ABSTRACT
INTRODUCTION: Pseudobulbar affect (PBA) is described as uncontrolled, unprovoked outbursts of laughing and/or crying not related to the patient’s underlying emotion or experience. PBA may be overlooked or underdiagnosed and so an effective method of evaluation is important. The Center of Neurologic Study-lability Scale (CN-LS) is such a self-report Likert scale consisting of seven questions on laughing and crying.
METHODS: The study was done to carry out the validity and reliability studies in Turkish for this test and validate it for multiple sclerosis (MS). For the content validity of the scale, one measurement and evaluation expert and five experts on the subject were interviewed. After a pilot study to evaluate the clarity of the scale, the reliability of the test was determined by sampling 154 normal and 56 MS patients, testing and retesting.
RESULTS: Test–retest correlations were high (Pearson correlation coefficient = 0.99, n = 56, p = .000). Also, there was no statistically significant difference between the means by using dependent sample t-test analysis (t = 0.40, n = 210, p = .69). In order to reveal the internal consistency of the test, the Cronbach alpha coefficient, another reliability test, was calculated and found to be high (α = 0.83, n = 210). For validation of the scale for MS, a separate study was conducted on 63 patients and ROC (receiver operating characteristic) analysis for criterion validity was performed. To define the cut-off score at or above which an individual with MS can be diagnosed as having PBA, one physician performed the test while the other independently stated his impression on the presence of the condition and the two scores were compared using the ROC analysis. The ROC curve analysis for the criterion validity was found to be very high (area under the curve = 0.976, p = .000). Using sensitivity and specificity calculations, the best acceptable cut-off score turned out to be 14.5 as being positive for PBA in Turkish MS patients.
CONCLUSION: Timely and appropriate evaluation of PBA is important in MS and the CNS-LS is a robust test instrument that has been used to assess PBA in different neurological conditions including MS. This condition has been known to influence the quality of life of patients. So after this study of validity, reliability, and validation, we hope it will be routinely used for evaluation.
Introduction
Multiple sclerosis (MS) is one of the most important chronic, non-traumatic neurological condition that can affect relatively young people during their most productive ages [Citation1]. Its prevalence is not distributed evenly throughout the world but is estimated to affect approximately 2.5 million people worldwide [Citation2]. It can diminish the quality of life by interfering with the daily lives of the patients by various ways, including the psychological, behavioural, cognitive, and affective problems [Citation3–5]. Patients with MS may have reactive psychiatric problems as well as problems caused from the lesions themselves, i.e. “organic” causation of psychopathology [Citation6,Citation7]. While psychiatric mood disorders are characterized by alterations of emotion or its expression, affective disorders that may be seen in MS are disorders of expression not representative of the underlying emotion [Citation8]. Sometimes even other psychopathologies may be confused with MS [Citation9]. One affective problem that some MS patients may have is the so-called pseudobulbar affect (PBA) [Citation10].
PBA is a clinical entity characterized with uncontrolled, easily provoked or unprovoked outbursts of laughing and/or crying [Citation11] that are often inappropriate with the social situation and with the patient’s underlying emotion/experience [Citation12]. Such outbursts can occur spontaneously or in response to provocative stimuli such as questions or events [Citation13]. Other terms have been used, including “emotional lability,” “emotional dysregulation,” “pathological laughing and crying,” “involuntary emotional expression disorder,” “emotional lability,” “affective lability,” “convulsive weeping and laughing,” and “emotional incontinence” [Citation14]. It may sometimes be confused with disorders of mood or other psychiatric diseases, but technically it is classified as a disorder of affect. PBA may be overlooked or underdiagnosed and so an effective method of evaluation is important.
In many cases, this clinical display of affect is dissociated from the patient’s underlying mood or emotions [Citation13]. It has been associated with a number of neurological diseases and brain injuries [Citation15] one of which is MS. It may also be encountered with motor neuron disease, traumatic central nervous system injury, brain tumours, Alzheimer’s disease, parkinsonism and other extrapyramidal disorders, stroke, and some cerebellar disorders [Citation16–21]. It has even been shown to occur with human immunodeficiency virus infection [Citation22]. Whatever its origins are, this clinical picture usually has serious social consequences, depending on the frequency and intensity of the laughing and/or crying, primary problem being the stigma attached to the loss of emotional control [Citation23].
