ABSTRACT
OBJECTIVES: Attention Deficit Hyperactivity Disorder (ADHD) is a neurobehavioral disorder that begins in early childhood, and many factors play role in its etiology. Many studies have been conducted to identify the causes of ADHD, but the exact factors are still unknown. Although cerebellar dysfunction in the etiology of ADHD was shown in different studies, the possible causes of dysfunction and the role of neuroinflammation among these causes has not been clarified yet. Anti-Yo is an antibody against the antigens in the cytoplasm of purkinje cells and indicates cerebellar degeneration, and Anti-Hu and Anti-Ri are antibodies against cellular nuclear antigens of purkinje cells. This study aimed to evaluate the role of neuroinflammation that is a potential cause of cerebellar dysfunction, which is thought to be an important factor in the development of ADHD.
METHODS: This is a cross-sectional and descriptive study that aimed to evaluate the potential association between ADHD and cerebellar neuroinflammation by comparing the serum anti-purkinje cell antibody measurements between case and control groups. The cases were recruited at the Gazi University Child Psychiatry Department, and laboratory analyses were performed at the Ankara Numune Research and Training Hospital Medical Microbiology Department. Sixty children and adolescents with ADHD, and 60 healthy controls were planned to be included in the study. Cases that admitted with ADHD symptoms were given Conners teacher forms according to routine procedure; then the cases with scores over the cut-off of Conners teacher form were evaluated clinically for a diagnosis of ADHD, and after clinical evaluations they were asked to participate the study if they met the eligibility criteria. If they accept to participate the study, informed consents were given to cases and parents, and meanwhile, Turkish version of Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version (K-SADS-PL) was also applied to cases. A 5-ml serum was spared from the blood samples that obtained for routine test during diagnosis. Control group was planned to be formed from the blood samples of 60 healthy children that admitted to child psychiatry clinic for counselling. At the time of analyses, diluted according to the directions of manufacturers, incubated with fluorescent staining including antibodies (Anti-Hu, Anti-Yo, and Anti-Ri), and evaluated under immunofluorescent microscope by three specialists.
RESULTS: Sixty healthy volunteers and 60 cases with ADHD were included in the study. Some of the samples were excluded from the study due to the damage to laboratory tubes during transport. Assessments were conducted with 52 ADHD and 52 healthy control samples. The male/female ratio was 41/11(78.8%/21.2%) in the patient group and 35/17 (67.3%/32.7%) in the control group (p = 0.185). Average age was 9.81 ± 2.41 in the patient group and 9.46 ± 2.14 in the control group (p = 0.442). No positive results were obtained for anti-Purkinje antibody in ADHD or control groups.
CONCLUSIONS: No evidence regarding the potential role of cerebellar neuroinflammation in the etiology of ADHD was determined in this study. But these results need replication in larger samples and different methods.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a neurobehavioral disorder that begins in early childhood, and a number of factors play role in its etiology [Citation1]. Several studies have been conducted to identify the causes of ADHD but the exact factors are still unknown [Citation2]. Cerebellar dysfunction was shown in the etiology of ADHD in various studies. At first, cerebellum plays a critical role in cognitive function, especially in learning, attention and emotional regulation [Citation3]. In this context, cerebellum cognitive and affective syndrome (CCAS) may be remembered for what cerebellar impairment contributes [Citation4]. Additionally, ADHD semptom severity has strongly related with cerebellar circuits dysfunction [Citation5] and cerebellar volume [Citation6,Citation7] especially vermis [Citation8]. On the other hand, distruption of cerebellar GABAergic neurons in juvenile preterm guinea pigs are associated with cognitive and behavioural problems [Citation9] and treadmill exercise and MPH alleviated the ADHD-induced the decrease of balance and the number of calbindine-positive cells, and the increase