ABSTRACT
AIM: In the present study, we aimed to determine the volume differences in brain regions involved in cortical-striatal-thalamic-cortical circuit (CSTC) between healthy subjects and obsessive–compulsive disorder (OCD) patients. We also evaluated the potential relationship between volumes of region of interest and various illness parameters (duration and current severity OCD, and the influence of drug treatment).
METHODS: We examined the volumetric differences in dorsolateral prefrontal cortex (DPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), thalamus and striatum between OCD patients (n = 21) and healthy controls (HCs) (n = 25).
RESULTS: Patients with OCD had significantly larger total, right, and left DLPFC, and OFC volumes compared to HCs. Total, and left ACC, total, and left striatum volumes were significantly smaller in OCD patients than in HC. The thalamus volumes were not different between two groups. The most of volumetric correlations in HCs disappeared among OCD patients. Only, the correlation between the volumes of left striaum and left ACC volume remained significant. Fisher's r-to-z transformation tests indicated that correlation coefficients of brain volumes significantly differed between both groups for right ACC and left (z = 2.17, p = .03) and right OFC (z = 2.00, p = .04); left ACC and right OFC (z = 2.41, p = .01); right ACC and left (z = 2.94, p = .003), and right striatum (z = 2.43, p = .01).
CONCLUSIONS: Our findings indicate the impaired connectivity of ACC, OFC, and striatum in the pathophysiology of OCD. Further research is needed to explore precisely which brain regions nuclei are specifically involved in the occurence of OCD symptoms.
Introduction
Obsessive–compulsive disorder (OCD) is a common psychiatric disorder with a life time prevalence of 2.0–3.0% that is characterized by obsessions and/ or compulsions [Citation1,Citation2]. Multiple investigations suggest that neurobiology plays a significant role in the aetiology and pathogenesis of OCD [Citation3–5]. A number of functional and structural neuroimaging studies have demonstrated abnormalities in functions of cortical-striatal-thalamic-cortical (CSTC) circuits linking orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), basal ganglia, and thalamus in the neuropathophysiology of OCD [Citation5–14]. In OCD, the existence of a lateral orbitofrontal loop was suggested, involving projections from the OFC to the striatum, then to the thalamus, and finally returning from the thalamus to the OFC [Citation15]. OCD symptoms may emerge from disturbed connectivity between brain regions related to hypoactivity in frontal-striatal circuits, or from increased activity/connectivity in the circuit [Citation16,Citation17]. Although volume changes in cortical and subcortical brain regions, and the impaired connections between these brain regions are previously demonstrated in OCD patients, the precise relationship between distinct brain regions in the dysregulation of the CSTC has yet to be well-characterized. Because of a great heterogeneity in the anatomical definition of brain regions, and in clinical characteristics such as medication status, or the severity of OCD may lead to quite different results. Identification of brain alterations is therefore still critical to the understanding of the pathophysiology and the aetiology of this disorder. In the present study, we investigated the volume differences in DLPFC, OFC, ACC, thalamus, and striatum between OCD patients and healthy controls (HCs). In addition, we evaluated the potential relationship between volumes of region of interest and various illness parameters (duration and current severity OCD, and the influence of drug treatment).
Methods
Subjects
The study sample consisted of 21 patients who met OCD diagnosis according to Structured Clinical Interviews for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I Disorders (SCID-I) [Citation18,Citation19] and had a score of 16 or higher on the Yale-Brown Obsessive–Compulsive Scale (Y-BOCS) [Citation20,Citation21] on the day of MRI, and 25 age, and sex matched HCs. The patients had no lifetime SCID-I diagnoses of bipolar disorder, schizophrenia, mental retardation, or substance abuse. Since comorbid depression can also influence brain volume in OCD patients [Citation22], the patients who had a current diagnosis of major depression obtained by SCID-I were also excluded. All HCs were screened using SCID Non-Patient edition (SCID-NP) to confirm the lifetime absence of psychiatric illnesses. All participants recruited from an university hospital were right-handed, and had no current or past neurological disorders. The subjects with cardiac pacemakers or other metallic implants or artefact were not included in the sample. There were fourteen patients using medications (6 sertraline, 1 citolopram, 1 paroxetine, 2 venlafaxine, 4 fluvoxamine, 2 klomipramine, and 1 aripiprazol, 1 olanzapin). Of the seven patients who were not receiving psychotropic medications, three had received them in the past, and four were treatment naive. The dose equivalents of antidepressants have been calculated according to the criteria defined in a previous study [Citation23].
