prospective cohort study is to evaluate and compare the rates of smoking cessation following different therapies in the first, third, sixth months and first and second year in a fairly large sample from Konya, Turkey.
Methods
Participants and study protocol
This is a longitudinal prospective cohort study (descriptive, and a follow-up study), and was conducted at the Selcuk University School of Medicine’s Smoking Cessation Outpatient Clinic. The protocol of this study was reviewed and approved by the Ethics Committee of Selcuk University (2014-332). Nearly 3322 applicants presented to the smoking cessation outpatient clinic to quit smoking between March 25 2011 and July 15 2014 and received a smoking cessation support individually accompanied by behaviour therapy (BT) and medication therapy. Smoking cessation patients were followed up on cessation day, after 7 days, 15 days, 1 month, and 3 months at the outpatient clinics, and then followed up with phone calls by researchers (it was free outdoor phone line) in the sixth month, first year, and during the second year following behavioural therapy and recommended medication treatment.
Clinical evaluation
Sociodemographic characteristics of individuals presented to quit smoking such as age, gender, existing complaints, and diagnosed medical diseases were questioned. The presence of any chronic medical illnesses, the risk, and presence of major depressive disorder were reviewed during the baseline visit. Physical examination was performed and carbon monoxide in the breath was measured. The Fagerström test for nicotine dependence (FTND) was administered to evaluate the dependence in each individual case. Participants who were diagnosed with major depressive disorder and with other psychiatric disorders during the baseline examination were referred to the psychiatry clinic. These individuals did not receive smoking cessation treatment. The patients admitted to our department were reviewed and examined in accordance with the proposals declared by the FDA (Food Drug Administration) and the Tobacco Control Department of Ministry of Health in Turkey, and they were recommended one of the most appropriate medication treatments such as varenicline (days 1–3: 0.5 mg once per day, days 4–7: 0.5 mg twice per day, day 8 to end of treatment: 1 mg twice per day), bupropion (150 mg in the morning for three days, then increased to 150 mg twice per day), NRT (doses vary and should be tapered as therapy progresses, heavy smokers: 21 mg per day (initial dosage), Light smokers, or those weighing less than 100 lb (45 kg): 10–14 mg per day (initial dosage) accompanied by behavioural changes therapy after their Fagerström scores were evaluated [Citation11]. The individuals who did not wish to receive medication treatment or inappropriate for medication treatment received only behavioural therapy.
Carbon monoxide measurement
CO measurements were performed in the expiration air by piCO Smokerlyzer Breath CO Monitor Bedfront Scientific England UK device. PiCO Smokerlyzer Breath device measures the level of CO in the expiration air between 0 and 100 ppm.
Fagerström test for nicotine dependence
The Fagerström Tolerance Questionnaire was developed by Karl-Olov Fagerström. The FTND is a standard instrument for assessing the intensity of physical addiction to nicotine [Citation12]. The test was designed to provide an ordinal measure of nicotine dependence related to cigarette smoking. It contains six items that evaluate the quantity of cigarette consumption, the compulsion to use, and dependence. In scoring the FTND, yes/no items are scored from 0 to 1 and multiple-choice items are scored from 0 to 3. The items are summed to yield a total score of 0–10. The higher the total Fagerström score, the more intense is the patient’s physical dependence on nicotine. In the clinic, the Fagerström test may be used by the physician to document indications for prescribing medication for nicotine withdrawal. 0–2 points indicate the degree of nicotine dependence very low, 3–4 points low, 5 points moderate, 6–7 points severe, over 8 points very severe () [Citation16].
Table 1. The Fagerström test for nicotine dependence.
Statistical analysis
The data were analysed using the Statistical Package for the Social Sciences Version 16.0 for Windows. Descriptive statistics such as frequencies and percentages were obtained. Student’s t-test, analysis of variance, Pearson’s correlation, and Chi-square test were used during the analyses. Findings were statistically considered as significant at p < .05 level.
Results
Sociodemographic characteristics, carbon monoxide levels, FTND scores, smoking characteristics, and administered treatments in the study are presented in .
