ABSTRACT
OBJECTIVES: Bipolar disorder (BD) carries a high rate of morbidity and mortality, and the clarification of its aetiology continues to be an important field of research. In recent studies, the clinical characteristics of BDs have been explained through a number of underlying factors, including cytokines, steroids, neurotrophins, mitochondrial energy generation, oxidative stress, and neurogenesis. In this study, we aimed to investigate potential associations between BDs and inflammatory processes.
METHODS: Patients with mania or in remission who attended outpatient clinics of the Department of Psychiatry of the Ankara Numune Training and Research Hospital, and who were diagnosed with BD according to the DSM-V criteria, were included in the study. IBM SPSS Statistics 23 software was used for statistical analyses of the data. The normality of the distribution of continuous and discrete numerical variables was tested with a Shapiro–Wilk Test.
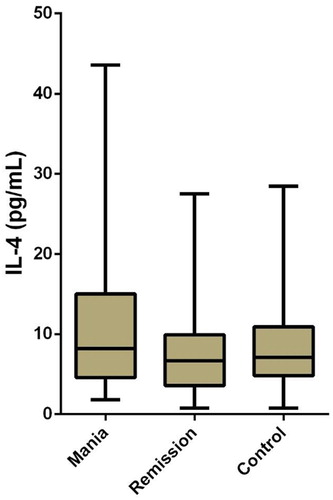
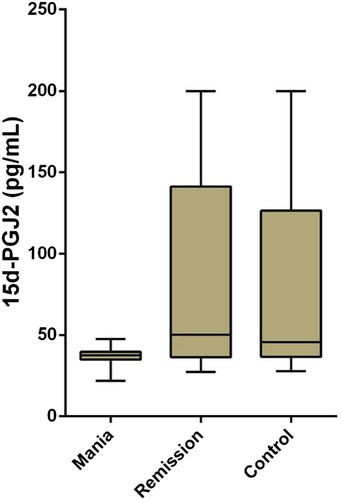
RESULTS: In this study, the measurements and statistical analyses revealed significantly lower 15-deoxy delta12, 14-prostaglandin J2 (15d-PGJ2) and Peroxisome Proliferator-Activated Receptor-Gamma (PPARγ) levels in patients with mania in comparison to healthy controls and patients in remission. Group comparisons did not reveal any significant differences between IL-4 levels.
CONCLUSIONS: This study was not a longitudinal study evaluating the same patients during both relapse and remission periods; no group of patients in a depressive episode of BD were included in the group comparisons either. Although this evidence is not adequate for a definite conclusion, the available evidence suggests that anti-inflammatory markers such as 15d-PGJ2 and IL-4 and nuclear PPARγ receptors are potential biomarkers to clarify the aetiology of BD, and these markers may be included among the therapeutic targets for future pharmacological modulations. Further studies are required in this area.
Introduction
Bipolar disorder (BD) is a psychiatric disorder with high mortality and morbidity rates [Citation1,Citation2]. There are new quests in the treatment of BD due to frequent side effects of the current treatments and the presence of resistant cases [Citation3]. Studies to understand the neurobiological changes underlying BD present a growing body of evidence that inflammation contributes to the pathophysiology of BD [Citation4].
Alterations in inflammatory markers are presented in many cases of psychiatric disorders in the literature including unipolar depression, BDs, and especially schizophrenia [Citation5–7]. Various hypotheses on pathophysiological mechanisms of schizophrenia involving inflammatory processes, caused both by external and endogenous factors, have been proposed [Citation8]. According to recent meta-analysis studies, favourable effects of add-on classic, non-steroidal anti-inflammatory drugs to antipsychotics on total, positive, and negative symptoms in schizophrenia are reported [Citation9,Citation10].
The role of inflammatory processes has been investigated in the pathophysiology of BD [Citation11,Citation12]. It is also reported in the literature that patients with BD have demonstrated alterations in the levels of peripheral members of inflammatory processes [Citation6]. Episodes of BD, depression and mania, are associated with the activation of inflammatory pathways as indicated by the increased levels of pro-inflammatory cytokines, positive acute-phase proteins [Citation13–16].
