ABSTRACT
OBJECTIVE
In this study we investigated the stimulant methylphenidate (MPH) effects in Attention deficit hyperactivity disorder (ADHD) from neuroimaging and neurophysiological perspective by simultaneous recording functional near infrared spectroscopy (fNIRS) and electroencephalography (EEG) during attention task.
METHODS
Using fNIRS we obtained frontal cortex hemodynamic responses and using event related potentials (ERP) we obtained amplitude values of P3 component of 18 children with ADHD and gender matched 18 healthy controls performing an oddball task. Same recordings were repeated 3 months after extended-release MPH (OROS-MPH) administration for ADHD group. Prefrontal cortex oxygenation and P3 amplitude were compared between control and pre-MPH ADHD groups and between Pre-MPH and post-MPH ADHD groups.
RESULTS
fNIRS indicated that the healthy controls exhibited higher right prefrontal activation than pre-MPH children with ADHD. Reduced P3 amplitude values were found in children with ADHD compared the control group. Reduced right prefrontal activation and P3 amplitude was normalized in ADHD group after MPH therapy.
CONCLUSION
Recently multimodal neuroimaging which combine signals from different brain modalities have started to be considered as a potential to improve the accuracy of diagnosis. The current study provides MPH effect assessment in children with ADHD using multimodal EEG/fNIRS system for the first time. This study suggests combination of neuroimaging and electrophysiological parameters is a promising approach to investigate MPH effect assessment in children with ADHD.
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent neurodevelopmental disorder in school-age children with prelavence of 5–10% [Citation1]. It is related to inattentiveness, impulsivity and hyperactivity that can negatively affect the academic activities, social behaviour and personality development of child and can continue into adulthood [Citation2]. Early diagnosis of ADHD and appropriate treatment is critical to achieve better patient outcomes and improve quality of life. The psychostimulant methylphenidate (MPH) which enhances the activation of the striatum and prefrontal cortex, is one of the widely used psychostimulant to treat ADHD [Citation3]. Approximately 70% of children with ADHD show improvement in attention, behaviour, cognitive functions and social skills as response to MPH [Citation4–6]. However; it has been shown that 10–30% of patients are not respond to MPH therapy [Citation4]. Currently, diagnosis of ADHD and effects of medication entails subjective evaluation of phenotypes listed in the diagnostic criteria [Citation7] that is required ratings by parents or teachers of the children. Thus, objective approaches based on bio-markers which are capable enough to confirm the effectiveness of medication treatment can be extremely valuable.
Recent neuroimaging studies have demonstrated new approaches for evaluating the effectiveness of medication treatment in children with ADHD. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies have shown that use of MPH, increased extracellular dopamine levels and dopamine transporters levels and claimed these parameters can be used as biomarkers of treatment response to MPH [Citation8]. Rubia et al. reviewed MPH effects on children with ADHD with functional magnetic resonance ımaging (fMRI) studies and showed MPH therapy significantly enhanced activation in frontal cortex [Citation9]. There are some limitations of functional neuroimaging studies in ADHD patients especially in children: long measurement time can cause deficits in attention during cognitive tasks and children with ADHD are susceptible to body motion. Becasue of motion artefacts, more severely afflicted children are often excluded from fMRI studies [Citation10]. Recently a new way of accessing the effects of MPH treatment on children with ADHD is proposed by using an optical imaging method called; functional near infrared spectroscopy (fNIRS). fNIRS provides advantages due to its safety, accessibility, cost-effectiveness and tolerance to body motion and thus is considered suitable for the clinical evaluations of psychiatric disorders [Citation11]. fNIRS measures hemodynamic activity of the brain with concentration changes of oxygenated and deoxygenated haemoglobin (oxy-Hb and deoxy-Hb). Prefrontal cortex (PFC) blood oxygenation is expected to increase during cognitive tasks [Citation12]. Various fNIRS studies have sought to assess the hemodynamic changes of PFC during a cognitive task and showed children with ADHD exhibit diminished oxy-Hb increase compared to healthy controls [Citation13]. Recently, right prefrontal activation measured by fNIRS was served as an objective biomarker to show MPH treatment effects on children with ADHD [Citation14].