The study was approved by the Local Ethical Committee (25.10.2018-2018/1504) and all subjects signed informed consent, and written informed consent was obtained from each subject. Research technicians were blind to diagnostic status of participants.
MRI examination
Magnetic resonance examination (MRI) was performed on a 1.5-Tesla Philips Achieva MR Scanner (Philips Medical Systems, Best, The Netherlands) with a 16-channel SENSE-type head coil. Axial and sagittal T2 weighted images were acquired using Turbo Spine Echo sequence with repetition time/echo time = 5800/110 ms, field-of-view = 23 × 23 cm2, matrix size = 256 × 256, number of excitation = 1 and slice thickness = 5 mm. The participants were instructed to keep their eyes closed, remain relaxed, and not to fall asleep. All participants underwent an approximately 8 min resting-state scan. We did not use any methods to characterize the motion artefacts in this study.
Data acquisition
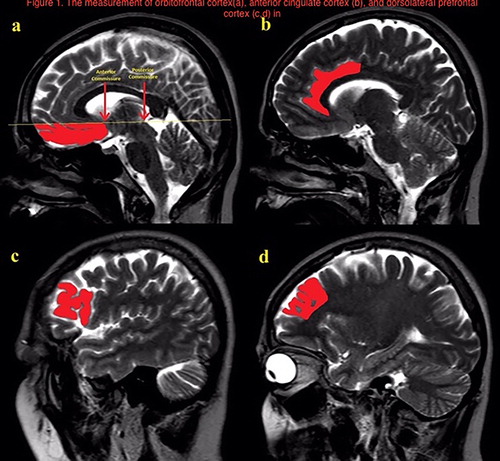
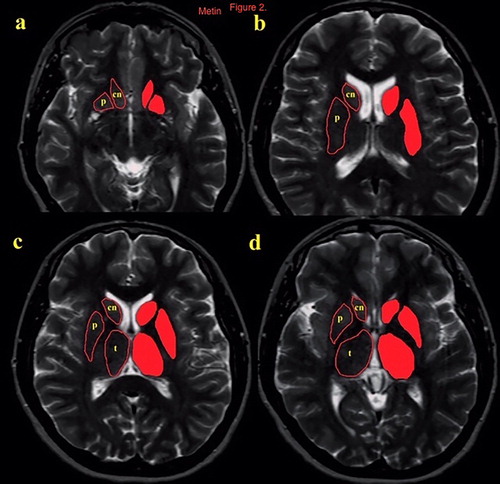
Volume measurements were made by free-hand tecnique with Ekinoks advanced CT and MRI imaging Workstation software (Telemed-Ekinoks software, Bogazici University Technopark, Istanbul, Turkey, version 1.7, 2017). The measurements of the interested brain regions were made blinded to the diagnosis of the patients. The brain regions consisted of the OFC, DLPFC, ACC, thalamus, and striatum (nucleus caudatus and putamen) on the basis of anatomic landmarks. The boundaries of the evaluated structures were determined on the axial and the sagittal MR images according to standard brain atlases [Citation24–28]. While the measurements of the OFC, DLPFC and the ACC were performed on sagittal images, the striatum and the thalamus measurements were performed on axial images ( and ). In order to determine the superior boundary of the OFC, a posterior-anterior line was drawn passing through the anterior and posterior commissure. The olfactor sulcus was determined as the posterior boundary, the most inferior aspect of the cortex as the inferior boundary, the most lateral egde of the cortex as the lateral boundary, and the longitudinal fissure as the medial boundary for the OFC. The cingulate sulcus indicated the anterior and the superior limit of the ACC. Posterior boundary of ACC was defined as the anterior commissure. DLPFC volumes were measured with the following boundaries: prefrontal sulcus as posteriorly, frontal pole as anteriorly, inferior frontal sulcus as ventrolaterally, and parasingulate gyrus as medially. The caudate and the putamen nucleuses were traced on axial slices. Tracing commenced when the structure could be seen with the naked eye and ended when the structure was no longer discernible. To define the anterior and the posterior boundaries of the thalamus on axial slices, the sagittal plane was used as a reference line. The main tracing was performed on the axial plane, in which the boundaries of the thalamus were based on the detailed guidelines of Portaset al. [Citation26]. The internal capsule, laterally; the third ventricle and the habenular nucleus, medially; the lateral ventricle and the crux fornix, dorsally; and the zona inserta and the red nucleus, ventrally.
