ABSTRACT
OBJECTIVES: Stimulants are recommended as the first-line pharmacotherapy in attention deficit/hyperactivity disorder (ADHD). Methylphenidate (MPH) is the most used stimulant. Medikinet Retard has modified-release capsules of MPH (MRC-MPH). In this study, we aimed to report observations on a sample of outpatients, who had been previously treated with other agents, but switched to MRC-MPH treatment. These observations focus on the treatment course, efficacy, side effects, and switching reasons.
METHODS: We included 20 out of the 163 patients with ADHD, who were previously treated with other medications, and switched to MRC-MPH. Turgay DSM-IV Based Child and Adolescent Behavior Disorders Screening as diagnosing tool and Rating Scale, Barkley’s Stimulants Side Effects Rating Scale for screening side effects and Clinical Global Impression Scale-Severity and -Improvement were administered.
RESULTS: Patients’ ages ranged between 9 and 17 years. Mean Clinical Global Impression Scale-Severity (CGI-S) score before the MRC-MPH treatment was 3.2, whereas after treatment it was 3.15. CGI-S scores were not significantly different (p = .593). Loss of appetite (n = 4, 20%) and drowsiness (n = 4, 20%) were the most common adverse events during the MRC-MPH treatment.
CONCLUSIONS: We did not observe significant difference between other treatment options and MRC-MPH with respect to efficacy. In terms of side effect profile, Osmotic Release Oral System-MPH was observed to be more problematic than immediate-release MPH and MRC-MPH formulations, while these two regimens did not differ significantly.
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a persistent condition with inattention and/or hyperactivity-impulsivity that interferes with functioning or development, with symptoms in two or more settings (e.g. at home, school, or work; with friends or relatives; in daily activities), and affects social, academic, and occupational functioning negatively [Citation1]. It is one of the most common childhood psychiatric disorders with a worldwide prevalence rate of 5.3% [Citation2]. When the patients with ADHD are left without treatment, long-term outcomes are poor relative to non-ADHD controls. On the other hand, the treatment of ADHD is associated with better academic and social life outcomes for individuals with ADHD [Citation3].
Proven effective psychopharmacological treatments are mainly divided into two groups: stimulants and non-stimulants [Citation4]. Possible pharmacologic mechanisms of stimulants are (1) increasing extracellular levels of dopamine via blocking dopamine transporters and (2) potentiation of dopamine neurotransmission via enhancing the binding of dopamine in the prefrontal cortex of the brain [Citation5]. The non-stimulant group includes atomoxetine (ATX), clonidine, and guanfacine. Clonidine and guanfacine are not used widely in the treatment of ADHD in Turkey while ATX is prescribed frequently. ATX is a selective noradrenaline reuptake inhibitor, acting by increasing the amount of noradrenaline in synaptic gaps via inhibition of noradrenaline transporter in the prefrontal cortex [Citation4].
Stimulants are mostly recommended as the first-line treatment [Citation6] and the methylphenidate (MPH), a stimulant agent, is the most frequently prescribed and the best-studied medication in the treatment of ADHD [Citation7]. The MPH treatment improves symptoms, functionality, and life quality of patients with ADHD, while causing various frequent side effects that cause problems but do not treat the life of the patients [Citation8].
MPH has immediate- and extended-release formulations. These formulations contain immediate- and extended-release components that allow both rapid onset of action and continuous effect throughout the day. The use of extended-release MPH (ER-MPH) formulations has increased considerably in recent years [Citation7]. There are two types of ER-MPH products in Turkey: Concerta® (Janssen-Cilag Ltd, High Wycombe, UK) and Medikinet Retard® (MEDICE Pharma GmbH and co. KG, Iserlohn, Germany).
The cover of Concerta® dissolves within 1–2 h and releases 22% of the total dose of MPH. The remaining 78% of the dose is gradually released in 10 h throughout the day using the Osmotic Release Oral System (OROS®) [Citation9,Citation10]. Medikinet Retard® has modified-release capsules of MPH (MRC-MPH), which contains equal proportions of immediate- and extended-release MPH [Citation10], providing 8 h of efficacy.
A multi-site, randomized, double-blind, crossover trial comparing the effects of OROS-MPH and MRC-MPH concluded that children and adolescents with ADHD could be treated with a lower daily dose of MRC-MPH (which has a similar IR component as OROS-MPH) without resulting in clinically relevant worse effect during school time [Citation9].
In this study, we aimed to explore the efficacy and side effect profile of Medikinet Retard® in the sample of ADHD patients previously treated with OROS-MPH, immediate-release MPH (IR-MPH), or ATX medications.