Recently, some endogenous balancing mechanisms activated in response to inflammatory stimuli have begun to be investigated in the sense of neuropsychiatric disorders [Citation17]. One of these mechanisms is the activation of peroxisome proliferator-activated receptors (PPARs). PPARs that were thought to have an inflammation-reducing effect have previously been studied in neuropsychiatric disorders such as schizophrenia, multiple sclerosis, and Alzheimer’s [Citation18–20]. PPARs are a group of nuclear receptor proteins that modulate gene expression and play a role in the modulation of transcription factors [Citation21].
Three subtypes of PPARs have been identified: alpha, beta, and gamma (α, β, and γ) [Citation22]. Recent studies show that PPARs (especially the gamma isoform, PPARγ) are potential targets for the treatment of various neuropathological conditions, including the main regulators of brain physiology and stress-related conditions [Citation17,Citation20,Citation23–25].
PPARγ is a nuclear receptor of 15d-PGJ2 which is one of the cyclooxygenase (COX)-derived products. 15d-PGJ2 is the endogenous ligand of PPARγ activated by the peroxisome proliferator, a transcription factor, which alleviates inflammation by primarily suppressing the expression of pro-inflammatory mediators [Citation25]. The 15d-PGJ2/PPARγ pathway regulates inflammatory processes through endogenous stabilization mechanism. This pathway is pharmacologically inducible and not only a potential biomarker, but is also involved in the pathophysiology of inflammation, which is an important new candidate therapeutic target in neurological and neuropsychiatric diseases [Citation26].
The role of inflammatory processes in the pathophysiology of BD is through many different pathways [Citation27]. Cytokines are also involved in the pathophysiology of the disease by providing a link between the peripheral immunity and the central nervous system [Citation6]. Today cytokines are also being used in diagnosis, treatment, and prognosis of BD [Citation28]. There are several studies in the literature that present different results with regard to IL-4 levels in patients with mania [Citation28–30]. Therefore, further research is needed to clarify IL-4 levels in patients during manic episodes (ME). Another motivation for our study is that there are conflicting findings in the literature about IL-4 in bipolar patients as well.
We claim that the levels of IL-4, PPARγ, and 15-d-PGJ2 in ME and remission are lower than compared to healthy controls. The aim of our study is to investigate the relationship between PPARγ, 15d-PGJ2, and IL-4 levels and the severity of the disease during ME in patients with BD.
Subjects and methods
Subjects
The study was designed to include patients with mania or in remission who attended the outpatient clinics of the Department of Psychiatry of the Ankara Numune Education and Research Hospital. The patients included in the study were diagnosed with BD according to the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-V) criteria [Citation31]. The study was approved by the ethics committee of Ankara Numune Training and Research Hospital, Ankara, Turkey (E-15-435, date: February 18, 2015), and an informed consent was given by all subjects recruited. The patients who applied to the clinic between February 2015 and October 2015 were included in the study. The control group included subjects without any known psychiatric or neurological disease history.
Methods
Each subject was interviewed by an experienced clinician before they participated in the study. A socio-demographic background questionnaire was filled out by a clinician. The Young Mania Rating Scale (YMRS) was used to assess the severity of their disease. The Hamilton Depression Rating Scale (HAM-D) was used to evaluate whether or not the patients were in an episode of depression. HAM-D and YMRS were also used to support the diagnoses of remission (i.e. HAM-D score <7 or YMRS score ≤4) and mania (i.e. YMRS score >20).
Venous blood samples (5cc) from antecubital veins were collected at 8:00 am after fasting overnight from each participant. Blood cells were separated from the serum. The serum samples were stored in a −80°C freezer. Biochemical analyses (PPARγ, 15d-PGJ2, and IL-4) were performed on these samples. Laboratory tests were applied to all patients, including high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), cholesterol, fasting blood glucose, C-reactive protein (CRP), and insulin measurements. Height, weight, waist, hip circumferences, systolic, and diastolic blood pressures of the patients were measured. The height, weight, waist, and hip circumferences of the patients were measured. The gender and age of each subject were recorded. When collecting data in our study, the amount of cigarette consumption was obtained as packs per year for each subject. Mental retardation, pregnancy, previous electroconvulsive therapy history, having any systemic and metabolic disease, acute chronic renal disease, nephrotic range proteinuria, rheumatic diseases, coronary heart disease, heart failure, peripheral artery disease, cerebrovascular event, malignancy, known inflammatory disease, diabetes mellitus, use of immunosuppressive drug, untreated hypertension, untreated dyslipidemia, and liver diseases were regarded as exclusion criteria. All subjects were examined and their medical history was recorded to evaluate if they met our exclusion criteria. According to medical examinations and anamnesis of the subjects, all subjects met the exclusion criteria.
