ABSTRACT
OBJECTIVE: Data on the effect of anticholinergic cognitive burden (ACB) in older adults with subjective cognitive decline (SCD) are limited. We aimed to study whether ACB increases the future risk of dementia in older adults with SCD.
METHODS: The retrospective cohort analysis was carried out on 1496 older adults. Out of those, 109 older patients with SCD followed up over 36 months were studied. They were divided into two groups according to cognitive status at last visit: group I included the subjects with SCD who did not progress to dementia and group II included those who progressed to dementia. The drugs with anticholinergic effects that were received by subjects three months or more were identified from records. The drugs were categorized as having absent (ACB = 0), possible (ACB = 1), and definite (ACB = 2) anticholinergic properties based on an ACB scale. ACB was calculated for each subject by adding the score of each drug and classified as no or low ACB (ACB ≤ 2) and high ACB (ACB ≥ 3).
RESULTS: The mean age of all subjects was 72.5 ± 6.3 years and 66.1% of the sample was female. The median follow-up time for all subjects was 75 months (range, 36–185). Fifteen (13.8%) of 109 participants with baseline SCD developed dementia. High ACB was present in 12 subjects (12.8%) in group I and 7 subjects (46.7%) in group II (p = .001). The 75–84 and 85+ age groups (hazard ratio (HR) = 3.595; CI: 1.117–11.574; p = .032 and HR = 12.203; CI: 2.889–51.537; p = .001, respectively), hypertension (HR = 7.835; CI: 1.020–60.189; p = .048), and high ACB (HR = 4.312; CI: 1.563–11.899; p = .005) were found to be possible risk factors for dementia among subjects with SCD in the univariate model. In the final multivariate Cox regression model, subjects with high ACB had a 4.2-fold the risk of the development of dementia. Metoprolol (28.6%), trazodone (21.4%), and trospium (12.9%) were leading used drugs with anticholinergic properties. Among subjects with a total ACB score ≥ 3, the majority were on trospium (29.0%), followed by metoprolol (16.2%), paroxetine (16.2%), and trazodone (16.2%).
CONCLUSION: We found that high ACB increases 4.2-fold the risk of the development of dementia in older adults with SCD in long-term follow up. The results of our study are promising, however, the effect of ACB on cognitive status among subjects with SCD is still lacking. To clarify the association between ACB and the risk of dementia, large and longer prospective studies are needed in this population.
Introduction
The numbers of older people has risen gradually worldwide due to the continuing increase in average life expectancy. Correspondingly, physicians have been started to face with an increase in memory complaints in this population in recent decades. Subjective cognitive decline (SCD) is one of the common health problems encountered in older people. It’s prevalence varies between 11% and 56% due to the different study groups and differences in making the diagnosis of SCD [Citation1,Citation2]. Although there is still not any consensus on its definition, SCD is commonly defined as individual’s perception of decline in memory and other cognitive abilities [Citation3]. This condition, which can be ignored by both patients and clinicians and which patients may even avoid disclosure, has been shown to be a potential early predictor of a possible dementia, particularly Alzheimer’s disease (AD) in several studies [Citation4,Citation5]. Recently, SCD has been reported as a risk factor for a future diagnosis of dementia, claiming that SCD may not be a phenomenon associated with age alone [Citation4]. Zwan et al. detected an increased beta amyloid burden, which plays a role in physiopathology of AD, in cases of SCD [Citation6].
Use of anticholinergic medications in chronic conditions such as hypertension, arrhythmia, depression, anxiety disorders, urinary incontinence, and psychotic diseases is common in older adults [Citation7]. Most anticholinergic adverse effects including cognitive impairment, falls, urinary retention, constipation, nausea, dry eyes, blurred vision, dry mouth, decreased sweating, and increased heart rate occur frequently in older adults, because the passage of those into central nervous system is quite easy, but on the contrary, their elimination is difficult [Citation8]. Moreover, the cumulative effect of taking one or more anticholinergic medications is described as the anticholinergic burden. Physicians prescribe these medicines for their therapeutic effects, but often do not take into consideration the cumulative effect due to drug burden [Citation9].