The association of PBA with MS has been known for many years (it may even be the first manifestation of MS) [Citation24]. Diagnosis of the condition is usually by clinical examination, and as stated above, the condition may be overlooked, underdiagnosed, or misdiagnosed, effective methods of evaluation are needed. The Center of Neurologic Study-Lability Scale (CN-LS) described by Moore et al. is such an effective self-report scale consisting of seven questions on laughing and crying [Citation13,Citation25]. Validation studies of this test have been done MS and amyotrophic lateral sclerosis (ALS) in USA [Citation25].
Before using this tool, it is important to evaluate the instrument to overcome the probable differences in different neurological conditions and different cultures. Thus, the purpose of this study was to carry out validity and reliability investigations in Turkish for this scale and validate it for Turkish MS patients.
Materials and method
The CNS-LS was used in this study (). This scale has been developed by Moore et al. is an effective self-report scale consisting of seven questions on laughing and crying. While the test was originally being developed, factor structure was also investigated. Fifty-six potential questionnaire items were examined (developed from responses provided by physicians familiar with both affective lability and ALS) by using principal components analysis with varimax rotation and three factors emerged, two of which were relevant for the scale, namely crying and laughing [Citation13]. Eventually, the present scale was developed, consisting of four questions on labile laughter and three on tearfulness.
Table 1. Original version of CNS-LS SCALE.
Translation of the scale to Turkish was done by a bilingual neurologist (). A back translation to English was done by an English Language and Literature specialist. The resulting minor differences were edited and two academicians knowing both languages were consulted about equivalence. After all this, it was observed that there was no difference in the last reconstructed back translation. Thus, the final Turkish version of the text was created. This version was given to MS patients and normal volunteers. Before participating in the study, all subjects were given information about the nature and purpose of the study, every participant was asked to sign a standard voluntary consent form and the patients were given assurance that the patients’ identifying information would remain confidential. The IRB has been provided by Haydarpasa GATA Training Hospital (February 26 2015/34)
Table 2. Turkish version of CNS-LS scale.
Pilot study
A pilot study was conducted with randomly selected 15 MS patients to evaluate the clarity of the scale. During this part of the study, patients both with PBA and those thought to be free were included and the scale was not given sequentially to patients in order to ensure oversampling.
Content validity
One measurement and evaluation specialist and five experts on the subject were interviewed for the validity of the scale. Each and every one gave their positive approval as evidence related to the content validity of the scale.
Reliability
The reliability of the test was determined by the test–retest method sampling 154 normal and 56 MS patients and scoring twice. Thus, the consistency of the scores was determined. The patients were randomly selected MS patients who had at least 1 year of disease and who had been followed up for at least one year in our clinic and volunteered to participate in the study. Both relapsing-remitting and progressive patients were included in the study. The mean age was 42 with 60% female, 40% male. Clinically definite MS patients were selected. The age and gender proportions of the normal subjects were similar (namely, 34 female MS patients, 22 male patients, 92 healthy normal females, 62 males).
Validation
For the validation of the scale for MS, the test was performed on a separate sample of 63 patients (for demographic information of these patients, see ) and Receiver Operating Characteristic (ROC) analysis for criterion validity was performed. For this analysis, one physician performed the test on 63 MS patients while another physician independently stated his impression whether the patient had PBD or not after a detailed interview/evaluation. If the answer was yes, the patient was identified as “pseudobulbar” (1), and if the answer was no, the patient was identified as “non-pseudobulbar” (0).
Table 3. Demographic information of the MS patients.
Results
Content validity
Content validity was provided by expert opinion, as described in the Section “Materials and methods.”
Reliability
After testing and retesting the sample and scoring twice, dependent samples t-test analysis was performed and there was no statistically significant difference between the means (t = 0.40, n = 210, p = .69). Also, Pearson correlation coefficient was calculated between means of test and retest as 0.99 (n = 56, p = .000).