Table 2. Demographic characteristics of the participants and nicotine dependence at the beginning, treatment choice, and pack-years.
As seen in , the average age of the users of the varenicline + BT were significantly higher than those received bupropion + BT, NRT + BT, and only behavioural therapy (p < .001). In general, although the average age of those using the varenicline is statistically higher, generally the age is over 60 years in more than 90% in all treatment arms when we categorize the ages ().
Table 3. Smoking characteristics according to treatment options.
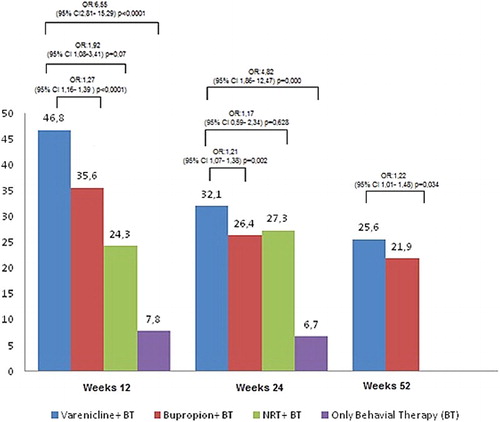
FTND scores of the cases using varenicline were significantly higher (p < .001). When we categorized and evaluated FTND scores, the prevalence of the very severe addiction score of varenicline + BT users was observed to be significantly higher than those who used bupropion + BT, NRT + BT, and behavioural therapy (p < .001). When the rate of quitting smoking in the first month is evaluated; the success rate in the varenicline + BT users (63.5%) was significantly higher than in the bupropion + BT users (49.9%), those using NRT + BT (53.2%) and only behavioural therapy (17.1%) (p < .001) ().
Table 4. The success rate of the patients quitting smoking in the first, third, sixth months, first year, and second years.
When the rate of smoking cessation was evaluated in the third month; the success rate of the cases using varenicline + BT was significantly higher with 46.8% than those using bupropion + BT (35.6%), NRT + BT users (24.3%), and only behavioural changes (7.1%) (p < .001) (). The success rate of the varenicline + BT users in the 12th week was 1.27 times (95% CI 1.16–1.39, p < .001) higher than those using bupropion + BT, and 1.92 times greater (95% CI 1.08–3.41, p = .07) than the users of NRT + BT, and 6.55 times (95% CI 2.81–15.29, p < .001) greater than those receiving behavioural therapy (). When rate of smoking cessation was evaluated in the sixth month, the success rate of those using varenicline + BT was significantly higher with 34.4% than those using bupropion + BT (28.4%), NRT users (27.3%), and behavioural (6.7%) (p < .001) When the rate of smoking cessation was evaluated in the first year; the success frequency with 27.9% in those using vareniclin + BT was significantly higher compared with bupropion + BT users (24.2%) (p = .001) ().
The success rate of the varenicline + BT users in the 24th week was 1.32 times (95% CI 1.10–1.58, p = .002) significantly higher than those using bupropion + BT, and 1.40 times greater (95% CI 0.54–3.60, p = .48) than the users of NRT + BT, and 7.35 times (95% CI 2.64–20.42, p < .001) greater than those receiving behavioural therapy ().
When the rate of smoking cessation of the patients was evaluated in the second year; the success rate (19.9%) of the varenicline + BT users in the second year was significantly higher when compared with bupropion + BT users (16.0%) (p = .028) ().
In 52 weeks, varenicline + BT users were found to be significantly 1.30 times (95% CI 1.02–1.66, p = .027) higher than those using bupropion + BT ().
Smoking cessation success rates significantly increased in the cases using varenicline + BT after first and second year follow-up in this study when compared to the other age ranges after the age of 50 (34.2%, 28.3%, p = .003, p = .002). Still, while smoking cessation success rates significantly increased in the cases using bupropion + BT after first-year follow-up in this study when compared to the other age ranges after the age of 40, it was not statistically significant (24.6%, p = .434).
Smoking cessation success rates after second year of follow-up in the cases using bupropion + BT significantly increased in comparison to the other age ranges after 50 years old (19.5%, p = .020) ().