Statistical analysis
Statistical analysis was performed using the IBM SPSS version 21.0 for Windows system (SPSSInc. Chicago, Illinois, USA). Differences between two groups were examined by non-parametric Mann Whitney U test. Chi-square test was used for comparison of the categorical variables. Correlations of several variables were determined using Spearman Rank Correlation Test. We used Fisher r-to-z transformation test to assess the significance of the difference between two correlation coefficients, found in OCD patients and HCs. All statistical assessments were two tailed, and we considered results to be significant at p < .05.
Results
The sociodemographic and clinical description of the OCD and HC groups is given in . There were no differences between the two groups in terms of age, gender, and educational levels. The rates of lifetime diagnosis of major depression (p = .002), and being married (p = .006) were likely to be higher in OCD patients compared to HCs. shows the volume differences in brain regions between OCD patients and HCs. Patients with OCD had significantly larger total, right, and left DLPFC (p < .0001), and OFC volumes (p < .0001) compared to HCs. In contrast, total (p < .0001), and left ACC (p < .0001); total (p = .008), and left striatum (p = .003) volumes were significantly smaller in OCD patients than in HC. The thalamus volumes were not different between two groups.
Table 1. The sociodemographic and clinical comparison of obsessive–compulsive disorder patients and healthy controls.
Table 2. The comparison of brain volumes between obsessive–compulsive disorder patients and healthy controls.
gives the results of correlation analyses between brain volumes and duration of the illness, equivalent doses and YBOCS scores in OCD patients. The current age of the patients showed significant inverse correlations with total (r = −0.56, p < .005) and left striatum volumes (r = −0.57, p < .05). We have found significant correlations of total, and obsession scores of YBOCS, with total ACC (r = 0.57, p = .005; r = 0.58, p = .005, respectively), and left striatum volumes (r = 0.45, p < .05; r = p < .005, respectively). YBOCS compulsion scores were correlated with total ACC volumes (r = 0.57, p < .005). We have found a slight correlation between the duration of the ilness and right ACC volumes (r = 0.46, p < .05). As showed in , there were several significant positive correlations between the volumes of distinct brain regions pointing out the structural connectivity in healthy subjects. We have found that the most of these volumetric correlations disappeared among OCD patients. Only, the correlation between the volumes of left striaum and left ACC volume remained significant (r = .54, p < .05) (). Fisher's r-to-z transformation tests () showed that correlation coefficients of brain volumes significantly differed between both groups for right ACC and left (z = 2.17, p = .03) and right OFC (z = 2.00, p = .04); left ACC and right OFC (z = 2.41, p = .01); right ACC and left (z = 2.94, p = .003), and right striatum (z = 2.43, p = .01).
Table 3. The correlations between brain volumes and clinical variables in OCD patients (n = 21).
Table 4. The correlations between brain volumes in healthy controls (n = 25).
Table 5. The correlations between brain volumes in OCD patients (n = 21).
Table 6. Fisher r–z transformation test results between OCD patients and healthy controls (z/p values).
Discussion
The abnormalities within CSTC circuits have been hypothesized to play a key role in the pathophysiology of OCD [Citation29–31]. It was suggested that these circuits are associated with a relatively specific functional role, based on the connections within each circuit. In the present study, we examined the volumetric differences in brain regions within CTSTC loop between OCD patients and HCs. We also assessed at what levels the associations between distinct brain regions in HCs were impaired in OCD patients. We obtained some findings showing volumetric abnormalities in the brain regions involved in OCD pathophysiology and posssible impairments in connectivity within CSTC loop in patients with OCD. Our results indicated that the bilateral volumes of OFC and DLPC were significantly larger than HCs. In contrast, total and left ACC and striatum volumes were significantly reduced in OCD patients compared to HCs. The current ages, duration and severity of OCD and, equivalent doses of antidepressant doses were correlated with volumes of some of the brain regions. We have found several intercorrelations between distinct brain areas in HCs suggesting the connectivity within CSTC circuits. Further analyses indicated that there were significant decreases in some of these correlations in OCD patients. Particularly, the differences in the correlations of ACC with OFC and striatum appeared to be significant following Fisher r-to-z transformation analysis. Therefore, our results might suggest that hyperactivity in DLPFC, and OFC may lead to neurotoxic changes resulting in brain tissue volume reduction in left striatum and ACC.