Hamilton Depression Rating Scale (HAM-D)
Hamilton (1960) designed the original version of the HAM-D containing 17 items, each scored from 0 to 4 for a maximum total score of 53. Williams (1988) later developed the Structured Interview for HAM-D-21 as another version of the HAM-D that improved its inter-rater reliability [Citation32]. The Turkish version of the scale was found to be valid and reliable [Citation33]. In our study, patients were evaluated complying with the 17-item version.
Young Mania Rating Scale (YMRS)
YMRS is an 11-item diagnostic questionnaire used to measure the severity of ME. Each item measures five degrees of severity; seven items are answered by using a 5-point Likert-type scale, while four items are answered by using a 9-point Likert-type scale. The Turkish version of the scale was found to be both valid and reliable [Citation34].
Biochemical assessments
Study and control group PPARγ levels were measured using ELISA (enzyme-linked immunosorbent assay) technique, using a BioTek Synergy device (China, 201-125622) with Human PPARγ (SunRed, Lot No.201512, Ref No. DZE201124528) kits. All measurements were performed on the same day. The minimum measurable PPARγ level was 2,554 ng/mL.
Serum 15d-PGJ2 levels were measured using ELISA technique, using a BioTek Synergy device (China, 201-125622), with 15d-PGJ2 (SunRed, Lot No.201512, Ref No. DZE201125622) kits. All measurements were performed on the same day. The minimum measurable 15d-PGJ2 level was 0.725 ng/L. According to ELISA kit Instruction data, low, medium, and high intra-assay values for PPARγ and 15d-PGJ2 are CV < 10% and inter-assay values for PPARγ and 15d-PGJ2 are CV < 12%.
Serum IL-4 levels were measured using ELISA technique using a BioTek Synergy device and Boster (USA, EKO 404) with Human IL-4 (Lot No.12611921008, Ref No. PRD-001629) kits. All measurements were performed on the same day. The minimum measurable IL-4 level was 1.5 pg/mL. Low, medium, and high intra-assay IL-4 CV% values are respectively 4.5, 4.1, and 5.9. Low, medium, and high inter-assay IL-4 CV% values are, respectively, 6.5, 6.4, and 7.1.
Statistical analysis
Statistical Package for Social Sciences (SPSS) for Windows 23 (IBM SPSS Inc., Chicago, IL) program was used for the statistical analysis. The normality of the distribution of continuous and discrete numerical variables was tested with a Shapiro–Wilk test. Descriptive statistics for continuous and discrete numerical variables were given as mean ± standard deviation or median (interquartile range) and nominal variables were given as case numbers and percentages.
A Student’s t-test or analysis of variance (ANOVA) was used to assess whether differences in mean values between groups were significant. As the PPARγ, 15d-PGJ2, and IL-4 were not normally distributed, the Kruskal–Wallis tests were conducted to compare these parameters and non-parametric values shown as median (interquartile range). The Mann–Whitney U test and Dunn test were performed to test the significance of pairwise differences. Bonferroni correction was used to adjust the data set for multiple comparisons. Nominal variables were tested using a Pearson’s chi-square test. A Spearman’s correlation test was used to assess whether correlations between continuous variables were statistically significant. P < .05 was considered as significance levels.