Materials and methods
Participants
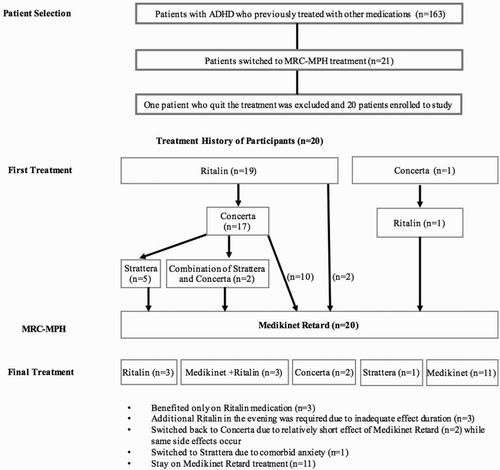
This report of case series was conducted in Disruptive Behavior Disorders Clinic of Ege University Child and Adolescent Psychiatry Department. Among 163 patients with ADHD admitted to this outpatient clinic and following up there in between January 2010 and April 2016, there were 21 patients who switched to MR-MPH treatment. Among them, one patient quits the treatment and was excluded from the study. The remaining 20 patients enrolled to the study were observed regarding efficacy, side effects, and switching reasons.
Their ADHD diagnoses were made via clinical assessment according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) [Citation11] by senior resident under the supervision of professor of child and adolescent psychiatry. Turgay DSM-IV Based Child and Adolescent Behavior Disorders Screening and Rating Scale (T-DSM-IV-S) was routinely applied to patient’s parents and teachers as screening and diagnostic tool in initial assessment, whereas the Clinical Global Impression Scale (CGI) was used as follow-up instrument.
Inclusion criteria were as follows: consent for participating in the study, switching to MRC-MPH treatment while previously receiving MPH/ATX medications for ADHD other than MRC-MPH. Exclusion criteria were comorbid diagnosis of bipolar disorder, psychosis, seizure disorder, recent history of drug or alcohol abuse, mental retardation, typical autism, arrhythmia or serious hypertension and serious suicidal ideation determined by the child psychiatrist.
Measurements and scales
Turgay DSM-IV Based Child and Adolescent Behavior Disorders Screening and Rating Scale (T-DSM-IV-S): This is a widely used diagnostic tool for ADHD and disruptive behaviour [Citation12,Citation13]. In the present study, it was used to evaluate the inattention, hyperactivity, and disruptive behaviour symptoms of the children. The T-DSM-IV Scale was developed by Turgay, and was translated/adapted to Turkish by Ercan et al. [Citation13]. It is based on the DSM-IV diagnostic criteria, and assesses hyperactivity/ impulsivity (9 items), inattention (9 items), opposition/defiance (8 items), and conduct disorder (15 items). The symptoms are scored by assigning a severity estimate for each symptom on a four-point likert-type scale (namely: 0 not at all, 1 just a little, 2 quite a bit, and 3 very much). In our study, we used hyperactivity/ impulsivity (9 items) and inattention (9 items) parts of this scale to determine the severity and subtype of ADHD.
The Clinical Global Impression Scale (CGI): This scale was developed for the use in clinical trials to provide general information of the patient’s global functioning according to clinician’s decision before and after the initiation of a medication [Citation14]. The CGI has two components; the CGI-Severity, which rates illness severity, and the CGI-Improvement, which rates the change from the initiation (baseline) of the treatment.
The Clinical Global Impression Scale-Severity (CGI-S): The CGI-S asks the clinician how mentally ill the patient is at the time of assessment, and the answer is rated as follows: 1 = normal, not at all ill; 2 = borderline mentally ill; 3 = mildly ill; 4 = moderately ill; 5 = markedly ill; 6 = severely ill; and 7 = among the most extremely ill patients. This rating is based on the observation and history of patient’s symptoms, behaviour, and function in the past seven days.
The Clinical Global Impression Scale-Improvement (CGI-I): The CGI-I is an instrument used by clinicians to compare patient’s clinical overall condition between the baseline and post-treatment visits. The CGI-I is rated on a seven-point scale: 1 = very much improved since the initiation of treatment; 2 = much improved; 3 = minimally improved; 4 = no change from baseline (the initiation of treatment); 5 = minimally worse; 6 = much worse; and 7 = very much worse since the initiation of treatment.
In our study, the CGI-S and the CGI-I were administered just before the initiation of MRC-MPH treatment and after using the effective dose.