Anterior cingulate cortex
Abnormalities in the ACC is supposed to contribute to the pathogenesis of OCD through impaired connectivity with other cortical and subcortical brain regions. Some studies found a reduced connectivity of dorsal striatum and medial dorsal thalamus to rostral and dorsal ACC in OCD patients [Citation32–38]. Our results indicated that total and left ACCs volume were decreased in OCD patients compared to HCs, and the connectivity of right ACC with bilateral OFC, and striatumwere significantly reduced in patients with OCD. Additionally, the connectivity between left ACC and right OFC were found to be impaired in OCD group. The smaller ACC and striatal volumes, and reduced correlations of ACC with OFC and striatum might suggest a damage and hypofunctionality in ACC and striatum in OCD patients. The increased volume in the OFC may indicate the hyperactivity in this pathway of the circuit. These findings converge with prior studies that implicate functional and structural abnormalities in the ACC, OFC and striatum in the pathophysiology of OCD [Citation38]. In contrast to previous studies which indicated that YBOCS compulsion scores had significant correlations with right ACC volume [Citation39,Citation40], we have found positive correlations between YBOCS total, and compulsion scores and left ACC volume. This finding might indicate that the smaller volumes of ACC were not related to the current severity of OCD. However, the negative correlation between the duration of OCD and right ACC volume might demonstrate that volume reduction in ACC is related to the longer duration of the illness.
Orbitofrontal prefrontal cortex
The OFC is a key region in the CSTC circuitry. The exact direction of increases and decreases in volumes of the OFC in patients with OCD relative to controls has been unclear. There are reports of increased [Citation41–43] or decreased volume in the OFC [Citation8–11,Citation44,Citation45] or no volumetric changes [Citation46] than those of normal controls, suggesting that a degenerative process might be implicated in the pathophysiology of OCD. Our results indicated a bilateral increase in OFC volume compared to HC. In addition, we have found that the connectivity ofbilateral ACC toright OFC was significantly impaired in OCD patients. Volumetric decrease in connectivity of right ACC to left OFC was also reduced. We supposed that increasing OFC volumes in OCD patients may play an inhibitory role on ACC. The functional and structural abnormalities of the OFC may disturb the feedback system, and may lead to dysfunctions of emotional and behavioural inhibitions in OCD [Citation40,Citation43].
Striatum
The basal ganglia are also important in the pathophysiology of OCD. Hypotheses on the etiopathogenesis of OCD suppose that a defective [Citation47,Citation48], imbalanced [Citation29], or hypertonic [Citation49] striatum produces OCD symptoms by impairing the fronto-subcortical mechanisms in the CTSC loops connecting the OFC and ACC to the striatum. Dysfunction in striatum might disinhibit the thalamus resulting in OCD symptoms [Citation49]. Considering the role of basal ganglia in OCD, our findings revealed that the striatal volume was significantly smaller in OCD patients than in HCs. The decreases in correlations between bilateral striatum and ACC volumes may suggest an abnormal anatomical connectivity between these regions in patients with OCD. A possible explanation of this relationship might be that hyperacitivity in DLPFC and OFC may lead to neurotoxic changes resulting in brain tissue volume reduction in striatum and ACC.
Dorsolateral prefrontal cortex
The functional neuroimaging and structural MRI studies provide evidence that brain regions involved in OCD pathophysiology are not limited to orbitofronto-striatal circuits. The involvement of DLPFC in OCD has been reported in some previous studies [Citation5]. The DLPFC has been considered as an important region, equally to the ACC, in higher cognitive functions such as attention and executive function [Citation50], which might be impaired in OCD. A volume change in DLPFC implicates damage in the CSTC. Some studies showed reduced volume in the DLPFC whereas other studies reported no difference in volume [Citation51] or increased volume [Citation43] in the DLPFC among OCD patients. In our study, although bilateral DLPFC volumeswere larger in OCD patients than in HC, there was no finding indicating an impairment in connectivity of DLPFC to other brain including OFC, ACC, and thalamus. This conclusion is not consistent with previous studies which found volume reductions in the DLPFC and in reciprocally connected regions as a possible sign for altered anatomical connectivity in fronto-subcortical circuitry [Citation17,Citation43,Citation52]. Therefore, we can not suggest that DLPFC is involved in OCD in contrast to previous findings [Citation11].