Along with neuroimaging studies, numerous neurophysiological studies, mainly focused on EEG and ERP based measures, were conducted to predict clinical response to stimulants in patients with ADHD [Citation15]. ERP that measure brain response during a visual, auditory or somatosensory stimuli, have been widely used for diagnosis of ADHD and for evaluating treatment effects of stimulants in children with ADHD [Citation16]. The interpretation of cognitive processing in ADHD using ERP mainly focuses identification of the latency and amplitude of P3 component that is correlated with attention [Citation17]. Several ERP components, particularly the P3 amplitude, have been evaluated for prediction of stimulant medication response [Citation18]. It has been demonstrated that MPH treatment raises P3 amplitudes [Citation19,Citation20]. This present study concerns auditory evoked potentials which reflect neural activity of the brain arising from acoustic stimulation. Auditory P3 amplitude has been investigated before and after treatment to predict response to stimulants in patient with ADHD.
Among with treatment effects, EEG and recently fNIRS have been widely used for objective and accurate diagnosis of ADHD. Most consistently prolonged P3 latencies, lower P3 amplitudes [Citation21] and reduced prefrontal activity were observed in children with ADHD compared to controls [Citation13]. In this present study ADHD diagnosed by two experienced child psychiatrists according to evaluation of phenotypes listed in the DSM-IV. Our clinical diagnosis was supported by neuroimaging methods. EEG and fNIRS data confirmed our diagnosis.
Previous studies that investigate effectiveness of MPH in ADHD treatment have largely focused on single neuroimaging or neurophysiological modalities. In this study we used a multimodal system and investigated effectiveness of MPH treatment both EEG and fNIRS modalities to obtain more robust results. To our knowledge this is the first report that uses multimodal EEG-fNIRS based measures as predictors of response to MPH stimulant.
Material and methods
Participants
Subjects comprised 18 children with ADHD-combined, before treatment (mean age = 9.55 ± 1.87, range 7–12 years) and 18 healthy children (mean age = 9.88 ± 1.99, range 7–12 years). Controls underwent a standard clinical assessment comprising neurological, endocrine and psychiatric evaluations. The ADHD patients were referred from Children Psychiatry Department of Erciyes University, Medical Faculty Hospital. Children with ADHD who have any central nervous system diseases such as epilepsy, cerebral palsy, developmental delay were not included in the study. All participants had normal hearing functions and were right-handed. The hearing functions tested with Rinne and Weber test. Intelligence Quotient (IQ) scores of participants were all >80 according to The Wechsler Intelligence Scale for Children-Revised (WISC-R). WISC-R is a general test of intelligence which measure verbal and performance abilities. The verbal test contains six subsets (information, vocabulary, arithmetic, comprehension, similarities and digit span), and performance test also contains six subtests (picture completion, block design, object assembly, picture arrangement, coding and mazes). So IQ score is obtained from WISC-R’s completely 12 subtests. In this study we used Turkish version of WISC-R whose standardization and norms were adapted for Turkish sample by Savasir and Sahin [Citation22]. The study was approved by the ethics committees of Erciyes University and written consent was obtained from the parents. Demographic characteristic of the subjects is shown in . There were not significant differences between controls and children with ADHD in terms of age, years of education, gender and IQ.
Table 1. Clinical characteristics of the participants.
MPH treatment
After the first assessment of children with ADHD, OROS-MPH at a dose of 0.5 mg/kg/day was prescribed to patients (mean 25.71 mg/g, min 18 mg/d, max 36 mg/d). A Dose of OROS-MPH was titrated up to 1.2 mg/kg/day with increments at 2-week intervals. The time to reach optimal dose (mean 41.14 mg/g, min 27 mg/d, max 54 mg/d) was generally 4–6 weeks; thus, effects of therapy was assessed 3 months after starting therapy.