Barkley’s Stimulants Side Effects Rating Scale (BSSERS): Side effects of treatment agents were measured using BSSERS [Citation15] by a clinician via asking each side effect in the questionnaire to the patients and their parents. This is a 17-item scale including frequently reported behavioural and physical symptoms during the treatment in children with ADHD. BSSERS includes insomnia, appetite, physical symptoms such as head and stomach aches, and emotionality and irritability on a scale of zero to nine (0 = absent; 9 = serious). Although this tool can be used to measure the side effect’s severity, we only used the side effect titles in the scale to monitor them.
Procedure
Enrolled 20 patients who were planned to start on MRC-MPH treatment were evaluated before and during the treatment regarding treatment history, side effects, efficacy, and switching reasons. Observations were recorded, and CGI-S and CGI-I were administered. After effective dose of MRC-MPH treatment according to clinician’s decision, patients were reassessed and CGI-S and -I were re-administered. The study ended with recording the last treatment option according to individual features and clinical decisions.
The study protocol was approved by the Ege University Faculty of Medicine, Child and Adolescent Psychiatry Department Academic Committee. All parents and children were informed about the study and the study procedure. Informed consent was obtained from the mothers of the patients and the children also verbally assented to the study.
Data analysis
Statistical analyses of this study were carried out by Medical Statistics Department of Medicine Faculty using IBM SPSS Statistics Version 20.0 program. Descriptive statistics were used to present the participant characteristics and follow-up data. The patients’ scores of CGI-S and CGI-I were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk’s test) to determine whether or not they are normally distributed. Descriptive analyses were presented using medians and interquartile range (IQR) for the non-normally distributed variables. Since the CGI-S and CGI-I scores were not normally distributed; non-parametric tests were conducted to compare these parameters. The Wilcoxon test was used to compare the change in CGI-S and CGI-I scores between baseline and after the MRC-MPH treatment. A p-value of less than .05 was considered as the indicator of a statistically significant result.
Results
Socio-demographic and clinical characteristics of participants
There were 17 boys (85%) and 3 girls (15%) in the study. The current mean age of the participants was 13.4 years (SD = 2.25, min = 9, max = 17.5).
Current DSM-IV-TR diagnoses
According to T-DSM-IV-S, 15 of the patients (75%) were diagnosed with combined type of ADHD and the remaining 5 patients (25%) had inattentive type of ADHD. The comorbid DSM-IV-TR diagnoses of our sample are shown in .
Table 1. Comorbid diagnoses of patients.
Treatment of participants
Treatment histories of 20 patients, who were prescribed with MRC-MPH, are presented in .
IR-MPH was prescribed as the first-line treatment in 19 patients while one patient was on 27 mg OROS-MPH. The mean dose of IR-MPH was 28.42 mg/day (SD: 8.5 mg, min: 15 mg, max: 40 mg). Treatment response, side effects, and reasons for drug switch from the first-step treatment are summarized in .
Table 2. Summary of the first-line treatment data.
As the second treatment choice, OROS-MPH was used in 17 patients. The mean dose of OROS-MPH was 36.00 mg/day (SD: 12.32 mg, min: 18 mg, max: 54 mg). Also, MRC-MPH was administered in two patients while 20 mg IR-MPH was used in one patient. Similarly, the summary of the second-line treatment data is demonstrated in .
Table 3. Summary of the second-line treatment data.
Only seven patients used ATX (n = 5) and combination of OROS-MPH and ATX (n = 2) prior to the MRC-MPH treatment. The mean dose of ATX was 43.29 mg/day (SD: 25.11 mg, min: 18 mg, max: 85 mg). The details of these patients’ treatment are summarized in .
Table 4. Summary of the third-line treatment data.
Twenty of 163 patients were prescribed with MRC-MPH treatment after all previous treatment agents. The mean dose of MRC-MPH was 33.5 mg/day (SD: 7.45 mg, min: 20 mg, max: 40 mg). Side effects observed during this treatment are listed in .
Table 5. Side effects of MRC-MPH treatment.
Scores of severity (CGI-S) and improvement (CGI-I) were rated both before the initiation of and after the MRC-MPH treatment. The mean CGI-S score before the MRC-MPH treatment was 3.2, whereas after the treatment it was 3.15. The CGI-S scores were not significantly different according to the Wilcoxon test before and after the MRC-MPH treatment (p = .593).
The mean CGI-I score before the MRC-MPH treatment was 2.75, whereas after 3 months of the treatment, it was 2.85. The CGI-I scores were not found significantly different, either, according to the Wilcoxon test before and after the MRC-MPH treatment (p = .564).