Thalamus
The thalamus acts as a relay centre that transmits and processes neuronal information from the basal ganglia to cortical areas. Within the thalamus, the mediodorsal and ventral anterior thalamic nuclei are of great interest in OCD because both nuclei send intense projections to the ACC and OFC [Citation53–Citation55]. Hence, volume changes observed in cortical areas may be related to volume changes in the thalamus. In the present study, we could not find a volume difference in thalamic volume between OCD patients and HC, in contrast to previous studies which have shoerwn that patients OCD patients have a greater thalamic volume relative to HC [Citation8,13,41,47,52,54]. However, our findings indicated that the association of left thalamus with right OFC was impaired in OCD patients. A previous study reported that thalamic and OFC volumes are inversely and spesifically related in OCD patients [Citation54]. It is likely explained by the fact that the majority of the patients in our study were receiving anti-obsessive treatments which may influence brain volumes. In our study, the association of thalamus to ACC in HC seemed to be strikingly impaired in OCD patients. This finding may permit the establishment of a causal relationship between thalamic and ACC volume changes, therefore is contradictory to previous suggestions which argue that thalamo-frontal alterations in OCD may be limited to the thalamo-orbitofrontal pathways [Citation54]. Because the thalamus sends glutamatergic projections to the cerebral cortex, a reduction in ACC volume could be related to neurodegeneration induced by glutamate neurotoxicity, as described for other neuropsychiatric disorders, and supported by the glutamatergic hyperactivity described in OCD [Citation56].
Limitations of the study
The major limitation of the present study is that the small sample size of the current study groups limits the generalizability of the study findings. Another potential limitation of this study is the lack of current mood and anxiety disorders symptom scores. Although subjects with major depressive disease were excluded, it is possible that this study included subjects with depressive symptoms that did not meet criteria for an axis mood disorder. Most of the patients included in our study were receiving anti-obsessive treatment, which may contribute to volume variations, even though the sample had clinically significant OCD symptoms. The inclusion of patients with lifetime major depression, and the lack of assessments for insight, hoarding disorder, and tic disorder are the other limitations of the current study.
Conclusions
In conclusion, our findings provide challenging evidence for future research on the volumetric alterations in OCD patients compared to HCs indicating the impaired connectivity in spesific brain areas involved in the pathology of OCD. Our findings support and extend previous neuroimaging studies that have demonstrated the involvement of frontosubcortical circuits originating within the ACC and OFC in the pathophysiology of OCD. Taken together the reported findings would suggest that morphometric alterations in ACC, OFC, and the striatum might be pathogenic for OCD. However, further research is needed to explore precisely which brain regions nuclei are specifically involved in the occurence of OCD symptoms, and to establish a relationship between the structural relationship of ACC with OFC and striatum. Further studies using drug-naïve patients are needed to confirm our results.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Bilge Dogan http://orcid.org/0000-0001-7895-9738
Ersen Ertekin http://orcid.org/0000-0001-7182-0725
References
- Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:743–758. doi: 10.1016/S0193-953X(18)30205-3
- Abramowitz JS, Taylor S, McKay D. Obsessive-compulsive disorder. Lancet. 2009;8(374):491–499. doi: 10.1016/S0140-6736(09)60240-3