Stimuli and protocol
EEG-fNIRS recordings were collected simultaneously while participants performed the auditory “oddball” paradigm. In this study 160 auditory stimuli were applied in a random order that consists 128 standard (2000 Hz) and 32 target (1500 Hz) stimuli. The interstimulus intervals (ISI) were randomized between 1250 and 2500 ms. Prior to the first run of the experiment, the participants were instructed to press the button when they hear target stimulus presented through headphones. During the experiment participants sat in a comfortable chair in a half supine position. The experiment conducted in a sound and light attenuated electrically shielded room. The participants instructed not to move, speak, or blink too much in order to avoid noise and stabilize the blood flow in fNIRS channels.
Instrumentation and data acquisition
fNIRS data acqusition
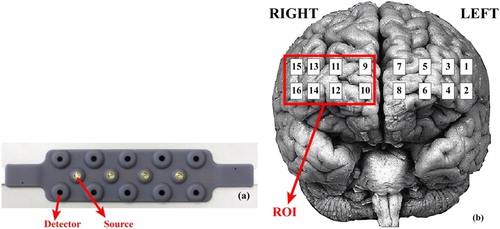
In this study, a 16 channel (CH) continuous wave fNIRS system has been used that has 4 sources with 2 different wavelengths (730 and 850 nm) and 10 photo detectors ((a)). (b) shows the CH locations on the fNIRS probe. The sampling frequency was 2 Hz. Heamodynamic changes were calculated using Beer–Lambert law [Citation23]. First, the raw fNIRS intensity measurements were low-pass filtered with a cut-off set to 0.14 Hz [Citation24] to remove noise derived from movement artefacts, heart pulsation and respiration. Then data of each channel were averaged across correctly identified target responses for each subject. Target responses identified 3 s before the target stimuli onset to 10 s after the target stimuli.
EEG data acquisition
EEG was recorded from Fz, Cz, Pz, and Oz locations using international 10–20 system through Ag/AgCl electrodes. The ground electrode was positioned on the right ear while the reference electrode was on the left ear. All electrode impedances were below 5 kΩ. The sampling frequency was 2500 Hz. EEG amplitudes greater than 100 µV raised from eye movements, eye blinks or muscle artefacts [Citation25] were rejected, then a band pass filter (0.05–100 Hz) and a Notch filter (with 45–50 Hz range) was applied to remove additional noise.
Data analysis
fNIRS measures and monitors changes in the concentrations of oxy-Hb and deoxy-Hb. In this study because of its high sensitivity, reliability and high signal to noise ratio we focused on oxy-Hb signals [Citation26,Citation27]. Moreover, because right PFC activation was served as an objective biomarker to show MPH treatment effects on children with ADHD [Citation14,Citation28] in this present study we selected this area as region of interest (ROI) as demonstrated in (b). We calculated the average of the integral value of oxy-Hb of right prefrontal channels for 18 ADHD (before and after treatment) and 18 control group. A sample fNIRS signal of right channels and grand average is demonstrated in (a,b) for a control participant.
Figure 2. fNIRS signal of right prefrontal channels including CH 9,10,11,12,13,14,15,16 for a control participant (b) grand average of these channels (c) AEP signal that is derived from EEG for the same participant.
Note: fNIRS and EEG signals obtained simultaneously.
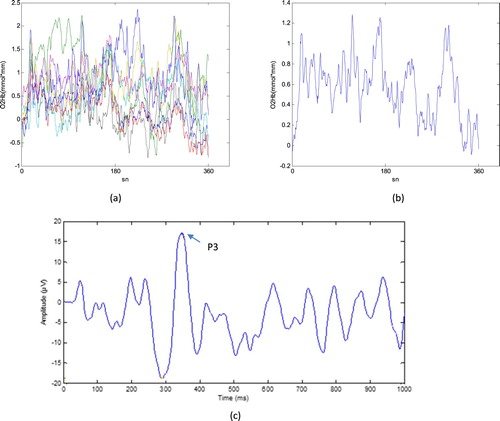
In addition to the hemodynamic values, we investigated the P3 component of ERP signals. The P3 component is correlated with attention and widely used in evaluating cognitive deficits in ADHD patients and medication effects on treatment. ERPs obtained from ongoing EEG with grand-average method [Citation29] that decreases the noise level ((c)). ERP signals were created according to this method by averaging of correctly identified target responses. The amplitude of P3 determined by two experienced physiologist and included the study.