Dizziness, confusion, sedation, and delirium are cognitive adverse effects associated with these medications [Citation7,Citation10]. Today, anticholinergic cognitive burden (ACB) caused by these drugs can be measured with Anticholinergic Cognitive Burden Scale [Citation11]. The relationship of increased ACB and cognitive impairment has been proved in several studies [Citation12–14]. Pasina et al. showed that dosage-dependent anticholinergic burden constitutes a risk factor for cognitive limitations and decreased physical functionality [Citation12]. Fox et al. monitored a large study group consisting of 13,004 people with and without cognitive impairment for 2 years and an extra drop of 0.33 point in Mini Mental State Examination (MMSE) score was found in patients who had ACB compared with those who did not use this type of medications [Citation15]. However, there was not any relationship between ACB and cognitive decline in subjects with significant cognitive limitation.
This study was based on the hypothesis that the use of medications with anticholinergic properties will increase the risk of dementia in older patients with SCD. To the best of our knowledge, this is the first study which investigates the effects of the relationship between ACB and SCD on the development of dementia.
Materials and methods
Study design and samples
We investigated the relationship between ACB and risk of dementia with a retrospective cohort design, using the database of the Geriatrics Outpatient Clinic of Gulhane Training and Research Hospital. All procedures performed in our study were in accordance with the ethical standards of the institutional and/or national research committee and with the revised Helsinki declaration in 2013. The study was approved by the local ethics committee (1491-52-16/1648.4-75).
The retrospective cohort analysis was done among 1496 subjects ≥65 years. Of those subjects, 109 older adults with SCD followed up over 36 months were enrolled into study (). The enrolled subjects were divided into two groups according to cognitive status at last visit: group I (the control group) contained the subjects with SCD who did not progress to dementia and group II (the study group) contained those who progressed to dementia.
Figure 1. Sample selection of the study. Note: SCD, subjective cognitive decline and ACB, anticholinergic cognitive burden.
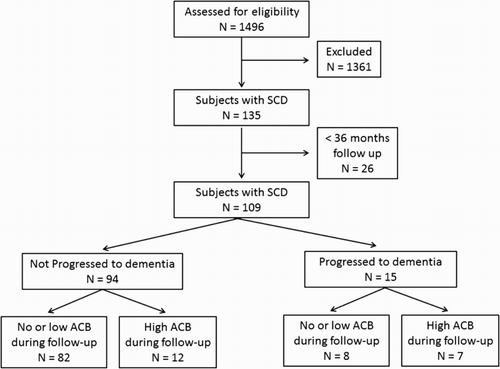
The index date for each subject was the date when it was first diagnosed with SCD. Subjects in study group were followed up from the index date until the date when dementia was developed. The last visit for subjects in control group was stated as the date when a patient admitted to outpatient clinic last time.
Exclusion criteria included inadequate data in the medical records, a follow up shorter than 36 months, a diagnosis of cognitive impairment including mild cognitive impairment and dementia, cognitive decline due to depression (pseudodementia), active malignancy, and central nervous system disease. One thousand three hundred and eighty-seven older adults were found to be ineligible for enrolment ().
Cognitive assessments
Cognitive functions were assessed by a neuropsychologist in all subjects using the MMSE and the short form of Yesavage Geriatric Depression Scale (YGDS) at index date and last visit [Citation16–18]. MMSE is a 30-point questionnaire (higher scores indicates better cognitive functioning) that is used to assess cognitive functions including orientation, registration, attention, calculation, recall, language, and constructional praxis [Citation16]. The short form of YGDS is a 15-item self-report scale (higher scores indicates more depressive symptoms) that is used to identify depressive symptoms and screen for depression among older adults [Citation18].
SCD was defined as follows: (a) reporting forgetfulness for a long time, (b) admission to the outpatient clinic due to memory problems, (c) having memory complaints and concerns every day, (d) minor difficulty in activities of daily living, (e) the belief of that worse memory compared to other person at similar age, (f) having no cognitive impairment at index date and last visit based on assessment of cognitive status by a geriatrician [Citation19,Citation20].
Clinical assessment of dementia included history taking, general physical examination, blood tests, neuropsychological tests, and radiographic studies (computed tomography or magnetic resonance imaging) if needed. The final diagnosis in patients with dementia was made by an expert panel based on the “Diagnostic and Statistical Manual of Mental Disorders Definition” criteria include following [Citation21,Citation22]: (a) cognitive impairments in one or more cognitive domains (complex attention, executive function, learning and memory, language, perceptual-motor, and social cognition), (b) cognitive impairments associated functional decline in everyday activities, (c) cognitive impairments do not occur only during the course of delirium, and (d) cognitive impairments are not better explained better by another mental disorder (e.g. major depressive disorders, and schizophrenia).