Results
There were 139 subjects included in the study. Fifty of them were patients with BD in mania episode, 45 of them were patients with BD in remission, and 44 of them were healthy controls. Seventy of the subjects were female (46.7%) and 69 were male (46%). The mean age of our subjects was 38.11 years (±10.70), and no statistically significant age differences were found in ANOVA test between the mania (mean age = 39.46 ± 11.31 years), remission (the mean age = 36.69 ± 10.35), and control groups (mean age = 38.02 ± 10.37) [F (2,136) = 0.79, p > .05]. No significant differences were found between the mania, remission, and control groups in gender ratios (χ2 (2) = 4.33, p = .115) and smoking (χ2 (2) = 2.43, p = .989). Basic analyses were used to test how mania, remission, and control groups differed from each other regarding PPARγ, 15d-PGJ2, and IL-4 levels. Based on Kruskal–Wallis test results, the differences in the mean ranks of PPARγ (p < .001) and 15d-PGJ2 (p < .001) values among the three groups were significant, but there were no statistically significant differences in IL-4 values between the groups ().
Table 1. Comparison of mania, remission, and control groups in terms of age, gender, smoking, IL-4, PPARγ, and 15d-PGJ2.
In comparisons of mean ranks between the groups, the mean rank of PPARγ values in the mania group (mean rank: 42.96) was found to be significantly lower than both those of the remission (mean rank: 90.74) and the control (mean rank: 79.51) groups (). In a similar way, the mean rank of 15d-PGJ2 values in the mania group (mean rank: 50.07) was found to be significantly lower than both those of the remission (mean rank: 81.16) and the control (mean rank: 81.24) groups (). The differences between the remission group and the control group were not statistically significant regarding levels of PPARγ and 15d-PGJ2. The mean ranks of these three groups were not compared in terms of IL-4, since differences in IL-4 levels between the groups were not statistically significant ().
Figure 1. Independent-samples Kruskal–Wallis test for PPARγ in mania, remission, and control groups.
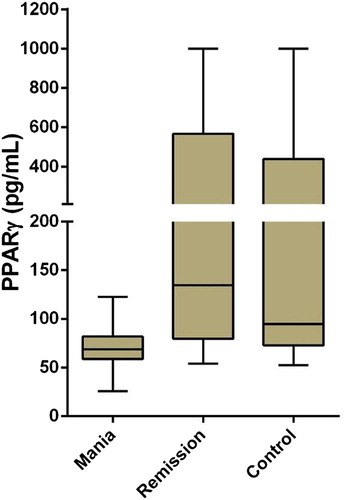
Figure 2. Independent-samples Kruskal–Wallis test for 15d-PGJ2 in mania, remission, and control groups.
When the mania and remission groups were compared in terms of socio-demographic and disease characteristics, two groups did not differ from each other in marital status, monthly income, comorbidity, and family history of medical conditions. There were no significant differences in the number of depressive episodes, ME, duration of disease, and mood-stabilizing medication use between these two patient groups. However, the rate of antipsychotic medication use was significantly higher in the mania group (χ2 (1) = 9.684, p = .002). The mania group were significantly more hospitalized than the remission group (p = .017). LDL (p < .001) and total cholesterol (p = .029) was significantly higher in the remission group than the mania group. Waist circumference was significantly higher in the mania group than in the remission group (p = .009). YMRS scores (p < .001) was found significantly higher in the mania group ().
Table 2. Comparison of mania and remission groups in terms of body measurements, blood values, blood pressures, and disease characteristics.
The correlations of PPARγ, 15d-PGJ2, and IL-4 with YMRS scores, body measurements (height, weight, waist circumferences, and hip circumferences), age, disease-related variables (duration of disease, number of hospitalizations, number of ME, YMRS score), levels of insulin, and CRP are presented separately for the mania and remission groups. YMRS score was found to have a significant negative correlation with 15d-PGJ2 (p = .001; rho: −0.462) and IL-4 (p = .047; rho: −0.232) in the mania group. Age was also found to have a significant negative correlation with PPARγ (p = .001; rho: −0.467) and 15d-PGJ2 (p = .025; rho: −0.334) in the remission group. There was also a significant negative correlation between weight and 15d-PGJ2 (p = .025; rho: −0.334) in the remission group. The insulin and 15d-PGJ2 (p = .006; rho: −0.406) have also a significant negative correlation in the remission group ().
Table 3. Correlations of PPARγ, 15d-PGJ2, and IL-4 levels with age, young total score, disease duration, number of hospitalization, number of ME, height, weight, waist and hip circumferences, insulin, and CRP in mania and remission groups.