Statistical analyses
We compared the variables between the pre-MPH ADHD and the control group using a student's t-test and the variables between pre-MPH ADHD and post-MPH ADHD groups using a two-tailed paired t-test with a statistical threshold of 0.05. Shapiro–Wilk normality test was used to confirm the distribution normality of variables. All the statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS Inc.; Chicago IL, ABD) Version 16.0.
Results
Pre-MPH comparison of variables between controls and ADHD patients
We compared pre-MPH ADHD and control groups in terms of integral value of oxy-Hb signals and P3 amplitude values of Fz, Cz, Pz, and Oz with independent two sample t-test. ADHD patients showed significantly reduced increases in oxy-Hb concentration as compared to healthy controls for average of channels in the right hemisphere. In the ADHD group P3 amplitudes were significantly smaller compared to healthy controls in Fz region. However, no significant difference in terms of P3 amplitude for Cz, Pz, Oz sensors was observed. shows concentration change of integral values of oxy-Hb and P3 amplitudes for each group among with group statistics. Descriptive values were expressed as mean ± S.D. (standard deviation).
Table 2. Statistical tests of medication effects with several potential confounding variables.
Pre-MPH vs. post-MPH comparison of variables
We investigated whether the oxy-Hb level and P3 amplitude increase by the MPH treatment. We compared the variables between pre-MPH and post-MPH group with paired sample t-test and observed significant MPH effect on oxy-hb increase (post-MPH > pre-MPH) in right prefrontal area. We also observed increased P3 amplitude values after treatment. Although P3 amplitude increased in all EEG locations (Fz, Cz, Pz, Oz) after treatment, we observed significant increase between groups only in Fz channel (). We also examined the effect of MPH by Conners’s Parent Rating Scale-Revised Short form (CPRS-RS). CPRS-RS is a standard instrument for the assessment of ADHD. CPRS-RS evaluate parent’s observations about the children or adolescents behaviour. The test evaluates inattention, hyperactivity/impulsivity, learning problems/executive functioning, aggression, and peer relations. In this study Turkish version of CPRS-RS that was adapted by Dereboy et al. [Citation30] was used to assess behavioural problems of children with ADHD. There was a significant decrease in Conners scores after treatment that means there was a decrease in ADHD symptoms ().
Discussion
This study was designed to investigate effectiveness of MPH medication treatment in children with ADHD by using information from changes in the concentration of oxygened haemoglobin and neuronal activity of frontal lobe during auditory oddball paradigm. We have previously shown that MPH reduced reaction times to stimuli and attenuated omission errors during the task in ADHD group [Citation31]. The data presented here extend this finding to the prediction of robust treatment response to the MPH by multimodal EEG/fNIRS system. The present study has three main findings; (1) Control children had greater right prefrontal activation with oddball paradigm at baseline compared to children with ADHD, (2) P3 amplitudes were reduced in children with ADHD, and (3) In children with ADHD both P3 amplitude and right prefrontal activation was normalized after three months of methlyphenidate therapy.
Prefrontal areas, specifically the dorsolateral PFC, is associated with attention to demanding cognitive tasks and the level of alertness [Citation32]. Various structural and functional neuroimaging studies indicated that ADHD is associated with abnormal neurodevelopmental process especially in PFC [Citation33]. Thus, with fNIRS we focused on PFC functions to evaluate cognitive effects of stimulants on children with ADHD. We obtained frontal cortex hemodynamic responses and amplitude values of P3 component of children with ADHD, performing an auditory oddball task before and 3 months after OROS-MPH administration. Ardic et al [Citation34] showed OROS-MPH treatment is more effective and safe compared to immediate release methylphenidate (IR-MPH) treatment.