- Aouizerate B, Guehl D, Cuny E, et al. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004
- Whiteside SP, Port JD, Deacon BJ, et al. A magnetic resonance spectroscopy investigation of obsessive-compulsive disorder and anxiety. Psychiatry Res. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006
- Menzies L, Chamberlain SR, Laird AR, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005
- Atmaca M, Yildirim B H, Ozdemir B H, et al. Volumetric MRI assessment of brain regions in patients with refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1051–1057. doi: 10.1016/j.pnpbp.2006.03.033
- Jung WH, Kang DH, Kim E, et al. Abnormal corticostriatal-limbic functional connectivity in obsessive-compulsive disorder during reward processing and resting-state. Neuroimage Clin. 2013;3:27–38. doi: 10.1016/j.nicl.2013.06.013
- Atmaca M, Yildirim H, Ozdemir H, et al. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. ProgNeuropsychopharmacol Biol Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008
- Choi JS, Kang DH, Kim JJ, et al. Left anterior subregion of orbitofrontal cortex volume reduction and impaired organizational strategies in obsessive-compulsive disorder. J Psychiatr Res. 2004;38:193–199. doi: 10.1016/j.jpsychires.2003.08.001
- Kang DH, Kim JJ, Choi JS, et al. Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2004;16:342–349. doi: 10.1176/jnp.16.3.342
- Pujol J, Soriano-Mas C, Alonso P, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720
- Moon CM, Kim BC, Jeong GW. Associations of neurofunctional, morphometric and metabolic abnormalities with clinical symptom severity and recognition deficit in obsessive-compulsive disorder. J Affect Disord. 2018;227:603–612. doi: 10.1016/j.jad.2017.11.059
- Gilbert AR, Moore GJ, Keshavan MS, et al. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Arch Gen Psychiatry. 2000;57:449–456. doi: 10.1001/archpsyc.57.5.449
- Matsumoto R, Ito H, Takahashi H, et al. Reduced gray matter volume of dorsal cingulate cortex in patients with obsessive-compulsive disorder: a voxel-based morphometric study. Psychiatry Clin Neurosci. 2010;64:541–547. doi: 10.1111/j.1440-1819.2010.02125.x
- Huey ED, Zahn R, Krueger F, et al. A psychological and neuroanatomical model of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2008;20:390–408. doi: 10.1176/jnp.2008.20.4.390
- Lochner C, Fouché JP, du Plessis S, et al. Evidence for fractional anisotropy and mean diffusivity white matter abnormalities in the internal capsule and cingulum in patients with obsessive-compulsive disorder. J Psychiatry Neurosci. 2012;37:193–199. doi: 10.1503/jpn.110059
- Zarei M, Mataix-Cols D, Heyman I, et al. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 2011;70:1083–1090. doi: 10.1016/j.biopsych.2011.06.032
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM IV). 4th ed. Washington (DC): American Psychiatric Press; 1994.
- Çorapçıoğlu A, Aydemir Ö, Yıldız M. DSM-IV Eksen I bozukluklarına Göre Türkçe Yapılandırılmış Klinik Değerlendirmenin Güvenirliği. İlaç ve Tedavi Dergisi. 1999;12:33–36.
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown obsessive-compulsive scale: development, use and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007
- Tek C, Ulug B, Rezaki BG, et al. Yale-Brown obsessive compulsive scale and US National Institute of Mental Health Global obsessive compulsive Scale in Turkish: reliability and validity. Acta Psychiatr Scand. 1995;91:410–413. doi: 10.1111/j.1600-0447.1995.tb09801.x
- Cardoner N, Soriano-Mas C, Pujol J, et al. Brain structural correlates of depressive comorbidity in obsessive-compulsive disorder. Neuroimage. 2007;38:413–421. doi: 10.1016/j.neuroimage.2007.07.039
- Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179–184. doi: 10.1016/j.jad.2015.03.021
- Jackson GD, Duncan JS. MRI anatomy: a new angle on the brain. New York (NY): Churchill Livingstone; 1996.
- Patel VH, Friedman L. MRI of the brain: normal anatomy and normal variants. Saunders; 1997.