In patients with BD (mania and remission together), PPARγ and 15d-PGJ2 (p = .000; rho: 0.745) were correlated. PPARγ had a negative correlation with YMRS score (p = .000; rho: −0.580), waist circumference (p = .005; rho: −0.289), and insulin (p = .023; rho: −0.233). IL-4 had a negative correlation with insulin (p = .032; rho = −0.220). 15d-PGJ2 and IL-4 (p = .001; rho: 0.274) were correlated. 15d-PGJ2 had negative correlations with YMRS score (p = .000; rho: −0.485) and insulin (p = .001; rho: −0.334) (). No significant correlations were observed in patients with BD in terms of number of mania attacks, fasting blood sugar, blood lipid, blood pressure, education level, age, weight, height, hip circumference, and cigarette consumption (packs per year) with IL-4, PPARγ, and 15d-PGJ2.
Table 4. Correlations of PPARγ, 15d-PGJ2, and IL-4 with PPARγ, 15d-PGJ2, IL-4, insulin, waist circumference, and young total score in patients with BD (mania and remission together).
Independent groups Kruskal–Wallis test (p < .05) were used to determine whether PPARγ, 15d-PGJ2, and IL-4 levels and YMRS score of the case groups differed in their distribution according to the mood-stabilizing medication (lithium, valproic acid/sodium valproate, carbamazepine, non-antipsychotic, and non-antipsychotic medication) and antipsychotic medications. The patient groups either the mania or remission group did not present different distributions in terms of PPARγ (χ2(4) = 4.60, p = .331), 15d-PGJ2 (χ2(4) = 2.43, p = .657), IL-4 (χ2(4) = 1.18, p = .770) and mania scores (χ2(4) = 2.617, p = .624) according to mood-stabilizing medications. When the antipsychotic use was considered, there was no difference in the blood values, but the average rank order of YMRS scores of the cases was significantly different. The rank order of mania ratings of cases who use antipsychotics is significantly higher than cases who do not use antipsychotics (Mann–Whitney U = 312.00, Z = 2.69, p = .007).
Discussion
In our study, we examined the anti-inflammatory status of patients with two different episodes of BD (mania and remission). A study on the role of inflammatory processes in schizophrenia showed lower levels of 15d-PGJ2 and its nuclear receptor PPARγ in patients with schizophrenia [Citation18]. García-Álvarez et al. reported that there were no differences between patients with schizophrenia and healthy controls in terms of anti-inflammatory parameters containing PPARγ and 15d-PGJ2 [Citation35]. They claim that this result of the study is associated with the activation of PPARγ-dependent endogenous counterbalancing mechanisms in schizophrenia. The same study also presented that higher levels of PPARγ were related to lower cognitive impairment. Similarly, other studies in the literature reported a relationship between inflammation and poor cognitive performance [Citation36].
Schizophrenia and BD are thought to share similar features in cellular and molecular neuropathology. This suggests that abnormalities in the immune system and stress response system in both diseases are mechanisms that cause pathogenesis [Citation37,Citation38]. For this reason, it is important that the result of our study gave similar results to those of the study done in schizophrenia group in acute period. Based on measurements and statistical analyses performed in our study, 15d-PGJ2 and PPARγ levels in patients with mania were found to be significantly lower, compared to those of patients in remission and healthy controls. Lower PPARγ and higher 15d- PGJ2 levels were found in patients with BD than patients with schizophrenia and healthy controls [Citation35].
According to our results, YMRS score was found to have a negative correlation with 15d-PGJ2 and IL-4 levels for the mania group. The YMRS score is correlated with the severity of the disease during ME. 15d-PGJ2 and IL-4 were found to have a negative correlation with YMRS score. This result could also be interpreted to show that there is an inverse correlation between the severity of the disease and anti-inflammatory markers. In one study, celecoxib, an anti-inflammatory drug and a selective inhibitor of COX-2, was added as an adjuvant therapy to valproate treatment in patients with mania. As a result, it is detected to reduce the symptoms of mania and YMRS scores significantly [Citation36Citation37 Citation38–Citation39]. This may show that investigating agonists of these markers (15d-PGJ2 and IL-4) in the future could be helpful to reduce the severity of the symptoms in patients in mania period. Agonists of PPARγ, especially pioglitazone (seen in both major depressive disorder and BD), were found to have a probable effect on the treatment of patients with major depression [Citation40]. Thiazolidinones such as rosiglitazone or pioglitazone are listed as potent agonists of PPARγ [Citation41]. Therefore, they may also be used in the treatment of the neurocognitive deficits associated with mood syndromes [Citation42].