Characteristics
Information on characteristics at the index date was collected from the records. Demographics included age, gender, and mean follow-up time (months). Lifestyle factors were smoking status (never, former, and current), alcohol status (never, former, and current), and physical activity (none, moderate (3 days in a week), heavy (everyday)). Social factors were living status (spouse and other) and education (years). Variables related to medical history were hypertension (yes or no), diabetes mellitus (yes or no), and depression (yes or no). Subsequently we categorized smoking (yes (current) and no (former and never)) and physical activity (yes (heavy and moderate) and no (none)) into two groups.
The medication list including name, dose, frequency, and quantity were obtained from each subject in the outpatient clinic and recorded. Detailed data on all receiving medications by each subject at index date were extracted from the medical records by an expert physician. Polypharmacy was defined as six or more drug use at index date.
Anticholinergic cognitive burden
The drugs with anticholinergic effects that were received by subjects three months or more were identified from records. Anticholinergic Cognitive Burden Scale was used for calculating the anticholinergic burden score for each subject [Citation23]. The drugs were categorized as having absent (ACB = 0), possible (ACB = 1), and definite (ACB = 2) anticholinergic properties based on ACB scale [Citation24]. A total score of ACB for each subject was obtained by adding the score of each drug and classified as no or low ACB (ACB ≤ 2) and high ACB (ACB ≥ 3). A total score of 3 or more was considered to show a potential harmful ACB.
Statistical analysis
The data were analysed using SPSS Statistics for Windows (2013, Version 22.0. Armonk, NY: IBM Corp) statistical program. Results were given as number or percentage or mean ± SD or median (minimum–maximum). The Kolmogorov–Smirnov test was used to evaluate normal distribution for continuous variables. Independent samples t-test and Mann–Whitney U test were used for comparisons between two groups for continuous variables (). Categorical variables were analysed using Chi-square test (). The effects of possible factors on the development of dementia in patients with SCD were evaluated by using clinically important variables or for which p value was <.20 in the first analyses. Identified independent variables were examined by univariate Cox regression analysis and multivariate Cox regression analysis using backward selection (). Differences were considered to be significant when p < .05.
Table 1. Initial characteristics of all subjects included in the longitudinal study.
Table 2. Predictors of dementia in patients with SCDa based on univariate and multivariate Cox regression analysis.
Results
Descriptive initial characteristics of all subjects, overall and by the progression from SCD to dementia are presented in . The mean age of all subjects was 72.5 ± 6.3 years and 66.1% of the sample was female. The median follow up time for all subjects was 75 months (range 36–185).
The majority of subjects (n = 58, 53.2%) had an ACB score of 0, 22.9% (n = 25) had a score of 1, 6.4% (n = 7) had a score of 2, and 17.5% (n = 19) had a score of ≥3.
Subjects in group I was younger and higher MMSE scores than those in group II (p = .017 and p = .013, respectively). Hypertension, high ACB, and polypharmacy were prevalent in group II (p = .027, p = .001, and p = .026, respectively). Number of drugs in subjects with polypharmacy was higher in group II (p = .045). The other characteristics were similar among two groups ().
We evaluated whether clinically important variables or for which p value was <.20 in the first analyses were associated with dementia risk in all subjects. The 75–84 and 85+ age groups (hazard ratio (HR) = 3.595; CI: 1.117–11.574; p = .032 and HR = 12.203; CI: 2.889–51.537; p = .001, respectively), hypertension (HR = 7.535; CI: 1.020–60.189; p = .048), and high ACB (HR = 4.312; CI: 1.563–11.899; p = .005) were found possible risk factors for dementia in the univariate Cox regression model. Gender, smoking, diabetes mellitus, and polypharmacy were not reached statistical significance in the univariate Cox regression analysis ().