Table 5. Smoking cessation rates according to one year and two years in varenicline and bupropion users.
Discussion
This study was based on findings of a two-year follow-up period in Selcuk University, Turkey. In this present study, success rate in quitting smoking among varenicline + BT users in the first, third, sixth month, first and second year was found to be statistically significantly higher.
When the studies on this subject are reviewed; in a study where Gonzalae et al. [Citation17] carried out medical treatment for 3 months and evaluated the status of smoking cessation of the cases in the 18‒75 age range; they determined that the success rate was 44.0% in those using varenicline, and 3.9 times higher compared to those using placebo (17.7%), and 1.9 times higher than bupropion users (p < .001). Third-month success rates for bupropion were 29.5% and were two times higher compared with placebo (p < .001). The success frequency in the varenicline users was found 21.9% in the 52nd week, and this rate was 3.09 times higher than in the smokers who quit smoking using placebo (8.4%) (p < .001). The frequency of success is 16.1% in bupropion users in 52 weeks, and this rate is 1.5 times higher than the incidence of cessation with placebo; however, the significance was lost (p = .057). In a study conducted by Nides et al. [Citation18] in 2008, the individuals among the smokers ranging between 18 and 75 years old were classified in three groups, and one group received varenicline (n = 696) therapy, one group bupropion (n = 671), and the other group received treatment with placebo (n = 685). When the success rate of quitting smoking between 9 and 12 weeks was evaluated, the frequency of smoking cessation using varenicline (44.0%) was significantly higher than smoking cessation success using bupropion (29.7%) and placebo (17.7%) (p < .0001, p < .0001). The success rate of cessation in the 9‒24 weeks with varenicline was 29.6%, bupropion 20.4%, and with placebo was 11.8%. When the success rate of quitting smoking at 9‒52 weeks was evaluated; varenicline users (22.4%) were significantly higher than smoking cessation success using bupropion (15.4%) and placebo (9.3%) (p = .0008, p < .0001, respectively). Zincir et al. [Citation19] found after 12-week follow-up of 251 consecutive patients presented to a smoking cessation outpatient clinic in 2013 that smoking cessation success rate was 72.3% in cases using varenicline, 57.1% in cases using bupropion, and 54.8% in cases using NRT, and also the overall success was 61.8%. The group with higher score of FTND used varenicline and they stated that they found success rate of quitting in this group as a high. Uçar et al. [Citation20] found smoking cessation success rates in the first year in 2014 as 32.5%, 23%, 52.8%, respectively, in the patients using varenicline (n = 166), bupropion (n = 148), NRT (n = 108), and stated that the superiority of NRT therapy to the other therapies could be attributed to the use of NRT forms in combination (gum, spray, and tape). They also indicated that the overall high success rate in all groups could arise from the administration of behavioural changes therapy to all patients. Tonstad et al. [Citation21] reported in their randomized controlled study that smoking cessation rate was significantly higher for the varenicline group than for the placebo group for weeks 13–24 (70.5% vs 49.6%; [OR], 2.48; 95% [CI], 1.95‒3.16; p < .001) as well as for weeks 13–52 (43.6% vs 36.9%; [OR], 1.34; 95% [CI], 1.06‒1.69; p = .020). In this study, it was emphasized that five clinic examinations and four phone calls in 24 weeks had an effect on the higher smoking cessation rates in 24 and 52 weeks compared to the other studies. Ebbert et al. [Citation22] reported in a study conducted in 61 centres in 10 countries (Australia, Canada, Czech Republic, Egypt, Germany, Japan, Mexico, Taiwan, United Kingdom, and United States) between July 2011 and July 2013 that the success rate of varenicline (n = 760) for 24 weeks (32.1%) was significantly higher than placebo (n = 750) (6.9%), and the success rates for 52 weeks were 27% and 9.9%, respectively. They reported use of 18 clinical and 10 phone calls in 52 weeks in this study. Nakamura et al. [Citation23], in a comparative study between varenicline and placebo, reported that success rate of smoking cessation after 9‒12 weeks in the users of varenicline was significantly higher compared to the placebo [66.7%, 104/156, OR (95% CI): 3.23 (2.02–5.19); 39.0%, 60/154; p < .001). Tsai et al. [Citation24], in a study conducted in 2007, reported that the success rate for 9‒12 weeks in patients taking varenicline and placebo for 4 weeks was 59.5% in varenicline users and 32.3% in placebo users, and the success rate for 9‒24 weeks in patients taking varenicline and placebo for 12 weeks was 46.8% in varenicline users and 21.8% in placebo users. Boudrez et al. [Citation25], in 551 patients receiving varenicline therapy found the frequency of smoking cessation success for 12 weeks as 64.6% in 2011, and when they classified the cases who quit smoking according to their countries, they found that the prevalence of smoking cessation was 61.1% in Belgium, 70.4% in Greece, 64.5% in Hungary, and 50% in Slovenia.