There are arguments that PPARγ participated in lipid metabolism and PPARγ agonists can be used in the treatment of metabolic syndrome [Citation6,Citation43]. PPAR-γ ligands include glitazones which possess hypocholesterolemic effects and also improve lipid profile and decrease the levels of circulating inflammatory markers [Citation44]. In our study, total cholesterol and LDL values were significantly higher in the remission group than in the control group when the blood lipid values in the mania and remission groups were examined. Although the total cholesterol and LDL values were higher in the remission group, the PPARγ level was higher in patients in the remission group. This may be an indication of abnormal anti-inflammatory decrease during mania. In our study, it was found that there was more use of antipsychotics in the mania group than the remission group. In the literature, it was stated that patients in antipsychotic treatment such as olanzapine exhibit lower levels of anti-inflammatory markers [Citation45,Citation46]. Although the use of antipsychotics may have caused a decrease in anti-inflammatory activity during mania, there is a need for studies with untreated patients. In our study, the waist circumference was significantly larger in patients with mania than the patients during the remission period. The result of our study may also be affected by the relationship of abdominal obesity measured by waist circumference and low-grade inflammation [Citation47].
PPAR isoforms are found to have many effects and applications in the literature. Pioglitazone and rosiglitazone, which are also PPAR isoforms, are anti-diabetic, hypoglycaemic, and insulin-sensitizing agents [Citation48]. There is an important association found between rs1805192 and rs3856806 minor allele (G allele) of PPARγ and increased type 2 diabetes mellitus (T2DM) risk. An interaction analysis presented a combined effect of G-obesity interaction between rs1805192 and obesity for elevated T2DM risk [Citation49]. In our study, insulin was found to have a negative correlation with PPARγ, 15d-PGJ2, and IL-4 in patients with BD. This may indicate that the use of agonists of PPARγ could be beneficial in the treatment of diabetes and insulin desensitization for the patients with BD.
There are a limited number of studies on alterations of cytokines in patients with BD, and these studies present conflicting results [Citation50]. Several studies report that IL-4 levels during ME of BD were found to be higher during manic subjects when compared with control subjects [Citation12,Citation49,Citation51,Citation52]. Some studies have reported higher levels of IL-4 in patients in remission [Citation51–53], while others have reported no alteration or even lower levels of IL-4 [Citation52–54]. In our study, no significant differences in IL-4 levels were found between mania, remission, and healthy control groups. Considering that sleep disturbance and physical activity affect cytokine production [Citation55,Citation56], the result of our study may have been affected by these variables.
The most important finding of our study is that during ME, when the disease is active, a reduction in anti-inflammatory markers (PPARγ, 15d-PGJ2) is observed compared to remission and healthy control group. There was no significant difference between the two markers in the remission and control groups. This indicates that PPARγ and 15d-PGJ2 are state markers rather than trait markers. The absence of significant differences in levels of PPARγ, 15d-PGJ2, and IL-4 between remission and control groups could suggest that inflammatory processes are primarily associated with disease activity. This may indicate that 15d-PGJ2 and its nuclear receptor PPARγ are actively involved in the pathophysiology of ME of BD. This could lead to new pharmacological developments.
Conclusion
The identification of biological markers for BD is important for projecting the course of the disease and developing methods for the treatment of the disease. According to our knowledge, our study was the first that examines the relationship of decrease in anti-inflammation in the ME and the severity of the disease with PPARγ, 15d-PGJ2, and IL-4. Our findings present that there is a reduction in levels of PPARγ and 15d-PGJ2 during the mania period. This may pave the way for future research studies that examine whether PPARγ and 15d-PGJ2 could be protective against ME in BD. Although the evidence is not yet adequate, anti-inflammatory markers such as PPARγ and 15d-PGJ2 may be considered as potential biomarkers that could be useful in clarifying the pathophysiology of BD in the ME.