of GFAP expression and Bax/Bcl-2 ratio in the cerebellum of rats [Citation10]. Although dysfunction of cerebellum is well documented in ADHD, the possible causes of the dysfunction and the role of neuroinflammation in these causes have not been clarified yet [Citation10–12]. Potential roles of neuroinflammation and autoimmunity were adressed and assessed in many psychiatric diseases [Citation13]. For example, between 25% and 75% of lupus patients have psychiatric symptoms may include anxiety, mood and psychotic disturbances [Citation13]. Likewise, autoimmune encephalitides are characterized by an acute onset of psychiatric features and cognitive dysfunctions [Citation13]. PANDAS, which is characterized by acute manifestation of obsessive/compulsive symptoms and tics following group A β-hemolytic streptococcal infection, is known to include cross-reactivity between anti-streptococcal antibodies and basal ganglia proteins [Citation14]. Anti-Yo is an antibody against the antigens in the cytoplasm of Purkinje cells and indicates cerebellar degeneration; Anti-Hu and Anti-Ri are antibodies against cellular nuclear antigens, and they become positive in paraneoplastic conditions and neuropathies [Citation15]. There is only one pilot study in the literature that evaluated the anti-Purkinje antibodies (Anti-Hu, Anti-Yo and Anti-Ri) and cerebellar neuroinflammation [Citation16]. To the authors’ knowledge, there are no replication studies on the possible role of anti-Purkinje autoantibodies since 2013, the time of the pilot study, despite the accumulation of a significant amount of data indicating cerebellar dysfunction in ADHD.
This study aimed to evaluate the role of neuroinflammation as a potential cause of cerebellar dysfunction, which is thought to be an important aspect in the development of ADHD, in a large sample. The hypothesis of the study is that seropositivity for anti-Purkinje antibodies (anti-Hu, anti-Yo and anti-Ri) is higher in patients with combined-type ADHD compared with control group and that the cerebellar neuroinflammation is an important factor in the etiology of ADHD.
Material and methods
Subjects
This study, which has a analytical sectional case–control design, aimed at examining the possible role of cerebellar neuroinflammation in the etiology of ADHD by comparing the seroprevalence of autoantibodies in the case and control groups.
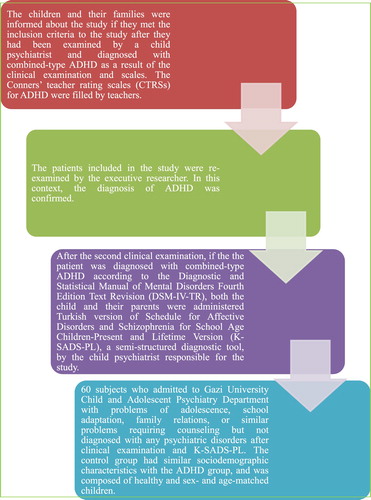
The study included the patients admitted to the outpatient clinic of Gazi University Child and Adolescent Psychiatry Department. The process of including subjects has been illustrated in . The number of subject for this study was determined by power analysis using with android application programme of P005 , when 0.05 has been accepted for type 1 error, 0.10 for type 2 error and 25% for the differences between two groups according to previous studies.
The patients in the study group were between the ages of 6 and 12 years, were diagnosed with combined-type ADHD only based on K-SADS-PL and DSM-IV-TR to homogenize the ADHD group, did not have any additional psychiatric disorders other than Oppositional Defiant Disorder (ODD), did not have any neurological/chronic diseases, and had a total IQ score greater than 90. The study protocol was approved by Clinical Research Ethics Committee of Gazi University, School of Medicine (Date: Dec 22, 2014; No: 570).
Data collection tools
Sociodemographic and clinical data collection form
A data form was prepared for the purpose of this study and filled in during the interview with the children and their parents.
Turkish version of schedule for affective disorders and schizophrenia for school age children-present and lifetime version (K-SADS-PL)
It was used to determine the diagnosis of the patients in the study group. The patients were diagnosed after interview of the patients and one of their parents by the responsible resident researcher, then by the clinical evaluation of the executive faculty researcher. Scale is a semi-structured interview form developed by Kaufman et al. to determine past and current psychopathologic disorders in children and adolescents (6–18 years of age) [Citation17]. Its validity and reliability in Turkish were studied by Gökler et al. [Citation18]. The child psychiatrist who performed the interviews has been trained and had certification to conduct this semi-structured interview.