Variables evaluated in the univariate Cox regression model were entered in a final multivariate Cox regression model. The 85+ age group (HR = 11.468; CI: 2.672–49.225; p = .001) and high ACB (HR = 4.179; CI: 1.430–12.212; p = .009) were found to be independent risk factors for dementia in older adults with SCD. The 75–84 age group, gender, smoking, hypertension, diabetes mellitus, and polypharmacy were not reached statistical significance in the multivariate Cox regression analysis ().
and list the frequencies of drugs with anticholinergic properties in all subjects and in subjects with a total ACB score ≥3, respectively. Overall, metoprolol (28.6%), trazodone (21.4%), and trospium (12.9%) were leading used drugs with anticholinergic properties (). Among subjects with a total ACB score ≥3, the majority were on trospium (29.0%), followed by metoprolol (16.2%), paroxetine (16.2%), and trazodone (16.2%) ().
Table 3. Frequencies of drugs with anticholinergic properties in all subjects (N = 109).
Table 4. Frequencies of drugs with anticholinergic properties in subjects with a total ACBa score ≥ 3 (N = 19).
Discussion
Our study showed that high ACB increases 4.2-fold the risk of the development of dementia in older adults with SCD in long-term follow up. The association between ACB and the development of dementia in this special group has not been previously studied. The results of this study provide important information’s on diminishing or eliminating of high ACB may reduce the future risk of cognitive impairment in advanced ages.
It has been recently reported that SCD is a possible risk factor for dementia [Citation4,Citation5]. In a 4-year follow-up cohort study of 758 participants, Waldorff et al. found an increased 2.27-fold risk in development of dementia in older population with SCD [Citation4]. However, information on the role of anticholinergic drug use in the progression of cognitive impairment in this population is limited in the literature. On the other hand, adverse effects of anticholinergic drugs on cognitive functions were shown in several studies [Citation10,Citation12,Citation15,Citation25]. According to the results of the REPOSI cross-sectional study of 1380 participants, the use of anticholinergic drug was demonstrated to be associated with deterioration in cognitive and physical status in older adults [Citation12]. A large prospective epidemiological study found that the use of anticholinergic drug increases the cumulative risk of cognitive decline and mortality [Citation15]. In addition, the use of anticholinergic drug was reported to have adverse effects on cognitive and physical function in a systematic review of 46 studies including 60,944 participants, mostly older adults [Citation10]. Furthermore, impairments in orientation to place and time, record, and recall were shown in patients with AD who had positive serum anticholinergic activity in a recent study [Citation25]. As a matter of fact the association between the risk of the development of dementia and high ACB is consistent with the current literature data that cognitive impairment can be affected and progressed by use of anticholinergic medication.
It is supposed that SCD could be an early clinical manifestation of cognitive impairment in future [Citation26]. In line with this, the risk of future dementia was found to be increased 6-, 3.2-, and 1.6-fold in cognitively normal older adults at 70, 75, 80 ages who reported subjective memory complaints, respectively [Citation27]. In addition, pathological changes in dementia including hippocampal atrophy, loss of entorhinal cortex volume, increase in subcortical white matter lesions, temporal atrophy, and increase in some cerebrospinal fluid biomarkers were shown to have occurred in subjects with SCD before starting the decrease in cognition [Citation26,Citation28,Citation29]. It can be suggested that anticholinergic drugs started in SCD subjects who are at risk for cholinergic neuron degeneration may promote the development of dementia by further increasing cholinergic deficiency.
Our study demonstrated that the majority of drugs with anticholinergic properties consisted of trospium, metoprolol, paroxetine, and trazodone in subjects with high ACB. It should be noted that the uses of trospium and paroxetine were increased in subjects with SCD who developed dementia compared to overall use of those ( and ), indicating high prescribing rate of these drugs despite to be present alternative treatment strategies and known risks to older adults [Citation30,Citation31]. Besides metoprolol and trazodone have low ACB, suggesting that the cumulative ACB by adding the drugs with low anticholinergic properties may emerge as a clinically risky condition for cognitive status in subjects with SCD. Most recently, one study reported that high ACB resulted frequently from a combination of low anticholinergic drug in older adults [Citation32]. For these reasons, it would be useful for clinicians to evaluate and review drugs with high anticholinergic properties and the cumulative ACB when seeing older patients and prescribing in outpatient settings. The clinical association between drugs with anticholinergic properties and cognitive impairment in subjects with SCD warrants further investigation.