Future controlled studies with larger study samples could contribute to our knowledge about the role of inflammatory processes in BD. Due to the insufficient number of cases, regression analysis could not be applied. Our study, however, has certain limitations related to these questions. This study was not a longitudinal study evaluating the same patients during both relapse and remission periods, and no group of patients were included during a depressive episode of BD. The patients in our study were under treatment, so there is a need for further studies with untreated patients. Another limitation of the study is the absence of blood lipid values, body measurement values, systolic and diastolic blood pressure levels, insulin, and CRP parameters of the healthy control group.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Gamze Erzin http://orcid.org/0000-0001-8002-5053
Bağdagül Çakır http://orcid.org/0000-0002-1252-0498
Erol Göka http://orcid.org/0000-0001-7066-2817
Additional information
Funding
References
- Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528
- Fagiolini A, Forgione R, Maccari M, et al. Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord. 2013;148:161–169. doi: 10.1016/j.jad.2013.02.001
- Rosenblat JD, Kakar R, Berk M, et al. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2016;18:89–101. doi: 10.1111/bdi.12373
- Kalelioğlu T, Genç A, Karamustafalıoğlu N, et al. Bipolar disorder and inflammation. J Mood Disord. 2017;7:54–64.
- Baumeister D, Russell A, Pariante CM, et al. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social and lifestyle factors. Soc Psychiatry Psychiatr Epidemiol. 2014;49:841–849. doi: 10.1007/s00127-014-0887-z
- Modabbernia A, Taslimi S, Brietzke E, et al. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007
- Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2013;144:16–27. doi: 10.1016/j.jad.2012.06.010
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;1(S1):147.
- Sommer IE, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73:414–419. doi: 10.4088/JCP.10r06823
- Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003
- Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001
- Kim YK, Jung HG, Myint AM, et al. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018
- Maes M, Bosmans E, Calabrese J, et al. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995;29:141–152. doi: 10.1016/0022-3956(94)00049-W
- Maes M, Meltzer HY, Bosmans E, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-L
- Maes M, Delange J, Ranjan R, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11. doi: 10.1016/S0165-1781(96)02915-0
- Wadee AA, Kuschke RH, Wood LA, et al. Serological observations in patients suffering from acute manic episodes. Hum Psychopharmacol. 2002;17:175–179. doi: 10.1002/hup.390
- Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771:1031–1045. doi: 10.1016/j.bbalip.2007.04.016
- Martínez-Gras I, Pérez-Nievas BG, García-Bueno B, et al. The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophr Res. 2011;128:15–22. doi: 10.1016/j.schres.2011.01.018
- Ferret-Sena V, Maia E Silva A, Sena A, et al. Natalizumab treatment modulates peroxisome proliferator-activated receptors expression in women with multiple sclerosis. PPAR Res. 2016;2016:1–5. doi: 10.1155/2016/5716415
- Kapadia R, Yi J-H, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802
- Gervois P, Fruchart J-C, Staels B. Drug insight: mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat Clin Pract Endocrinol Metab. 2007;3:145–156. doi: 10.1038/ncpendmet0397
- Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202
- Galea E, Heneka MT, Dello Russo C, et al. Intrinsic regulation of brain inflammatory responses. Cell Mol Neurobiol. 2003;23:625–635. doi: 10.1023/A:1025084415833
- García-Bueno B, Madrigal JLM, Lizasoain I, et al. Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry. 2005;57:885–894. doi: 10.1016/j.biopsych.2005.01.007
- Feinstein DL. Therapeutic potential of peroxisome proliferator-activated receptor agonists for neurological disease. Diabetes Technol Ther. 2003;5:67–73. doi: 10.1089/152091503763816481
- García-Bueno B, Bioque M, Mac-Dowell KS, et al. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2014;40:376–387. doi: 10.1093/schbul/sbt001
- Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. Int J Neuropsychopharmacol. 2011;14:997–1012. doi: 10.1017/S1461145710001410
- Ando T, Ishikawa T, Kawamura N, et al. Analysis of tumor necrosis factor-α gene promoter polymorphisms in anorexia nervosa. Psychiatr Genet. 2001;11:161–164. doi: 10.1097/00041444-200109000-00009
- Barbosa IG, Bauer ME, Machado-Vieira R, et al. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast. 2014;2014:1–9. doi: 10.1155/2014/360481
- Guloksuz S, Cetin EA, Cetin T, et al. Cytokine levels in euthymic bipolar patients. J Affect Disord. 2010;126:458–462. doi: 10.1016/j.jad.2010.04.027
- Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Washington, DC: American Psychiatric Pub; 2013.
