ABSTRACT
Pregabalin reduces central neuronal excitability and has gamma-aminobutyric acid-mimetic features, which was demonstrated in rats. Pregabalin is indicated for the treatment of neuropathic pain, post-herpetic neuralgia, fibromyalgia and partial seizures. However, pregabalin is widely used for off-label conditions. Euphoria is a frequent side effect of pregabalin and several cases of overdose from recreational use have been reported until recently. Our aim is to present a case of a patient with myoclonus after high dose nasal pregabalin misuse. A 23-year-old female patient presented to the emergency department with unconsciousness and involuntary movements in her arms. She used, 10–15 numbers of 300 mg pregabalin capsules by nasal inhalation for recreational purpose for the first time. Her vital signs were within normal limits. The patient's serum ethanol, drug and substance levels were negative. During the examination, myoclonus with 2–3 beats was occasionally observed in the upper extremities. Emergency brain computed tomography was normal. During the electroencephalography (EEG monitorization, one generalized spikewave activity was observed for a second. We started 800 mg valproic acid i.v. therapy and prescribed valproic acid 500 mg/day p.o. at discharge. One week after discharge, she had no myoclonic jerks even though she did not use the recommended antiepileptic drug. Control EEG was within normal limits. Pregabalin overdose was suggested to be relatively safe but myoclonic seizures should be taken into consideration as a side effect.
Introduction
Pregabalin was first introduced in 2004 and was approved by US Food and Drug Administration (FDA) for the treatment of neuropathic pain, post-herpetic neuralgia, fibromyalgia and partial seizures. However, pregabalin is widely used for off-label conditions such as substance use disorders, alcohol withdrawal syndrome, restless legs syndrome, migraine and vasomotor symptoms of menopause [Citation1,Citation2].
Pregabalin is an alkylated analogue of gamma-aminobutyric acid (GABA), which binds to the a2d subunit of voltage-gated calcium channels, reducing the central release of excitatory molecules. This mechanism is believed to contribute to their antinociceptive, anticonvulsant and anxiolytic properties [Citation1]. In Turkey, pregabalin is indicated for neuropathic pain, generalized anxiety disorder, fibromyalgia and partial epilepsy as add-on therapy at 150–600 mg/day doses. Euphoria is a frequent side effect of pregabalin and several cases of overdose from recreational use have been reported [Citation1,Citation2]. In the study of Zellner et al., seizure was defined as a rare intoxication symptom [Citation3].
Myoclonus is involuntary, jerky, shock-like movement due to muscular contractions or sudden lapses of muscle contraction in active muscles. There are various causes of myoclonus and pathophysiological mechanisms associated with this symptom [Citation4,Citation5]. Metabolic and toxic etiologies such as renal/hepatic failure, hyponatremia, hypoglycemia, hyperthyroidism, head trauma can cause myoclonus. Also many antiparkinsonian, antidepressant, anxiolytics, antiepileptic, analgesic, anesthetic drugs may induce myoclonus [Citation6]. Pregabalin was reported to increase spikewave activity in a dose-dependent way in an animal model study with rats. However, the mechanism of pregabalin-induced myoclonus remains unclear [Citation7]. Herein, we present a case of a patient with myoclonus after high dose nasal pregabalin misuse.