Discussion
We investigated the efficacy and side effect profile of MRC-MPH in outpatient clinic patients who had been medicated by other psychostimulants and/or ATX prior to the MRC-MPH treatment.
IR-MPH was used as the first-line treatment in our study except for one patient. It was observed that IR-MPH was effective for ADHD symptoms in most of the participants. In addition, side effects of IR-MPH treatment were not common and severe. However, the main limitedness of IR-MPH treatment was poor adherence to treatment, and it was the main reason for drug alteration in our sample.
OROS-MPH was prescribed to 18 patients as the first ER-MPH preparation. Response to OROS-MPH treatment was high, with 13 patients specified as responsive. However, side effects were observed in a substantial part of the patients, and the treatment was changed due to side effects. Among these side effects, loss of appetite was the most common side effect as it was observed in eight patients on OROS-MPH. Drowsiness, stomachache, thoracic pain, and nausea were the other frequent side effects in this group.
In another study about the efficacy of IR- and ER-MPH formulations, authors concluded that both presentations have the same effect on ADHD symptoms [Citation16]. In our study, we did not compare the efficacy of IR-MPH and OROS-MPH specifically. Nevertheless, we observed similar responses to treatment while there were substantially more side effects in patients using OROS-MPH. The findings of a recent review, which considers studies comparing IR- and ER-MPH formulations, indicated that long-acting forms of MPH had a modest effect on the severity of inattention/overactivity and hyperactivity/impulsivity according to parent reports, whereas the short-acting MPH was preferred according to teacher reports for hyperactivity [Citation17]. On the other hand, similar adverse events were observed with IR- and ER-MPH formulations in this study [Citation17].
In our study, ATX was prescribed to five patients, and it was combined with OROS-MPH in two patients. Inadequate treatment response and common side effects were observed, but these findings are not reliable given the small size of the group. Furthermore, ATX was prescribed to patients as the third choice in our sample. Therefore, these patients were relatively non-responsive and more likely to develop side effects. Consequently, we cannot imply that ATX is not as effective as MPH formulations in the treatment of ADHD. In a current review, which investigated the ATX treatment, it was concluded that ATX was significantly less effective than OROS-MPH and other extended-release mixed amphetamine salts. However, in this report, ATX was found non-inferior to IR-MPH regarding efficacy [Citation18]. A recent study indicated that the balance of benefits and risks of ATX in the treatment of ADHD was positive [Citation19].
In our clinical sample, 20 patients were prescribed with MRC-MPH as the third or fourth choice, after the previous treatment options were given up. For our sample, substitution with MRC-MPH was due to side effects of the other treatments rather than an insufficient response. We did not observe significant difference between other treatment options and MRC-MPH regarding efficacy. However, side effects were seen less frequently when compared to OROS-MPH and ATX or the combination of these two agents. Looking at the side effects of the MRC-MPH treatment, loss of appetite and drowsiness were the most frequent ones, as each of these symptoms was observed in four patients. Corresponding to our findings, in COMAC study which OROS-MPH and MRC-MPH were compared, it has been found that the average effects of MRC-MPH were greater than OROS-MPH during a typical school day (1.5–7.5 h post-dose) whereas OROS-MPH had significantly greater effect in an early evening. Therefore, according to this study, these agents did not differ notably in the total outcome and a clinician may select the most appropriate once-daily stimulant for each child with ADHD [Citation20]. Besides these data, in a recent systematic review authors concluded that no one long-acting MPH preparation is clearly superior to another [Citation10].
On the other hand, a study of MRC-MPH in the routine treatment of adolescents with ADHD stated a reduction of the total ADHD symptom severity in 78% of patients who were previously treated with different MPH formulations [Citation21].
Current evidence along with the findings of other studies suggests that different patients respond differently to various ADHD medications. Undoubtedly, certain children will benefit from the use of long-acting formulations, while others will only require immediate action doses, and still others a combination of both or ATX. It is difficult to interpret the diversity in response to treatment of ADHD and further research is required for an improved understanding.
This study has several limitations. First, the number of patients in our study is quite small to interpret efficacy and side effect profile of the utilized drugs. Therefore, our findings cannot be generalized. Second, our study was not designed as a comparison of these agents. It is just a summary of our clinical experience about Medikinet Retard®, which is a novel ER-MPH formulation in Turkey. Third, our outcomes were measured in outpatient clinic settings by the same clinician, and our findings are dependent on the information gathered from parents and teachers. The results could be different from a method that depends on direct observation or measurement of clinical symptoms. In order to reach more reliable and more generalizable results, these studies should be repeated with a larger sample and better designed controlled studies.