It has been observed in international and domestic studies that generally varenicline has been used in the cases with higher dependence score of FTND and with higher packages of smoking cigarette year, and bupropion has been used in cases generally presenting with depressive symptoms and less consumption of cigarettes and with lower FTND scores [Citation2,Citation19,Citation20,Citation22,Citation26]. In fact, also in our study, chronic diseases of the patients were questioned, and patients were informed about the smoking cessation drugs and it was observed that the cases with higher FTND scores and those with longer packet years were recommended varenicline preferred varenicline and varenicline was preferred. Indeed, in 81 provinces at 228 smoking cessation clinic between 2010 and 2011 in our country, the success rate of smoking cessation after one-year follow-up was 29.6% in 16,473 varenicline users, 25.1% in the bupropion users. The success rate of smoking cessation at the end of one year was 41.4% in the cases using varenicline with increased age, especially at the 50‒59 age bracket, and 42.3% in the cases over 60 years, and this was found to be significantly higher than in other age brackets [Citation27]. Also in this study, the success rate of smoking cessation at the end of one year was 31.8% in the cases using bupropion with increased age, especially at the 50‒59 age bracket, and this was found to be significantly higher than in other age brackets. Günay et al. reported the success rate of smoking cessation at the end of one year as 49.0% in their study conducted in 2011, which was significantly higher in the elderly patients (over 60 years) using varenicline and bupropion than in younger patients (below 60 years), which was 33.4% [Citation2]. In fact, still the success rate of smoking cessation did not differ significantly in the bupropion users over the age of 40 after first-year follow-up when compared to the other age brackets, and the success rate of smoking cessation in the bupropion users over the age of 50 after second year follow-up was significantly higher than in other age brackets in our study. This situation was consistent with the previous literature, and both the numbers of smoking cessation trials and associated comorbidities increased with aging, and therefore, it increased the success rate of smoking cessation in these cases [Citation27].
It can be concluded in this study that varenicline as a smoking cessation drug seems to be more effective and is tolerated better than other smoking cessation medications. This result was consistent with the results of the previous studies comparing the efficacy of bupropion and NRT with that of varenicline on smoking dependence. In conclusion, considering the principle that there is no disease but there are patients, and taking into account the patient’s previous experience of smoking cessation, medication uses, and methods, the patients should always be recommended personalized medicine, i.e. the most effective and safe medication. Appropriate medical treatment, phone calls, and clinical follow-up for two years accompanied by behavioural changes therapy in the management of the smoking cessation patients would increase the success rate.
Acknowledgement
The authors would like to thank the staff of the Family Medicine Clinic of at the Selcuk University School of Medicine Smoke Cessation Center and English lecturer Mustafa Taşbent for all their kind help and support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- World Health Organization. WHO report on the global tobacco epidemic 2013 [Updated 2013; cited 2014 Oct 30]. Available from: http://www.who.int/tobacco/global_report/2013/en/
- Gunay T, Pekel O, Simsek H, et al. Smoking habits and cessation success, what differs among adults and elderly? Saudi Med J. 2014;6:585–589.