In order to reveal the internal consistency of the scale, Cronbach alpha coefficient was calculated and found to be high (α = 0.83, n = 210) as additional reliability evidence.
Validation
For the validation of the test for MS, it was performed on a sample of 63 patients and ROC analysis for criterion validity was performed.
There was a statistically significant difference between the two groups identified as “pseudobulbar” and “non-pseudobulbar” when the total score averages of the groups were compared with the independent samples t-test (t = 9.043, p = .000). The mean of 20 patients evaluated to have PBA was 18, while the mean of 43 patients without was 10.6.
Then, ROC curve analysis for the criterion validity was calculated. Criterion validity is a statistical concept to measure the extent to which one measure predicts an outcome for another measure, e.g. how well a survey or scale can predict (or reproduce) an accepted standard assessment. In the present study, the “standard assessment” is the evaluation by the physician, while the other measure is the severity of affective disturbance.
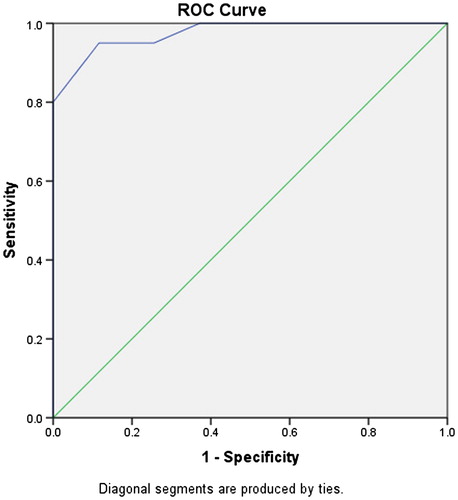
To define the cut-off score at or above which an individual with MS can be diagnosed as having PBA, patient’s score on the CNS-LS was compared to the second physician’s diagnosis using the ROC analysis. For each possible cut-off point, sensitivity and specificity were calculated. Sensitivity was defined as the proportion of patients evaluated to have PBA by the physician who has a positive CNS-LS, i.e. score higher than the cut-off point. Specificity was defined as the proportion of patients evaluated as non-PBA who score lower than the cut-off point (negative CNS-LS). and show the results of the ROC analysis; the graph is sensitivity versus 1-specificity. The curve and the analysis did not change when the result of one patient with the highest result (total 27) was removed from the analysis. In statistical analysis, points near the upper left corner are potential cut-offs with both high sensitivity and specificity. A curve that lies near the upper left corner, as is the case for this test in this study, indicates a relatively accurate diagnostic instrument. The area under the curve for ROC analysis measures the overall predictive accuracy of this instrument and it was found to be very high (area under the curve = 0.976, p = .000).
Table 4. Receiver operating characteristic (ROC) curve statistical analysis (also see ).
To calculate the cut-off value of the test: a curve that lies near the upper left corner indicates a highly accurate predictive or diagnostic instrument, as is the case here. The upper-leftmost points in correspond to CNS-LS scores of 13.5 and 14.5. Using each of these in turn as a cut-off produces sensitivities of 95% and 95% and specificities of 74.4% and 88.4%, respectively. Using a CNS-LS score above 14.5 as being positive for PBA seems to have acceptable sensitivity and specificity.
Table 5. Sensitivity and specificity levels of scores.
The positive predictive value of the test, i.e. the patients with a PBA diagnosis with a CNS-LS score above the cut-off is 78.2%, while the negative predictive value (patients scoring below the cut-off and not diagnosed to have PBA) was calculated to be 100%.
More than a third of the patients (35%) have points between 13 and 16 (average 14.5). The distribution of points obtained from CNS-LS is shown in .
Table 6. The average score points and percentages.