- Portas CM, Goldstein JM, Shenton ME, et al. Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry. 1998;43:649–659. doi: 10.1016/S0006-3223(97)00339-9
- Lacerda AL, Hardan AY, Yorbik O, et al. Measurement of the orbitofrontal cortex: a validation study of a new method. Neuroimage. 2003;19:665–673. doi: 10.1016/S1053-8119(03)00137-X
- Sass JO, Forstner R, Sperl W. 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase deficiency: impaired catabolism of isoleucine presenting as neurodegenerative disease. Brain Dev. 2004;26:12–14. doi: 10.1016/S0387-7604(03)00071-8
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23(3):563–586. doi: 10.1016/S0193-953X(05)70181-7
- Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive-compulsive disorder. Semin Clin Neuropsychiatry. 2001;6:82–101. doi: 10.1053/scnp.2001.21833
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28(2):343–347. doi: 10.1016/S0896-6273(00)00113-6
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046
- Radua J, van den Heuvel OA, Surguladze S, et al. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70
- Rotge JY, Guehl D, Dilharreguy B, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019
- Rotge JY, Langbour N, Jaafari N, et al. Anatomical alterations and symptom-related functional activity in obsessive-compulsive disorder are correlated in the lateral orbitofrontal cortex. Biol Psychiatry. 2010;67:e37–e38. doi: 10.1016/j.biopsych.2009.10.007
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/REVNEURO.1999.10.1.49
- Botvinick M, Nystrom LE, Fissell K, et al. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;11:179–181. doi: 10.1038/46035
- Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in obsessive-compulsive disorder: a diffusiontensor imaging study. Arch Gen Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782
- Venkatasubramanian G, Zutshi A, Jindal S, et al. Comprehensive evaluation of structure abnormalities in drug-naive, adult patients with obsessive-compulsive disorder: a surface-based morphometry study. J Psychiatr Res. 2012;46:1161–1168. doi: 10.1016/j.jpsychires.2012.06.003
- Alvarenga PG, do Rosario MC, Batistuzzo MC, et al. Obsessive-compulsive symptom dimensions correlate to spesific gray matter volumes in treatment-naive patients. J Psychiatr Res. 2012;46:1635–1642. doi: 10.1016/j.jpsychires.2012.09.002
- Kim JJ, Lee MC, Kim J, et al. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330
- Valente AA, Miguel EC, Castro CC, et al. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry. 2005;58(6):479–487. doi: 10.1016/j.biopsych.2005.04.021
- Christian CJ, Lencz T, Robinson DG, et al. Gray matter structural alterations in obsessive-compulsive disorder: relationship to neuropsychological functions. Psychiatry Res. 2008;164:123–131. doi: 10.1016/j.pscychresns.2008.03.005
- van den Heuvel OA, Remijnse PL, Mataix-Cols D, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–868. doi: 10.1093/brain/awn267
- Hoexter MQ, de Souza Duran FL, D'Alcante CC, et al. Gray matter volumes in obsessive-compulsive disorder before and after fluoxetine or cognitive-behavior therapy: a randomized clinical trial. Neuropsychopharmacology. 2012;37:734–745. doi: 10.1038/npp.2011.250
- Jenike MA, Breiter HC, Baer L, et al. Cerebral structural abnormalities in obsessive-compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Arch Gen Psychiatry. 1996;53:625–632. doi: 10.1001/archpsyc.1996.01830070073011
- Baxter LR. Neuroimaging studies of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:871–884. doi: 10.1016/S0193-953X(18)30215-6
- Modell JG, Mountz JM, Curtis GC, et al. Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1989;1:27–36. doi: 10.1176/jnp.1.3.340-a
- Szechtman H, Ahmari SE, Beninger RJ, et al. Obsessive-compulsive disorder: insights from animal models. Neurosci Biobehav Rev. 2017;76:254–279. doi: 10.1016/j.neubiorev.2016.04.019
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7
- Rosenberg DR, Keshavan MS. A.E. Bennett Research Award. Toward a neurodevelopmental model of of obsessive–compulsive disorder. Biol Psychiatry. 1998;1(43):623–640. doi: 10.1016/S0006-3223(97)00443-5
- Yoo SY, Roh MS, Choi JS, et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. J Korean Med Sci. 2008;23:24–30. doi: 10.3346/jkms.2008.23.1.24
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:368–404. doi: 10.1007/s00381-002-0604-1
- Rotge JY, Dilharreguy B, Aouizerate B, et al. Inverse relationship between thalamic and orbitofrontal volumes in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:682–687. doi: 10.1016/j.pnpbp.2009.03.011
- Taylor-Robinson SD, Weeks RA, Sargentoni J, et al. Evidence for glutamate excitotoxicity in Huntington's disease with proton magnetic resonance spectroscopy. Lancet. 1994;343:1170. doi: 10.1016/S0140-6736(94)90280-1
- Carlsson ML. On the role of cortical glutamate in obsessive-compulsive disorder and attention-deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatr Scand. 2000;102:401–413. doi: 10.1034/j.1600-0447.2000.102006401.x