Advanced age is a crucial risk factor for dementia. When looking at age groups, while the prevalence of dementia is 3% in the 65–74 age group and 18.7% in the 75–84 age group, the prevalence increases to 47.2% among subjects aged 85 years and older [Citation33]. There is no important difference in the incidence of dementia until 85 years old, but a significant increase (76.1 per 1000 person years) is seen after that age [Citation34]. Consistent with literature, we found that there was an 11-fold increase in the future dementia risk among subjects aged 85 years and older compared to those aged 65–74 years. On the other hand, the risk of dementia was significantly increased in the 75–84 age group in univariate analysis, but this relationship was lost in multivariate analysis. This finding could be explained with the small number of subjects in age groups. Moreover, we saw that the group developed dementia was significantly older than the other group. However, the risk of developing dementia in subjects under long-term high ACB, even when age-adjusted by Cox regression analysis, increased significantly.
The depression accompanied by cognitive impairment (predominantly deficits in information processing, memory, and executive dysfunction), which is more likely occur in older people with major depression, can be confused with dementia at advanced ages [Citation35,Citation36]. This comorbidity is just named as dementia syndrome of depression, which is previously known as “pseudodementia” [Citation35]. Antidepressant treatment is effective for older patients with this comorbidity to improve cognitive status, physical function, quality of life, and mood, while dementia patients do not respond to this treatment [Citation35]. To eliminate this confounding factor, we excluded cases with dementia syndrome of depression, so those included did not have cognitive impairments. Besides, the definitive diagnoses of cases in group II were dementia, in which there were no cases of dementia syndrome of depression or overdiagnosis. Moreover, depression status was not different among groups (p = .504). Lastly, depression ratio was lower in those with high ACB, while the risk of dementia was higher in the same group (data not shown). For these reasons, depression did not appear to be a risk factor in this special group of patients.
The results of this study reveal that SCD should not be considered to be clinically negligible, particularly in older adults under high ACB. In this regard, screening of anticholinergic drugs and calculation of ACB is of importance during routine geriatric assessment visits in older adults with SCD to reduce the development of cognitive impairment and dementia [Citation37]. In addition, it would be helpful to raise awareness about the risk of cognitive impairment and other comorbid conditions due to high ACB among doctors and other health care providers. So, it should be noted that, because of the central nervous system side effects, good clinical assessment are required before prescribing anticholinergic drugs in older adults with SCD.
The main strength of this retrospective cohort study was the long-term median follow-up over 6 years that allowed us enough time to observe cognitive changes. Another notable strength of this study was the use of the reliable and well-designed database of a geriatric centre. Thus, it provided us to examine better evaluation of the use of drug with anticholinergic properties. Moreover, the work on the same database reduces variation in all assessments by expert geriatricians and recorded data. However, our findings should be interpreted with caution as there are several important limitations of the study. Firstly, the retrospective and non-randomized design might introduce selection bias into results. Secondly, the number of cases who progressed to dementia was small, so could not analyse the interrelationship between ACB and the future risk of dementia subtype. Furthermore, our findings could not reflect the general older population because of small sample size of the study.
Conclusions
To the best of our knowledge, this is the first study which provided evidence that high ACB appears to impair cognitive status and increase the risk of dementia in older adults with SCD. The results of our study are promising, however the effect of ACB on cognitive status among subjects with SCD is still lacking. To clarify the association between ACB and the risk of dementia, large and longer prospective studies are needed in this population.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population- based studies. Int J Geriatr Psychiatry. 2000;15(11):983–991. doi: 10.1002/1099-1166(200011)15:11<983::AID-GPS238>3.0.CO;2-5
- Jorm AF, Christensen H, Korten AE, et al. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31(3):441–449.
- Pearman A, Storandt M. Self-discipline and self-consciousness predict subjective memory in older adults. J Gerontol B Psychol Sci Soc. 2005;60(3):153–157. doi: 10.1093/geronb/60.3.P153
- Waldorff FB, Siersma V, Vogel A, et al. Subjective memory complaints in general practice predicts future dementia: a 4-year follow-up study. Int J Geriatr Psychiatry. 2012;27(11):1180–1188. doi: 10.1002/gps.3765
- Abner EL, Kryscio RJ, Caban-Holt AM, et al. Baseline subjective memory complaints associate with increased risk of incident dementia: the PREADVISE trial. J Prev Alzheimers Dis. 2015;2(1):11–16.