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007
- Akdemir A, Türkçapar MH, Orsel SD, et al. Reliability and validity of the Turkish version of the Hamilton Depression Rating Scale. Compr Psychiatry. 2001;42:161–165. doi: 10.1053/comp.2001.19756
- Karadağ F, Oral T, Yalçin FA, et al. Reliability and validity of Turkish translation of Young Mania Rating Scale. Turk Psikiyatr Derg. 2002;13:107–114.
- García-Álvarez L, Caso JR, García-Portilla MP, et al. Regulation of inflammatory pathways in schizophrenia: a comparative study with bipolar disorder and healthy controls. Eur Psychiatry. 2018;1:47–50.
- Ribeiro-Santos A, Lucio TA, Salgado JV. Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr Neuropharmacol. 2014;12:273–280. doi: 10.2174/1570159X1203140511160832
- Thompson Ray M, Weickert CS, Wyatt E, et al. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048
- Drexhage RC, Knijff EM, Padmos RC, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10:59–76. doi: 10.1586/ern.09.144
- Arabzadeh S, Ameli N, Zeinoddini A, et al. Celecoxib adjunctive therapy for acute bipolar mania: a randomized, double-blind, placebo-controlled trial. Bipolar Disord. 2015;17:606–614. doi: 10.1111/bdi.12324
- Park EY, Cho IJ, Kim SG. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid X receptor heterodimer. Cancer Res. 2004;64:3701–3713. doi: 10.1158/0008-5472.CAN-03-3924
- Colle R, de Larminat D, Rotenberg S, et al. PPAR-γ agonists for the treatment of major depression: a review. Pharmacopsychiatry. 2017;50:49–55.
- Chappuis B, Braun M, Stettler C, et al. Differential effect of pioglitazone (PGZ) and rosiglitazone (RGZ) on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab Res Rev. 2007;23:392–399. doi: 10.1002/dmrr.715
- Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547
- Giannini S, Serio M, Galli A. Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest. 2004;27:982–991. doi: 10.1007/BF03347546
- Carrizo E, Fernández V, Quintero J, et al. Coagulation and inflammation markers during atypical or typical antipsychotic treatment in schizophrenia patients and drug-free first-degree relatives. Schizophr Res. 2008;103:83–93. doi: 10.1016/j.schres.2008.03.004
- Fan X, Pristach C, Liu EY, et al. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res. 2007;149:267–271. doi: 10.1016/j.psychres.2006.07.011
- Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509
- Derosa G, Sahebkar A, Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J Cell Physiol. 2018;233:153–161. doi: 10.1002/jcp.25804
- Lv X, Zhang L, Sun J, et al. Interaction between peroxisome proliferator-activated receptor gamma polymorphism and obesity on type 2 diabetes in a Chinese Han population. Diabetol Metab Syndr. 2017;9:7. doi: 10.1186/s13098-017-0205-5
- Kauer-Sant’Anna M, Kapczinski F, Andreazza AC, et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol. 2009;12:447–458. doi: 10.1017/S1461145708009310
- O’Brien SM, Scully P, Scott LV, et al. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J Affect Disord. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015
- Mota R, Gazal M, Acosta BA, et al. Interleukin-1β is associated with depressive episode in major depression but not in bipolar disorder. J Psychiatr Res. 2013;47:2011–2014. doi: 10.1016/j.jpsychires.2013.08.020
- Doganavsargil-Baysal O, Cinemre B, Aksoy UM, et al. Levels of TNF-α, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Hum Psychopharmacol. 2013;28:160–167. doi: 10.1002/hup.2301
- Hope S, Dieset I, Agartz I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45:1608–1616. doi: 10.1016/j.jpsychires.2011.08.003
- Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Med. 2001;31:115–144. doi: 10.2165/00007256-200131020-00004
- Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–204. doi: 10.1093/sleep/32.2.200