Conners’ teacher rating scale (CTRS) for attention deficit hyperactivity disorder
This scale was developed by Conners [Citation19], and revised by Goyotte et al. [Citation20]. Adaptation, validation, and reliability studies in Turkish were done by Dereboy et al. [Citation21]. The scale contains questions for teachers about hyperactivity, attention deficit, and behavioral problems of the child in school. In this study, this scale was used to analyze the probable relationships of the polymorphisms with clinical characteristics.
Wechsler intelligence scale for children-revised (WISC-R)
This scale was developed by Wechler in 1949, and revised in 1974 (WISC-R) to increase the age range of the scale between 6 and 16 years of age [Citation22]. Savaşır et al. have performed the reliability and validity studies in Turkish [Citation23].
Immunofluorescence microscopy
Serum samples were prepared in Gazi University Biochemistry Laboratory; after 30 min incubation period, samples were centrifuged at 1200 g for 3 min, and the sera were aliquoted into microcentrifuge tubes and stored at −80°C. Later, the samples were analyzed in the Microbiology Clinic at Ankara Numune Training and Research Hospital. Antibodies against Yo, Hu, and Ri were detected by indirect immunofluorescence assay (IFA). The assay was performed on cerebellum, nerves, intestine substrate of monkey and HEp-2 cells. Primate cerebellum and primate nerves are the standard substrates for the determination of various neuronal antibodies. The parallel use of primate intestine substrates permits the reliable differentiation from other autoantibodies (e.g. ANA) and makes it possible to distinguish between anti-Ri and anti-Hu (Euroimmun Germany). The presence of specific antibodies was determined by their specific reactions to the cerebellar neurons. The patients’ sera were first diluted 1:10 in PBS-Tween and then incubated with the substrate provided in the presence of fluoresceinlabelled anti-human IgG conjugate in order to allow binding of the antibodies to the specific antigens of the substrate. The antibodies against Yo stain exclusively the cytoplasm of the Purkinje cells in the cerebellum. In the case of antibodies against Hu and Ri all neuronal nuclei in the grey matter show a granular fluorescence. The anti-Hu antibodies react in the intestine with cell nuclei of the plexus myentericus, whereas anti-Ri antibodies do not. The immunofluorescence was visualized by means of a fluorescence microscope (EUROSTAR III , EUROIMMUN, Germany) at amagnification of 20× and 40x . All samples were evaluated with immunofluorescence microscope by three different experts and were sent to the Euroimmun center in Germany for reevaluation and confirmation.
Statistics
The statistical analysis of the data was done with SPSS 15.0 (SPSS Inc., USA). Chi square (χ2 9and Fisher’s Exact test were used to evaluate the relationships between the categorical/nominal variables (sex, proportion of anti-Yo, anti-Ri, and anti-Hu Ab positive/negative results). To test for the strength of agreement, the percent of inter-rater agreement was conducted. The alpha value for significance was 0.05.
Results
The study was initiated with 60 healthy subjects and 60 subjects with ADHD. However, some of the samples were excluded from the study due to the damage to laboratory tubes during transport. Assessments were conducted with 52 ADHD and 52 control samples. The male/female ratio was 41/11(78.8%/21.2%) in the patient group and 35/17 (67.3%/32.7%) in the control group (p = 0.185). Average age was 9.81 ± 2.41 in the patient group and 9.46 ± 2.14 in the control group (p = 0.442). The patient and control groups were similar regarding mean age and the gender distribution. No positive results were obtained for anti-Purkinje antibody in ADHD or control groups. The Percent Agreement between three microbiologists is 83.3%. Socio-demographic, clinical and laboratory variables have been described in .
Table 1. Sociodemographic and clinical characteristics.