Conclusion
The purpose of this study is to investigate the validity and reliability of the Turkish version of CNS-LS and validate the instrument for MS in the Turkish population. The study was carried out in healthy controls and MS patients who volunteered to participate in the study. The reliability study consisted of 56 patients and 154 normals, the validation study of 63 patients. The initial pilot study that was carried out during the development of the translation of the scale included 15 randomly selected MS patients. Content validity was provided by expert opinion. The reliability of the scale was determined by test–retest method of the 210 subjects to investigate the consistency of the scores. Also, correlations between the means of these two measurements were calculated and were found to be high (Pearson correlation coefficient = 0.99, p = .000). Dependent sample t-test analysis also revealed no statistically significant differences between the means. (t = 0.40, n = 210, p = .69). In the original study by Moore et al., test–retest reliability was also calculated and found to be high (0.88) [Citation13]. Internal consistency of the test was calculated by Cronbach alpha coefficient and was found to be high (α = 0.83, n = 210). Moore et al. also evaluated internal consistency by Cronbach alpha coefficient and they also found a similar level, i.e. 0.87 [Citation13]. For the validation of the instrument for MS, it was performed on a sample of 63 patients and ROC analysis for criterion validity was performed. There was a statistically significant difference between patients with “pseudobulbar” symptoms and those without. Afterwards, ROC curve analysis for the criterion validity was calculated. The area under the curve for ROC analysis measures the overall predictive accuracy and it was found to be very high (area under the curve = 0.976, p = .000). The cut-off value of the test (i.e. CNS-LS score above which is positive for PBA) was calculated to be 14.5 with a fairly acceptable sensitivity and specificity. More than a third of the patients (35%) have points between 13 and 16 (average 14.5). The positive predictive value of the test was calculated to be 78.2% and the negative predictive value 100%. Smith et al. also conducted a similar ROC curve analysis for criterion validity on their study of the test in MS patients and in their study, the area under the curve was 0.9472, also high. The cut-off value of the test in that study was calculated to be 17. Their positive predictive values for scores 16, 17, and 18 were 86%, 87%, and 94%, while the negative predictive values were 94%, 92%, and 86%. Basically, the cut-off value of this test for MS in the present study has been shown to be lower than the original United States study with similar robust statistical verification. This is probably due to cultural differences and/or characteristics. Of note, both scores are higher than the cut-off score of the original study by Moore et al. to develop the scale, namely 13 [Citation13]. This is the cut-off score for the presence of PBA or not in general and appropriate validity investigations were made. But this study was not made in MS patients but ALS patients; things are different in MS. The present study has some limitations. Namely, the effects of drugs used by the patients, age and gender, disability (EDSS) values, patient education, socio-economic status, and the presence of a caregiver were not taken to account and adjustments were not made. All of these may have some effect on the final score, although probably minor.
Although PBA is classified as a disorder of affect and not mood, it can be associated with disorders of mood, especially anxiety, due to the psychological consequences and the impact on social interactions [Citation23]. PBA also interferes with rehabilitation. Thus, timely recognition of this syndrome is important in the management of patients. Technically, PBA is a disinhibition syndrome with uncontrolled laughing and crying in which pathways involving serotonin and glutamate are disrupted.
The association of PBA with MS has been known for many years (it may even be the first manifestation of MS [Citation24], but in the recent years, the interest on this clinical entity has increased with a number of studies on its prevalence done. The range of estimates of prevalence in various neurological disorders is variable, ranging from 5% to over 50%, depending on the methods, and the studied patient populations. Prevalence rates for MS ranged from 9.4% to 37.5% in the large studies done in the USA [Citation26]. Vidović et al. have reported a rate of 41.8% in their population [Citation27].
An interesting but not too surprising point is that no relation has been shown between the physical disability evaluated with Expanded Disability Status Scale (EDSS) scores and the severity of PBA in MS [Citation28]. Yet in general, more patients with PBA have entered the chronic progressive stage of the disease and have more physical disability [Citation25]. Its timely diagnosis is important since it may be modulated–treated to an extent by some medications.
The relation between mood and affective lability is not clear in MS. Since this is a reliability and validity study, no attempt was made to investigate correlation with depression, and thus depression or mood was not evaluated. This may be noted as a limitation of the study. Our own clinical experience suggests that the uncontrolled affective outbursts may be a source of distress for the patients and may be the cause of depression. This has also been noted by Moore et al. [Citation13]. Further studies should be done on the subject with the present scale.