Case
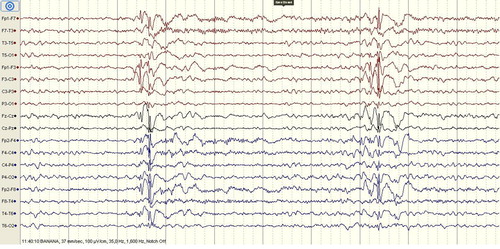
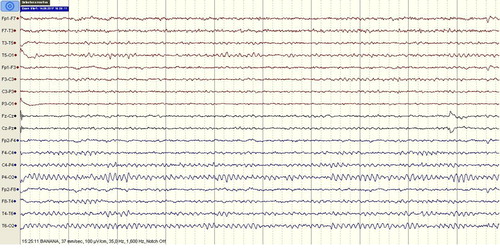
A 23-year-old female patient presented to the emergency department with unconsciousness and involuntary movements in her arms. In the detailed anamnesis obtained from her companions, she had no history of epilepsy or seizure and during the last night she used pregabalin for recreational purpose for the first time. She used 10–15 numbers of 300 mg pregabalin capsules by nasal inhalation until the morning hours. Two to three hours after the first pregabalin use, she had involuntary movements in her arms. After the last inhalation of pregabalin, the patient suffered from dizziness and myoclonic jerks. Her vital signs were within normal limits. She was lethargic and non-cooperate in the neurological examination. Pupils were isochoric and direct/indirect light reflexes were positive. No laterality was found on the motor examination. No flexor response was found in bilateral sole skin reflex. During the examination, myoclonus with 2–3 beats was observed in the upper extremities. Her brain computed tomography was normal. Her laboratory findings were within normal limits. A 32-channel scalp electroencephalography (EEG) was recorded and the basal activity was composed of waves of 9–10 Hz alpha rhythm. During the EEG monitorization, one generalized spikewave activity was observed for a second and the patient fell asleep after a while (). During the sleeping period, the generalized spikewave activity disappeared. Subsequently, for the treatment of myoclonic seizure, we started 800 mg valproic acid i.v. therapy and prescribed valproic acid 500 mg/day p.o. at discharge. She was evaluated by psychiatry in terms of substance use. She had a history of cannabis misuse but her last cannabis use was six months before. She had no history of suicide or psychiatric disorder. The patient's serum ethanol, drug and substance levels were negative. One week after discharge, she was evaluated in the neurology clinic and there were no myoclonic jerks even though she did not use the recommended antiepileptic drug. Control EEG was within normal limits (). Orally informed consent was obtained from the patient.
Discussion
Pregabalin reduces central neuronal excitability by binding to the a2d type 1 subunit of voltage-gated calcium channels. Although pregabalin does not bind to GABA receptors and not associated with GABA activity, it has GABA-mimetic features which was shown in rats [Citation2]. Over the therapeutic doses, sedation, dissociation, relaxation, pleasure, drowsiness, disinhibition, improved sociability, empathy or auditory and visual hallucinations may occur. Clinical trials provided evidence that pregabalin may cause euphoria 4% and up to 12% versus placebo. Euphoria seems to be a dose-dependent adverse effect of pregabalin. The experience of euphoria might be the key factor that incites some patients to misuse large doses of pregabalin [Citation1].
Pre-marketing clinical trials demonstrated low potential for abuse and limited dependence liability if misused. Pregabalin has been described as a superior psychotropic drug for recreational purposes to achieve specific alcohol/benzodiazepine-like effects mixed with euphoria, entactogenic feelings and dextromethorphan-like dissociation, and to cope with opioid withdrawal [Citation8,Citation9]. Pregabalin may have a higher addiction potential due to its rapid absorption with peak plasma concentration achieved within one hour. There are case reports of its misuse, particularly in cocaine users and prisoners [Citation10].
Misuse of pregabalin, at dosages up to 20 times higher than the maximal dosage indicated, mostly seems to occur orally, but intravenous, nasal insufflation, rectal (“plugging”), smoking and “parachuting” (emptying the content of the capsule into a pouch) have also been reported [Citation9]. Zellner et al. suggested that pregabalin is the fifth most frequently abused substance. In addition, pregabalin users consume additional substances [Citation3]. Studies specifically assessing patients with opioid use disorder demonstrated that pregabalin abuse varied widely, from 3% to 68%. Following the acute overdose, pregabalin appears relatively safe, most commonly causing hypotension, tachycardia and central nervous system effects at greater magnitude compared to the adverse effects reported with therapeutic doses [Citation1]. In the study published by Zellner et al., intoxication symptoms were defined as impaired consciousness, respiratory distress, agitation/aggressiveness, restlessness, hallucinations and seizures [Citation3]. In some cases it was shown that add-on therapy of pregabalin can induce myoclonus in patients with seizure history. In patients with renal failure pregabalin also can evoke myoclonic seizures [Citation11–13].