Discussion
This study has investigated the possible differences between combined-type ADHD cases and controls in terms of the seroprevalence of anti-Yo, anti-Ri and anti-Hu antibodies against cerebellar Purkinje cells. However, all subjects in the control and case group were found to be seronegative for the relevant antibodies. This suggests that anti-Purkinje autoantibodies are not involved in the development of cerebellar dysfunction in ADHD. Although Passarelli et al. have found clues for a possible association in their pilot study; we have found no relationship between anti-Purkinje autoantibodies and ADHD in our replication study [Citation16].
Although cerebellum occupies 10% of the area covered by all brain regions, it surely bears more tasks than its share [Citation24]. On one hand, it forms reciprocal connections with the cerebral cortex via the thalamus and the pontine nucleus; on the other hand, it has reciprocal pathways to the vestibular system and spinal cord [Citation25]. Cerebellum has regulatory, organizing, and optimizing functions in both motor tasks and cognitive and affective tasks [Citation26]. By creating a model with an internal regulation before the behavior emerges, it fine-tunes the motor, cognitive, and affective processes as well as allowing the relevant behavior/motor process to be automated over time [Citation27–29]. All these functions are provided by the pathways in the cerebellocortical closed circuit system [Citation26]. Injuries of the anterior lobe of the cerebellum more often lead to loss of sensorimotor functions such as dysmetria, ataxia, and dysdiadokinosis while posterior lobe injuries more often result in failure of cognitive and affective regulation such as cognitive dysmetry and cerebellar cognitive affective syndrome [CCAS] [Citation3]. Recently, structural and functional imaging studies allow detection of problems in cerebellocortical pathways and cerebellar problems in many neuropsychiatric disorders such as autism, ADHD, and dyslexia where cognitive and affective regulation are affected and abnormal and inappropriate behaviors are observed [Citation30].
Strong evidences from neuroimaging studies are the reduction in cerebellar volume, especially a reduction in the posterior lobe including lobes 8 and 9 [Citation31], the evidence of correlation with reduction in the posterior lobe volume in ADHD clinical features, and the association of ADHD symptomatology with cerebellar vermis [Citation8,Citation30,Citation31]. The significant improvement was observed in the cerebellar volume in the ADHD cases treated with methylphenidate (MPH) compared to those not treated with MPH and the significant improvement was observed in postural control in ADHD cases with MPH treatment implicate the cerebellum and cerebellocortical pathways in ADHD etiology [Citation32,Citation33]. In a study evaluating the role of atypical fronto-cerebellar circuit in the etiopathogenesis of ADHD, an increased neuronal activation was detected in the posterior cerebellar lobe [Citation11]. In another study, treadmill exercises were shown to increase dendritic plasticity in Purkinje cells in rats [Citation34]. In yet another study designed based on this finding, treadmill exercises were shown to contribute to the improvement of ADHD symptoms by reducing Purkinje cell loss and astrocytic activation in spontaneous hyperactive rats [Citation10]. A study by Bucci et al., the MPH treatment was shown to improve the decreased postural instability, which was increased as a clear sign of cerebellar dysfunction in children with ADHD compared with controls [Citation33]. However, it is not yet clear when and how cerebellar damage is initiated, what triggers the damage, and which pathophysiological processes are involved in ADHD. Significant association of ADHD with autoimmune diseases such as asthma and allergy is known [Citation35]. In this context, it was suggested that the autoantibodies against Purkinje cells might be the cause of cerebellar dysfunction. In the study by Passarelli et al. involving 30 patients with ADHD and 17 healthy controls, anti-Yo autoantibodies specific for cytoplasm of Purkinje cells were probed as possible biomarker for ADHD, and promising results were obtained with significant positive rates observed in patients with ADHD compared with the controls. Interestingly, a lower seropositivity was found in ODD and conduct disorder compared with ADHD in that study [Citation16]. The low number of samples was a major limitation of that study and has prevented the generalization of the results. In a study investigating GAD65 autoantibodies, which were seropositive for diseases such as chronic cerebellar ataxia, in neurodevelopmental disorders, seropositivity was found 27% in ADHD and 15% in autism. In the same study, sera of 53% of children with ADHD were reactivated with mouse cerebellar cells [Citation36]. In light of these findings, cerebellar damage as well as increased cellular activation to compensate for hypofrontality were evident in ADHD although not as severely and frequently as in otistic patients [Citation37]. In our study of ethiopathogenesis of cerebellar dysfunction, seronegativity of anti-Purkinje autoantibodies found in all patients with ADHD suggests that these autoantibodies have no role in pathogenesis and that other possible causes should be considered in Purkinje cell dysfunction.