The analyses of fNIRS revealed that before MPH treatment children with ADHD showed reduced increases in the concentration of oxy-Hb particularly for channels located in right PFC, compared to healthy controls. We observed MPH therapy significantly normalized the prefrontal cortex oxygenation in children with ADHD. Our results confirm previous fNIRS and other neuroimaging modalities finding of right prefrontal dysfunctions and MPH elicited recovery in children with ADHD [Citation28,Citation35]. In addition to fNIRS we also obtained EEG of participants simultaneously. We examined ERP that is produced from ongoing EEG and has been widely used for evaluating cognitive deficits in several psychiatric disorders, including ADHD [Citation36]. Johnstone et al. [Citation36] reviewed many of the ERP studies in ADHD and indicated interpretation of cognitive process of ADHD via ERPs mainly focuses on the latency and amplitude of P3 component that is correlated with attention. Consistent with previous studies, we observed smaller P3 amplitudes in the pre-MPH ADHD group compared with the control group [Citation36,Citation37]. Several ERP studies have examined the effects of MPH medication in ADHD treatment. It has been shown that P3 amplitude is an important parameter that predicts response to MPH. In these studies MPH enhanced the amplitude of the P3 peak especially in Fz, Cz, Pz locations [Citation38,Citation39]. In this study, we focused in these channels and Oz. Although P3 amplitude increased in all EEG locations (Fz, Cz, Pz, Oz) after treatment, we observed significant increase between groups only in Fz channel. This result confirms previous studies that were indicated frontal cortex is associated with cognitive processes such as planning, attentional control, response inhibition andworking memory [Citation32].
Previous studies that investigate effectiveness of MPH in ADHD treatment have largely focused on single neuroimaging or neurophysiological modalities. Most recent studies indicated that multimodal neuroimaging has the potential to enhance diagnosis of neuropsychiatric disorders [Citation40]. EEG measures electrical activity directly from the active neurons and fNIRS measures hemodynamic activity of the brain with concentration changes of oxy-Hb and deoxy-Hb. So EEG and fNIRS signals both reflected the brain activity with different parameters. We could not observe an increase in P3 amplitude after treatment in 5 of the 18 ADHD patients while there was an increase in oxy-Hb signal in four of them. Moreover, there were not increases in oxy-Hb signals in four patients of children with ADHD while three of them exhibited increased P3 amplitude. One of the patient could not show an improvement in P3 signal or oxy-Hb signal who is probably a non-responder to MPH treatment. Thus the combination of EEG and fNIRS provided robust results to examine MPH treatment effects in ADHD.
Clinical utility
Considering its high prevalence, large economic and societal costs, and the negative effects on the future lives of the children, early diagnosis of ADHD and appropriate treatment is important. Neuroimaging and neurophysiological studies have demonstrated new approaches for objective assessment of the ADHDs’ brain functions with underlying pathophysiology. Numerous research mainly focused on EEG and ERP signals to differentiate ADHD patients from controls. Loo et al. [Citation41] showed the clinical utility of EEG in ADHD in details. Recently fNIRS system has been applied in ADHD studies due to its compactness, tolerance to motion artefacts and and accessibility. In this study we used multimodal EEG/fNIRS modality that complement each other in spatial and temporal resolution, both noninvasive and easily accessible. Many studies have reported positive effects of MPH on a range of cognitive task including, the continuous performance test, the trail making test, verbal fluency tasks, go/no-go paradigms [Citation5]. Monden et al. [Citation42] claims alternating go/no-go blocks are more appropriate than one using a task and a rest period for children with ADHD because children with ADHD may exhibit unexpected movements and hyperactive behaviours during the rest period. Similarly we used standard auditory stimuli and did not adopt rest period. We selected an easy, short task contains standard and target paradigm with random sequence that can be appropriate for children with ADHD. Because long attentional tasks may not be appropriate for children with ADHD we choosed shorter auditory task that contains only 160 stimuli that would be more appropriate for children with ADHD. In short, this fNIRS/EEG based method offer clinical advantage for evaluating treatment effects in children with ADHD: It is simple, robust and applicable to children with ADHD as young as 7 years old. Moreover, there are patients who do not respond to MPH and side effects of treatment are observed in these patients [Citation43]. The proposed system will provide utility to identify MPH non-responders robustly by examining treatment effects both neuroimaging and neurophysiological perspective.