In our case, the patient had never used pregabalin before but had a history of substance abuse. As pointed before, pregabalin misuse is mostly associated with substance use disorders. Although it is known that patients misusing pregabalin prefer oral use, to the best of our knowledge, this is the first case of nasal pregabalin misuse reported in the literature. Pregabalin overdose is thought to be safe and complications are rare. In this case, after high dose nasal pregabalin inhalation, the patient had myoclonic seizure. In addition, control EEG, which was performed one week later, was within normal limits. Although it was shown that pregabalin use can induce myoclonus in elderly patients with renal failure, there is only one article demonstrating seizures as a result of pregabalin toxicity [Citation3]. Our case is the first report of myoclonus caused by nasal pregabalin inhalation.
The abuse potential of pregabalin is still controversial according to literature. Pregabalin is likely to be misused for its euphoric effect. Pregabalin misuse associated papers mainly emphasize that there is a history of drug or substance dependency in these patients. It is recommended to investigate the substance use history of a patient before prescribing pregabalin to identify patients with high-risk for misuse. Besides, in patients presenting with myoclonus, pregabalin misuse should be taken into consideration as an underlying etiology.
Acknowledgements
All authors have substantial contributions to conception and design (H. Mihrimah Ozturk), or acquisition of data, and analysis and interpretation of data (H. Mihrimah OZTURK and Gülin Morkavuk); drafting the article (H. Mihrimah Ozturk and Gülin Morkavuk) or revising it critically for important intellectual content (H. Mihrimah Ozturk and Gülin Morkavuk) and final approval of the version to be published.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403–426. doi:10.1007/s40265-017-0700-x.
- Schjerning O, Rosenzweig M, Pottegard A, et al. Abuse potential of pregabalin: a systematic review. CNS Drugs. 2016;30(1):9–25. doi:10.1007/s40263-015-0303-6.
- Zellner N, Eyer F, Zellner T. Alarming pregabalin abuse in Munich: prevalence, patterns of use and complications. Dtsch Med Wochenschr. 2017;142(19):e140–e147. doi:10.1055/s-0043-104228.
- Caviness JN. Treatment of myoclonus. Neurother. 2014;11(1):188–200. doi:10.1007/s13311-013-0216-3.
- Levy A, Chen R. Myoclonus: pathophysiology and treatment options. Curr Treat Options Neurol. 2016;18(5):447. doi:10.1007/s11940-016-0404-7.
- Janssen S, Bloem BR, van de Warrenburg BP. The clinical heterogeneity of drug-induced myoclonus: an illustrated review. J Neurol. 2017;264(8):1559–1566. doi:10.1007/s00415-016-8357-z.
- Knake S, Klein KM, Hattemer K, et al. Pregabalin-induced generalized myoclonic status epilepticus in patients with chronic pain. Epilepsy Behav. 2007;11(3):471–473. doi:10.1016/j.yebeh.2007.06.012.
- Hakkinen M, Vuori E, Kalso E, et al. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Sci Int. 2014;241:1–6. doi:10.1016/j.forsciint.2014.04.028.
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs. 2014;28(6):491–496. doi:10.1007/s40263-014-0164-4.
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European medicines agency’s “suspected adverse drug reactions” database. CNS Drugs. 2016;30(7):647–654. doi:10.1007/s40263-016-0359-y.
- Healy DG, Ingle GT, Brown P. Pregabalin- and gabapentin-associated myoclonus in a patient with chronic renal failure. Mov Disord. 2009;24(13):2028–2029. doi:10.1002/mds.22286.
- Hellwig S, Amtage F. Pregabalin-induced cortical negative myoclonus in a patient with neuropathic pain. Epilepsy Behav. 2008;13(2):418–420. doi:10.1016/j.yebeh.2008.04.006.
- Shimizu T, Yoshida T, Kitamura K, et al. Disturbance of consciousness and involuntary movements caused by pregabalin. BMJ Case Rep. 2012;2012; doi:10.1136/bcr-2012-007559.