Although the benefical results of anti-Purkinje autoantibodies on ADHD, there was a limitation of the current study. Patients with combined type ADHD without comorbidity other than ODD, could not reflect the universe properly as known ADHD is a highly comorbid condition and this resrict the generalization of our results.
Conclusion
The role of cerebellar dysfunction in ADHD is clear; however, it is not yet clear what the cause of cerebellar dysfunction in ADHD is. Although Purkinje cell damage emerged as a potential factor and a possible association has been suggested by a single pilot study; our study, which constitutes a replication of this study with a larger sample, indicated for the first time that there was no association between anti-Purkinje autoantibodies and ADHD. In this context, groups of more homogeneous cases should be formed firstly by treating the clinical findings that are indicative of cerebellar dysfunction as endophenotypes. Secondly, anti-Purkinje autoantibodies can be said to have lost their significance in the pathogenesis of cerebellar dysfunction in ADHD, and other possible hypotheses should be explored.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Association AP. Diagnostic and statistical manual of mental disorders [DSM-5®]: American Psychiatric Pub. 2013.
- Çetin FH, Işık Y. Attention deficit hyperactivity disorder and genetics. Curr Approaches in Psychiat. 2018;10(1):19–39.
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561
- De Smet HJ, Paquier P, Verhoeven J, et al. The cerebellum: its role in language and related cognitive and affective functions. Brain Lang. 2013;127(3):334–342. doi: 10.1016/j.bandl.2012.11.001
- Giedd JN, Blumenthal J, Molloy E, et al. Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci. 2001;931(1):33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x
- Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Jama. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740
- Bruchhage MM, Bucci M-P, Becker EB. Cerebellar involvement in autism and ADHD. Handb Clin Neurol. 2018;155:61–72. doi: 10.1016/B978-0-444-64189-2.00004-4
- Mackie S, Shaw P, Lenroot R, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164(4):647–655. doi: 10.1176/ajp.2007.164.4.647
- Shaw JC, Palliser HK, Dyson RM, et al. Disruptions to the cerebellar GABAergic system in juvenile Guinea pigs following preterm birth. Int J Dev Neurosci. 2018;65:1–10. doi: 10.1016/j.ijdevneu.2017.10.002
- Yun H-S, Park M-S, Ji E-S, et al. Treadmill exercise ameliorates symptoms of attention deficit/hyperactivity disorder through reducing Purkinje cell loss and astrocytic reaction in spontaneous hypertensive rats. J Exerc Rehabil. 2014;10(1):22–30. doi: 10.12965/jer.140092
- Rapin L, Poissant H, Mendrek A. Atypical activations of fronto-cerebellar regions during forethought in parents of children with ADHD. J Atten Disord. 2017;21(12):1050–1058. doi: 10.1177/1087054714524983
- Picazio S, Koch G. Is motor inhibition mediated by cerebello-cortical interactions? The Cerebellum. 2015;14(1):47–49. doi: 10.1007/s12311-014-0609-9
- Najjar S, Pearlman DM, Alper K, et al. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10(43):1–24.
- Swedo SE. Streptoccocal infection, Tourette syndrome, and OCD: is there a connection? Pandas: Horse or zebra? Neurology. 2010;74:1397–1399. doi: 10.1212/WNL.0b013e3181d8a638
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543–1554. doi: 10.1056/NEJMra023009
- Passarelli F, Donfrancesco R, Nativio P, et al. Anti-Purkinje cell antibody as a biological marker in attention deficit/hyperactivity disorder: a pilot study. J Neuroimmunol. 2013;258(1):67–70. doi: 10.1016/j.jneuroim.2013.02.018
- Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version [K-SADS-PL]: initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021
- Gökler B, Ünal F, Pehlivantürk B, et al. Okul Çaği Çocuklari İçin Duygulanim Bozukluklari ve Şizofreni Görüşme Çizelgesi-Şimdi ve Yaşam Boyu Şekli-Türkçe Uyarlamasinin Geçerlik ve Güvenirliği. Çocuk ve Gençlik Ruh Sağliği Dergisi. 2004;11:10–116.