Conclusion
From the observations in this series of cases, it can be concluded that MRC-MPH is an effective option in the treatment of ADHD; however, there is no significant difference compared to other treatments with regard to efficacy. In respect of adverse events, OROS-MPH seemed worse than IR-MPH and MRC-MPH formulations, while the latter two regimens did not differ significantly. Unfortunately, the available data do not permit us to address questions about the variability in treatment response and tolerability. It is assumed that pharmacokinetics, MPH isomer ratios in different formulations, comorbidity, or personal parameters (i.e., pharmacodynamic characteristics and genetics) may play a role in those variations. There is a great demand for future studies for finding out the reasons that underlie these variations in response and tolerability in different ADHD treatment options.
Acknowledgements
The authors would like to thank Mehmet N. Orman for his contribution in conducting the medical statistical analyses. We are also grateful to all the families that took part in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Uğur Tekin http://orcid.org/0000-0001-6720-4292
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013; DOI:10.1176/appi.books.9780890425596.
- Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:942. doi: 10.1186/1741-7015-10-99
- Treuer T, Gau SS-F, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23:179–193. doi: 10.1089/cap.2012.0093
- Frolich J, Banaschewski T, Spanagel R, et al. The medical treatment of attention deficit hyperactivity disorder (ADHD) with amphetamines in children and adolescents. Z Kinder Jugendpsychiatr Psychother. 2012;40:287–300. doi: 10.1024/1422-4917/a000185
- Pliszka SR, Crismon ML, Hughes CW, et al. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb
- Castle L, Aubert RE, Verbrugge RR, et al. Trends in medication treatment for ADHD. J Atten Disord. 2007;10:335–342. doi: 10.1177/1087054707299597
- Storebo OJ, Krogh HB, Ramstad E, et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ. 2015;351:h5203. doi: 10.1136/bmj.h5203
- Dopfner M, Ose C, Fischer R, et al. Comparison of the efficacy of two different modified release methylphenidate preparations for children and adolescents with attention-deficit/hyperactivity disorder in a natural setting: comparison of the efficacy of medikinet® retard and concerta® —a randomized, controlled, double-blind multicenter clinical crossover trial. J Child Adolesc Psychopharmacol. 2011;21:445–454. doi: 10.1089/cap.2010.0082
- Coghill D, Banaschewski T, Zuddas A, et al. Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry. 2013;13:942. doi: 10.1186/1471-244X-13-237
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR). 4th ed. Arlington (VA): American Psychiatric Association; 2000; DOI:10.1176/appi.books.9780890423349.
- Turgay A. Disruptive behavior disorders child and adolescent screening and rating scale for children, adolescents, parents, and teachers. West Blomfield (MI): Integr. Ther. Inst. Publ.; 1994.
- Ercan ES, Amado S, Somer O, et al. Development of a test battery for the assessment of attention deficit hyperactivity disorder. Çocuk ve Gençlik Ruh Sağlığı Derg. Çocuk ve Gençlik Ruh Sağlığı Derg. Turkish J Child Adolesc Ment Heal. 2001;8:132–144.
- National Institute of Mental Health. CGI (Clinic Global Impression) Scale. Psychopharmacol Bull. 1985;21:839–843.
- Barkley RA, McMurray MB, Edelbrock CS, et al. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192.
- Durand-Rivera A, Alatorre-Miguel E, Zambrano-Sanchez E, et al. Methylphenidate efficacy: immediate versus extended release at short term in Mexican children with ADHD assessed by Conners scale and EEG. Neurol Res Int. 2015: 2015:207801.
- Punja S, Zorzela L, Hartling L, et al. Long-acting versus short-acting methylphenidate for paediatric ADHD: a systematic review and meta-analysis of comparative efficacy. BMJ Open. 2013;3:e002312.
- Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11:203–226.
- Reed VA, Buitelaar JK, Anand E, et al. The safety of atomoxetine for the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a comprehensive review of over a decade of research. CNS Drugs. 2016;30:603–628. doi: 10.1007/s40263-016-0349-0
- Swanson JM, Wigal SB, Wigal T, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (The Comacs Study). Pediatrics. 2004;113:e206–e216. available at http://pediatrics.aappublications.org/content/113/3/e206.abstract. doi: 10.1542/peds.113.3.e206
- Sobanski E, Döpfner M, Ose C, et al. A non-interventional study of extended-release methylphenidate in the routine treatment of adolescents with ADHD: effectiveness, safety and adherence to treatment. ADHD Atten Deficit Hyperact Disord. 2013;5:387–395. doi: 10.1007/s12402-013-0113-y