- Zwan MD, Villemagne VL, Dore V, et al. Subjective memory complaints in APOƐE4 carriers are associated with high amyloid-β burden. J Alzheimers Dis. 2015;49(4):1115–1122. doi: 10.3233/JAD-150446
- Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x
- Gray SL, Joseph T. Hanlon. anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217–224. doi: 10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain – how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151–159. doi: 10.1111/bcpt.12140
- Fox C, Smith T, Maidment I, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age and Ageing. 2014;43(5):604–6615. doi: 10.1093/ageing/afu096
- Naples JG, Zachary AM, Perera S, et al. Concordance among anticholinergic burden scales. J Am Geriatr Soc. 2015;63(10):2120–2124. doi: 10.1111/jgs.13647
- Pasina L, Djade CD, Lucca U, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs Aging. 2013;30(2):103–112. doi: 10.1007/s40266-012-0044-x
- Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–159. doi: 10.1212/WNL.0b013e3181e7f2ab
- Shah RC, Janos AL, Kline JE, et al. Cognitive decline in older persons initiating anticholinergic medications. PLoS One. 2013;8(5): e64111. doi: 10.1371/journal.pone.0064111
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x
- Folstein MF, Folstein S, McHugh PR. “Minimental state”: a practical method for grading the cognitive state of patient for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6
- Cangoz B, Karakoc E, Selekler K. The norm determination and validity-reliability studies of clock drawing test on Turkish adults and elderly (ages 50 and over). Turk J Geriatr. 2006;9(3):136–142.
- Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–172. doi: 10.1300/J018v05n01_09
- Reisberg B, Prichep L, Mosconi L, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4(1):S98–S108. doi: 10.1016/j.jalz.2007.11.017
- Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5-6):471–485. doi: 10.1159/000096295
- American Psychiatric Association. Diagnostic and statistic manual of mental disorders. 4th ed. Printing Washington (DC): American Psychiatric Association; 1994.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Printing Arlington (VA): American Psychiatric Publishing; 2013.
- Campbell NL, Maidment I, Fox C, et al. The 2012 update to the anticholinergic cognitive burden scale. J Am Geriatr Soc. 2013;61(1):142–143.
- Zia A, Kamaruzzaman S, Myint PK, et al. Anticholinergic burden is associated with recurrent and injurious falls in older individuals. Maturitas. 2016;84:32–37. doi: 10.1016/j.maturitas.2015.10.009
- Konishi K, Hori K, Hachisu M, et al. Assessment of cognitive dysfunction caused by anticholinergic burden in Japanese Alzheimer’s disease patients, using the most commonly used scales in Japan. Nihon Shinkei Seishin Yakurigaku Zasshi. 2015;35(5-6):113–118.
- Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27(12):1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010
- Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52(12):2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x
- Norden AGWV, Fick WF, De Laat KF, et al. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology. 2008;71(15):1152–1159. doi: 10.1212/01.wnl.0000327564.44819.49
- Haley AP, Hoth KF, Gunstad J, et al. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am J Geriatr Psychiatry. 2009;17(11):976–985. doi: 10.1097/JGP.0b013e3181b208ef
- Gormley EA, Lightner DJ, Burgio KL, et al. American urological association; society of Urodynamics, female pelvic medicine & urogenital reconstruction. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6):2455–2463. doi: 10.1016/j.juro.2012.09.079
- Tedeschini E, Levkovitz Y, Iovieno N, et al. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(12):1660–1668. doi: 10.4088/JCP.10r06531
- Reppas-Rindlisbacher CE, Fischer HD, Fung K, et al. Anticholinergic drug burden in persons with dementia taking a cholinesterase inhibitor: the effect of multiple physicians. J Am Geriatr Soc. 2016;64(3):492–500. doi: 10.1111/jgs.14034
- vans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262(18):2551–2556. doi: 10.1001/jama.1989.03430180093036
- Ott A, Breteler MM, van Harskamp F, et al. Incidence and risk of dementia. The Rotterdam study. Am J Epidemiol. 1998;147(6):574–580. doi: 10.1093/oxfordjournals.aje.a009489
- Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345:36–46. doi: 10.1111/nyas.12669
- Gonda X, Pompili M, Serafini G, et al. The role of cognitive dysfunction in the symptoms and remission from depression. Ann Gen Psychiatry. 2015;14:32. doi: 10.1186/s12991-015-0068-9
- Covington LP, McCarrell J, Hoerster NS. Prevalence of anticholinergic medication use in the program of all-inclusive care for the elderly. Consult Pharm. 2016;31(3):168–174. doi: 10.4140/TCP.n.2016.168