Since PBA may be overlooked, underdiagnosed, or misdiagnosed, effective methods of evaluation are needed. The CNS-LS is a robust test instrument that has been used to assess PBA in different neurological conditions [Citation29,Citation30,Citation31]. This scale can easily be used in our society and confidently employed to evaluate this symptom in MS. It has to be validated for clinical entities other than MS like motor neuron disease/ALS, cerebrovascular diseases, extrapyramidal diseases, and Alzheimer’s disease. One can predict that it will be important in monitoring and regulating the quality of life in patients with these clinical pictures [Citation32,Citation33].
This scale (CN-LS) described by Moore et al. is such an effective self-report scale consisting of seven questions on laughing and crying [Citation13]. It has been used in a number of different clinical entities successfully in different cultures. This is a Likert measure which the patient responses personally, consisting of seven questions of the patient’s PBA episodes and the frequency, each question is rated between 1 and 5. In studies conducted in the United States with the scale, it was shown that patients with scores higher than a total 13 points may have symptoms of PBD [Citation28]. It evaluated both the frequency and severity and is relatively easy to answer. Validation studies of this test have been done for MS and ALS in the USA.
To cope with a chronic condition like MS, the individual must try to maintain normal daily activities as much as possible, stay connected to one’ s social environment, and pursue leisure activities, hobbies one enjoys and is able to do. But usually, there are problems and these problems interfere with the quality of life of the individual. The psychosocial and behavioural problems these individuals experience are due to different factors, some due to the actual illness itself and its neuropsychological effects, others are due to the social interaction that is disturbed as a result of the manifestations of the disease, one of which is PBA. As stated above, its evaluation and monitoring are valuable. MS patients with this condition, when diagnosed, may be treated with antidepressants (tricyclic’s, selective serotonin reuptake inhibitors), also with palliative drugs like dextromethorphan, levodopa, and TRH. Recently, a new treatment containing dextromethorphan plus quinidine have also been introduced [Citation34].
Thus, timely evaluation and diagnosis of this condition are valuable. It is usually accepted that physician diagnosis is correct for evaluating PBA, in some instances, it may be underdiagnosed, as stated in the literature [Citation25]. We hope that with this scale adapted/developed for Turkish, it will be routinely used, and new scientific research will be done based on it.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560
- Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63:2039–2045. doi: 10.1212/01.WNL.0000145762.60562.5D
- Achiron A, Barak Y. Cognitive impairment in probable multiple sclerosis. J Neurol Neurosurg Psychiatry. 2003;74:443–446. doi: 10.1136/jnnp.74.4.443
- Deloire MS, Salort E, Bonnet M, et al. Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:519–526. doi: 10.1136/jnnp.2004.045872
- Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/WNL.41.5.685
- Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. therapeutic advances in neurological disorders Gazioglu & Ozmenoglu. Psychiatric findings in multiple sclerosis. Turkiye Klinikleri J Neurol Special Topics. 2016;9(4):57–62.
- Gazioglu & Ozmenoglu. Psychiatric findings in multiple sclerosis. Turkiye Klinikleri J Neurol Special Topics 2016;9(4):57–62
- Rao SM, Reingold SC, Ron MA, et al. Workshop on neurobehavioral disorders in multiple sclerosis. diagnosis, underlying disease, natural history, and therapeutic intervention, Bergamo, Italy, June 25–27, 1992. Arch Neurol. 1993;50:658. doi: 10.1001/archneur.1993.00540060088026
- Citak S, Kenangil G, Ari BC. A case of conversion disorder, which was followed as multiple sclerosis. Klinik Psikofarmakoloji Bulteni – Bulletin of Clinical Psychopharmacology. 2014;24(Ek 1):S296.
- Vidović V, Rovazdi MČ, Kraml O, et al. Pseudobulbar affect in multiple sclerosis patıents. Acta Clin Croat. 2015;54(2):159–163.
- Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA): distinguishing PBA among disorders of mood and affect. CNS Spectr. 2005;10:1–16. doi: 10.1017/S1092852900026602
- Feinstein A, Feinstein K, Gray T, et al. Prevalence and neurobehavioral correlates of pathological laughter and crying in multiple sclerosis. Arch Neurol. 1997;54:1116–1121. doi: 10.1001/archneur.1997.00550210050012
- Moore SR, Gresham LS, Bromberg MB, et al. A self report measure of affective lability. J Neurol Neurosurg Psychiatry. 1997;63:89–93. doi: 10.1136/jnnp.63.1.89
- Dark FL, McGrath JJ, Ron MA. Pathological laughing and crying. Aust N Z J Psychiatry. 1996;30:472–479. doi: 10.3109/00048679609065020
- Schiffer R, Pope LE. Pseudobulbar affect including a novel and potential therapy. [review]. J Neuropsychiatry Clin Neurosci. 2005;17:447–454. doi: 10.1176/jnp.17.4.447
- Gallagher JP. Pathologic laughter and crying in ALS: a search for their origin. Acta Neurol Scand. 1989;80:114–117. doi: 10.1111/j.1600-0404.1989.tb03851.x
- Smith RA, Berg JE, Pope LE, et al. Measuring pseudobulbar affect in ALS. Amyotroph Later Scler Other Motor Neuron Disord. Sep 2004;5(Supplement 1):99–102. doi: 10.1080/17434470410020058
- Chang YD, Davis MP, Smith J, et al. Pseudobulbar affect or depression in dementia? J Pain Symptom Manag. May 2016;51(5):954–958. doi: 10.1016/j.jpainsymman.2015.12.321
- Starkstein SE, Migliorelli R, Teson A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59:55–60. doi: 10.1136/jnnp.59.1.55
- Hackett ML, Köhler SK, O’Brien JT, et al. Neuropsychiatric outcomes of stroke. Lancet Neurol. 2014;13(5):525–534. doi: 10.1016/S1474-4422(14)70016-X
- Kim JS. Post stroke emotional incontinence after small lenticulocapsular stroke: correlation with lesion location. J Neurol. 2002;249:805–810. doi: 10.1007/s00415-002-0714-4
- Almeida V, Mestre T, De Carvalho M. Pseudobulbar syndrome in two patients with human immunodeficiency virus infection. Amyotroph Lateral Scler. Mar 2010;11(1/2):220–222. doi: 10.3109/17482960902748694
- Tateno A, Jorge RE, Robinson RG. Pathological laughing and crying following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2004;16(4):426–434. doi: 10.1176/jnp.16.4.426
- Aguirregomozcorta M, Ramió-Torrentà Ll, Gich J, et al. Paroxysmal dystonia and pathological laughter as a first manifestation of multiple sclerosis. Mult Scler. Mar 2008;14(2):262–265. doi: 10.1177/1352458507082053
- Smith RA, Berg JE, Pope LE, et al. Validation of the CNS emotional lability scale for pseudobulbar affect (pathological laughing and crying) in multiple sclerosis patients. Mult Scler. Dec 2004;10(6):679–685. doi: 10.1191/1352458504ms1106oa
- Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483.
- Vidović V, MČ R, Kraml O, et al. Pseudobulbar affect in multiple sclerosis patients. Acta Clin Croat. June 1, 2015;54(2):159–163.
- Buckley R. No relation between physical disability scores and severity of PBA in MS. Peer-reviewed highlights from the 6th World Congress on Controversies in Neurology (CONy) Vienna, Austria, March 8–11, 2012, MD Conference Express.
- McCullagh S, Moore M, Gawel M, et al. Pathological laughing and crying in amyotrophic lateral sclerosis: an association with prefrontal cognitive dysfunction. J Neurol Sci. 1999;169(12):4348.
- Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the quality standards subcommittee of the American academy of neurology. Neurology. 2009;73(15):1227–1233. doi: 10.1212/WNL.0b013e3181bc01a4
- Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382–1387. doi: 10.1007/s00415-010-5550-3
- Greb E. Pseudobulbar affect May influence quality of life for patients with stroke. Neurology Reviews. May 2013;21(5):24.
- Kocer E, Koçer A, Yaman M, et al. Quality of life in multiple sclerosis patients: impact of depression and physical limitations? J Mood Disord. 2011;1(2):63–67. doi: 10.5455/jmood.20110419054303
- Pioro EP, Brooks BR, Cummings J, et al. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann. Neurol. Nov.2010;68(5):693–702. doi: 10.1002/ana.22093