Limitations
A limitation of this study was a small sample size that limits the generalizability of our results. With larger sample size it would be possible to investigate treatment effects between three subtypes of ADHD. Furthermore, one of the ADHD participants could not show an improvement in P3 signal or oxy-Hb signal after treatment. He was probably a non-responder to MPH treatment because we observed clinic global impression scale of this patient does not fall below 2 while other patient’s fall. With larger sample size it will be possible to evaluate non-responders. An additional limitation of this study is although we tested hearing functions with Rinne and Weber test, there would be difference in hearing threshold between subjects.
In conclusion, before treatment children with ADHD exhibited reduced prefrotantal activation and lower P3 amplitudes compared to controls. In this study we observed MPH significantly increase oxy-Hb signals in the right PFC and increases P3 amplitude. Our results suggest that, multimodal fNIRS/EEG system provide more robust examination of MPH effects on ADHD. The current study provides MPH effect assessment in children with ADHD using multimodal EEG/fNIRS system for the first time. The findings suggest that it might be appropriate to measure electrical activity as well as hemodynamic outcomes when investigating medication effects when treating ADHD.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Nazan Dolu http://orcid.org/0000-0002-3104-7587
Miray Altınkaynak http://orcid.org/0000-0002-0258-2804
Ayşegül Güven http://orcid.org/0000-0001-8517-3530
Sevgi Özmen http://orcid.org/0000-0002-7545-2824
Esra Demirci http://orcid.org/0000-0002-8424-4947
Meltem İzzetoğlu http://orcid.org/0000-0002-1528-0127
Ferhat Pektaş http://orcid.org/0000-0002-1862-9515
Additional information
Funding
References
- Farone SV, Sergeant J, Gillberg C, et al. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–113.
- Biederman J. Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248.
- Arnsten AF. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacolog. 2006;31:2376–2383.
- Spencer TJ. ADHD treatment across the life cycle. J Clin Psychiatry. 2004;65(Suppl 3):22–26.
- Coghill D, Gagliano A, Pedroso S, et al. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/ hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol Psychiatry. 2014;76:603–615.
- Yilmaz A, Gokcen C, Fettahoglu EC. et al. The effect of methylphenidate on executive functions in children with attention-deficit hyperactivity disorder. Bulletin of Clinical Psychopharmacology. 2013;23(2):162–170.
- American psychiatric association diagnostic and statistical manual of mental disorders. 4th ed Washington (DC): American Psychiatric Association; 1994.
- Skokauskas N, Hitoshi K, Shuji H, et al. Neuroimaging markers for the prediction of treatment response to methylphenidate in ADHD. Eur J Paediatr Neurol. 2013;17:543–551.
- Rubia K, Alegria AA, Cubillo AI, et al. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Biol Psychiatry. 2014;76:616–628.
- Durston S, Tottenham NT, Thomas KM, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878.
- Ehlis AC, Schneidera S, Dreslera T, et al. Application of functional near-infrared spectroscopy in psychiatry. NeuroImage. 2014;85(1):478–488.
- Izzetoglu K, Bunce S, Onaral B, et al. Functional optical brain imaging using near-infrared during cognitive tasks. Int J Hum Comput Interact. 2004;17(2):211–227.
- Moser SJ, Cutini S, Weber P, et al. Right prefrontal brain activation due to stroop interference is altered in attention-deficit hyperactivitydisorder – a functional near-infraredspectroscopystudy. Psychiatry Res Neuroim. 2009;173:190–119.
- Monden Y, Dan H, Nagashima M, et al. Clinically-oriented monitoring of acute effects of methylphenidate on cerebral hemodynamics in ADHD children using fNIRS. Clin Neurophysiol. 2012;123:1147–1157.
- Hermens DF, Cooper NJ, Kohn M, et al. Predicting stimulant medication response in ADHD: evidence from an integrated profile of neuropsychological, psychophysiological and clinical factors. J Integr Neurosci. 2005;4:107–121.
- Ogrim G, Aasen IE, Brunner JF. Single-dose effects on the P3no-go ERP component predict clinical response to stimulants in pediatric ADHD. Clin Neurophysiol. 2016;127:3277–3287.
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148.