- Global adult tobacco survey, Ankara, Turkey 2012 [cited 2016 Jan 30]. Available from: http://www.who.int/tobacco/surveillance/survey/gats/report_tur_2012.pdf?ua=1
- Turkish Statistical Institute. Global adult tobacco survey, Ankara, Turkey 2012 [Updated 2012; cited 2013 Oct]. Available from: http://www.tuik.gov.tr/PreHaberBultenleri.do?id=13425
- Ozdemir L, Kocoğlu G, Sümer H, et al. Frequency of some chronic diseases and risk factors among the elderly people in Sivas, Turkey. Çukurova Med J. 2005;27:89–94. Turkish.
- Yengil E, Çevik C, Demirkıran G, et al. Smoking among medical school students and attitudes against smoking. Konuralp Med J. 2014;6(3):1–7.
- Andreas S, Chenot FJ, Diebold R, et al. Effectiveness of varenicline as an aid to smoking cessation in primary care: An observational study. Eur Addict Res. 2013;1:47–54. doi: 10.1159/000341638
- Scallan C, Doonan RJ, Daskalopoulou SS. The combined effect of hypertension and smoking on arterial stiffness. Clin Exp Hypertens. 2010;6:319–328. doi: 10.3109/10641960903443558
- Sezer H, Akkurt I, Guler N, et al. A case control study on the effect of exposure to different substances on the development of COPD. Ann Epidemiol. 2006;1:59–62. doi: 10.1016/j.annepidem.2004.12.014
- Merdad LA, AlZahrani MS, Farsi JM. Smoking habits among Saudi female university students: prevalence, influencing factors and risk awareness. Ann Saudi Med. 2007;5:366–369. doi: 10.5144/0256-4947.2007.366
- Larzelere MM, Williams DE. Promoting smoking cessation. Am Fam Physician. 2012;6:591–598.
- U.S. Preventive Services Task Force. Counseling and intervention stop revent tobacco use and tobacco-caused disease in adults and pregnant women: U.S. preventive services task force: reaffirmation recommendation statement. Ann Intern Med. 2009;8:551–555.
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2005;2:CD001007.
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;2:CD001292.
- 2008 PHS Guideline Update Panel, Liaisons, and Staff. Treating tobacco use and dependence: 2008 update U.S. public health service clinical practice guideline executive summary. Respir Care. 2008;9:1217–1222.
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;11:763–765.
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;1:47–55. doi: 10.1001/jama.296.1.47
- Nides M, Glover ED, Reus VI, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav. 2008;6:664–675.
- Zincir SB, Zincir S, Kaymak E, et al. Comparison of the effectiveness of varenicline, extended-release bupropion and nicotine replacement therapy on the success and the maintenance of a smoking cessation program. BCP. 2013;3:224–230. doi: 10.5455/bcp.20130313045037
- Ucar E, Araz O, Yilmaz N, et al. Effectiveness of pharmacologic therapies on smoking cessation success: three years results of a smoking cessation clinic. Multidiscip Respir Med. 2014;1:9. doi: 10.1186/2049-6958-9-9
- Tonstad S, Tonnesen P, Hajek P, et al. Varenicline phase 3 study group, effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;1:64–71. doi: 10.1001/jama.296.1.64
- Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687–694. doi: 10.1001/jama.2015.280
- Nakamura M, Oshima A, Fujimoto Y, et al. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;6:1040–1056. doi: 10.1016/j.clinthera.2007.06.012
- Tsai ST, Cho HJ, Cheng HS, et al. A randomized, placebocontrolled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, a new therapy for smoking cessation in asian smokers. Clin Ther. 2007;6:1027–1039. doi: 10.1016/j.clinthera.2007.06.011
- Boudrez H, Gratziou C, Messig M, et al. Effectiveness of varenicline as an aid to smoking cessation: results of an inter-European observational study. Curr Med Res Opin. 2011;4:769–775. doi: 10.1185/03007995.2011.557718
- Okuyemi K, Nollen N, Ahluwalıa J. Interventions to facilitate smoking cessation. Am Fam Physician. 2006;2:262–271.
- Çelik İ, Yüce D, Hayran M, et al. Nationwide smoking cessation treatment support program-Turkey project. Health Policy. 2015;1:50–56. doi: 10.1016/j.healthpol.2014.11.017