- Conners CK. A teacher rating scale for use in drug studies with children. Am J Psychiat. 1969;126(6):884–888. doi: 10.1176/ajp.126.6.884
- Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners parent and teacher rating scales. J Abnorm Child Psychol. 1978;6(2):221–236. doi: 10.1007/BF00919127
- Dereboy C, Senol S, Sener S, et al. Validation of the Turkish versions of the short-form Conners’ teacher and parent rating scales. Turkish J Psychiat. 2007;18(1):48–58.
- Wechsler D. Manual for the Wechsler intelligence scale for children, revised: Psychological Corporation. 1974.
- Savasır I, Sahin N. Wechsler Cocuklar Için Zeka Olçegi [WISC-R] El Kitabı. Türk Psikologlar Dernegi Yayınları, Ankara. 1995.
- Villanueva R. The cerebellum and neuropsychiatric disorders. Psychiatry Res. 2012;198(3):527–532. doi: 10.1016/j.psychres.2012.02.023
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008
- Sokolov AA, Miall RC, Ivry RB. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci (Regul. Ed.). 2017;21(5):313–332. doi: 10.1016/j.tics.2017.02.005
- Akshoomoff NA, Courchesne E, Townsend J. Attention coordination and anticipatory control. Int Rev Neurobiol. 1997;41:575–598. doi: 10.1016/S0074-7742(08)60371-2
- Green AM, Meng H, Angelaki DE. A reevaluation of the inverse dynamic model for eye movements. J Neurosci. 2007;27(6):1346–1355. doi: 10.1523/JNEUROSCI.3822-06.2007
- Requarth T, Sawtell NB. Plastic corollary discharge predicts sensory consequences of movements in a cerebellum-like circuit. Neuron. 2014;82:896–907. doi: 10.1016/j.neuron.2014.03.025
- Semrud-Clikeman M, Hooper SR, Hynd GW, et al. Prediction of group membership in developmental dyslexia, attention deficit hyperactivity disorder, and normal controls using brain morphometric analysis of magnetic resonance imaging. Arch Clin Neuropsychol. 1996;11(6):521–528. doi: 10.1093/arclin/11.6.521
- Ashtari M, Kumra S, Bhaskar SL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57(5):448–455. doi: 10.1016/j.biopsych.2004.11.047
- Ivanov I, Murrough JW, Bansal R, et al. Cerebellar morphology and the effects of stimulant medications in youths with attention deficit-hyperactivity disorder. Neuropsychopharmacolo. 2014;39(3):718–726. doi: 10.1038/npp.2013.257
- Bucci MP, Stordeur C, Acquaviva E, et al. Postural instability in children with ADHD is improved by methylphenidate. Front Neurosci. 2016;10:63. doi:10.3389/fnins.2016.00163.
- González-Burgos I, González-Tapia D, Zamora DAV, et al. Guided motor training induces dendritic spine plastic changes in adult rat cerebellar purkinje cells. Neurosci Lett. 2011;491(3):216–220. doi: 10.1016/j.neulet.2011.01.043
- Najjar S, Pearlman DM, Alper K, et al. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10:43. doi:10.1186/1742-2094-10-43.
- Rout UK, Mungan NK, Dhossche DM. Presence of GAD65 autoantibodies in the serum of children with autism or ADHD. Eur Child Adolesc Psychiatry. 2012;21(3):141–147. doi: 10.1007/s00787-012-0245-1
- Salman MS, Tsai P. The role of the pediatric cerebellum in motor functions, cognition and behavior: a clinical perspective. Neuroimaging Clin N Am. 2016;26(3):317–329. doi: 10.1016/j.nic.2016.03.003