- Sangal RB, Sangal JM. Attention-deficit/hyperactivity disorder: use of cognitive evoked potential (P300) to predict treatment response. Clin Neurophysiol. 2006;117:1996–2006.
- Groom MJ, Scerif G, Liddle PF, et al. Effects of motivation and medication on electro-physiological markers of response inhibition in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;67:624–631.
- Pliszka SR, Liotti M, Bailey BY, et al. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:356–366.
- Senderecka M, Grabowska A, Szewczyk J, et al. Response inhibition of children with ADHD in the stop-signal task: an event-related potential study. Int J Psychophysiol. 2012;85(1):93–105.
- Savaşır I, ve Şahin N. Wechsler Çocuklar İçin zeka Ölçeği uygulama kitapçığı. Ankara: Türk Psikologlar Derneği; 1995.
- Cope M, Delpy DT. System for long-term measurement of cerebral blood flow and tissue oxygenation on newborn infants by infrared transillumination. Med Biol Eng Comput. 1988;26:289–294.
- Izzetoglu M, Izzetoglu K, Bunce S, et al. Functional near-infraredneuroimaging. IEEE Trans Neural Syst Rehabil Eng. 2005;13(2):153–159.
- Fisch BJ. EEG PRIMER: basic principles of digital and analog EEG. 3rd ed. Richmond: Elsevier Academic Press; 1999.
- Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40(4):511–520.
- Ehlis AC, Bahne CG, Jacob CP, et al. Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: a functional near-infrared spectroscopy (fNIRS) study. J Psychiatr Res. 2008;42:1060–1067.
- Monden Y, Dan H, Nagashima M, et al. Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: An fNIRS study. Neuroimage Clin. 2012;1(1):131–140.
- Davila CE, Srebro R. Subspace averaging of steady-state visual evoked potentials. IEEE Trans Biomed Eng. 2000;47(6):720–728.
- Dereboy Ç, Şener Ş, Dereboy İF. Adaptation of Conners’ parent rating scale in Turkish study X. Ankara: Ulusal Psikoloji Kongresi; 1998.
- Güven A, Altınkaynak M, Dolu N, et al. Effects of methylphenidate on reaction time in children with attention deficit / hyperactivity disorder. Arch Neuropsychiatry. (Article in Press). doi:10.29399/npa.22873.
- Miller EK. Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: A review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284.
- Ardic UA, Ercan ES, Ercan E, et al. Osmotic release oral system methylphenidate is more effective than immediate release methylphenidate: A retrospective chart review in turkish children with attention deficit hyperactivity disorder. Bulletin of Clinical Psychopharmacology. 2014;24:342–349.
- Rubia K, Halari R, Cubillo A, et al. Meth-ylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652.
- Johnstone SJ, Barry RJ. Clarke AR ten years on: a follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2013;124:644–657.
- Kratz O, Studer P, Malcherek S, et al. Attentional processes in children with ADHD: an event-related potential study using the attention network test. Int J Psychophysiol. 2011;81:82–90.
- Lopez J, Lopez V, Rojas D, et al. Effect of psychostimulants on distinct attentional para-meters in attentional deficit/hyperactivity disorder. Biol Res. 2004;37(3):461–468.
- Sawada M, Iida J, Ota T, et al. Effects of osmotic-release methylphenidate in attention-deficit/hyperactivity disorder as measured by event-related potentials. Psychiatry Clin Neurosci. 2010;64(5):491–498.
- Liu S, Cai W, Liu S, et al. Multimodal neuroimaging computing: a review of the applications in neuropsychiatric disorders. Brain Inform. 2015;2:167–180.
- Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics. 2012;9(3):569–587.
- Monden Y, Dan I, Nagashima M, et al. Individual classification of ADHD children by right prefrontal hemodynamic responses during a go/no-go task as assessed by fNIRS. Neuroimage Clin. 2015;9:1–12.
- Rapport MD, Denney C, DuPaul GJ, et al. Attention deficit disorder and methylphenidate: normalization rates, clinical effectiveness, and response prediction in 76 children. J Am Acad Child Adolesc Psychiatry. 1994;33:882–893.