ABSTRACT
OBJECTIVE: In this study, we aimed to study copper metabolism in schizophrenia, bipolar disorder, major depression compared with healthy control.
METHODS: This is a single-centered cross-sectional study. The patients with schizophrenia (n = 36), bipolar disorder (n = 37), major depression (n = 40), and healthy control (n = 32) were included in the study. All participants were initially evaluated by a clinical psychiatrist to confirm the appropriate diagnosis using the Structured Clinical Interview for Diagnostic and Statistical Manuel of Mental Disorders-IV (DSM-IV) Axis I Disorders (SCID-I). Serum copper level, ceruloplasmin mass, and ceruloplasmin-ferroxidase activity were measured. One-way ANOVA and Kruskal–Wallis Tests were performed for statistical analyses.
RESULTS: Serum ceruloplasmin-ferroxidase activity (χ2 = 9.11, p = 0.028) demonstrated a significant statistical difference in all groups compared with the control group. Serum ceruloplasmin-ferroxidase activity of the bipolar disorder group was significantly higher than the healthy control group (p = 0.012), major depression group (p = 0.027), and the schizophrenia group (p = 0.019). Erythrocyte sedimentation rate (ESR) (p = 0.028) and waist circumference (p = 0.005) in bipolar disorder group, and the C-reactive protein (CRP) (p < 0.001) and cholesterol (p = 0.043) in the schizophrenia group were found as the determinants of ceruloplasmin-ferroxidase activity.
CONCLUSION: In this study, ceruloplasmin-ferroxidase activity is higher in all groups in comparison to the healthy control. The significantly higher ceruloplasmin-ferroxidase activity was shown in bipolar disorder followed by the major depression and schizophrenia. The ceruloplasmin-ferroxidase activity was correlated with erythrocyte sedimentation rate in the bipolar disorder group and with C-reactive protein in the schizophrenia group. Therefore, the ceruloplasmin-ferroxidase activity may be an encouraging candidate in the neuro-immune modulation and become a reliable clinical tool for demonstrating the strong association of inflammation in these disorders.
Introduction
Copper (Cu) is one of the most abundant elements among the transition metals. It plays a critical physiological function as a component of proteins. It also serves as the cofactor for numerous critical metalloenzymes involving the dopamine and norepinephrine metabolism. Level of transition metals is tightly controlled, since their excessive or scarce levels may lead to several abnormalities [Citation1–3].
Ceruloplasmin (Cp) is a protein synthesized by the liver, and it regulates copper homeostasis by providing its delivery. Cp is also an acute phase reactant (APR), whose concentration rises in inflammation, infection, and trauma mostly as an antioxidant response. In addition, Cp is named as ferroxidase according to its ability for being an iron oxidase [Citation4]. It plays an essential role in iron homeostasis through Cp-ferroxidase activity. Unbound ferrous iron ions (Fe+2) are oxidized into ferric iron ions (Fe+3) and thus associates transferrin and delivered in the blood [Citation1,Citation2,Citation4,Citation5]. The oxidase activity of Cp also has a high affinity for Cu defining its status [Citation5]. Therefore, abnormal levels or insufficient functioning of Cp is found to be associated with iron and copper related pathologies [Citation6,Citation7].
Much evidence gathered on psychiatric disorders shows an inadequately functioning Cp as it checks iron oxidization state. Cp also serves as a bridge between the copper and iron metabolisms. Systemic Cp malfunction is the possible mechanism in which the deteriorated iron-associated redox processes lead to oxidative stress in schizophrenia (SZ), bipolar disorder (BD) and major depression (MD) patients. Therefore Cp disturbance is most likely to be the responsible factor in the malfunction of metal metabolism influencing mental disorders [Citation7]. As the serum Cu levels, Cp weight, and Cp-ferroxidase activity controlling for metabolic risk factors in SZ, BD, and MD, we hypothesized that some of these changes might be moderately estimated by the variation in either absolute or relative amounts of Cp-ferroxidase activity. To our knowledge, this is the first study comparing the Cp-ferroxidase activity between SZ, BD, and MD in the available literature.
Material-methods
Participants
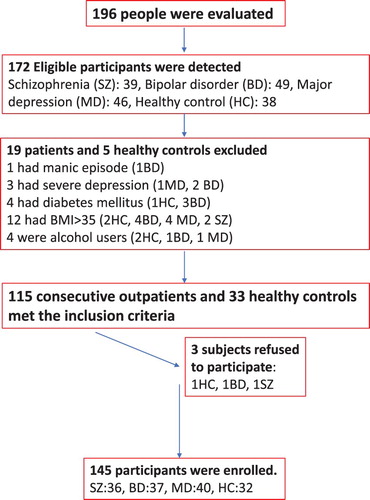
This is a cross-sectional, single-centered study. Patients with SZ (n = 36), BD (n = 37), and MD (n = 40) were enrolled into the study. First-degree relatives of the hospitalized patients to surgery clinics participated in the study as the healthy controls (HCs) (n = 32). The HC group was similar to the patient groups in terms of age, gender, and education. Healthy participants with a personal or family history of psychiatric disorders were excluded (n = 3). All participants were initially evaluated by a clinical psychiatrist to confirm the appropriate diagnosis using the Structured Clinical Interview for DSM-IV (Diagnostic and Statistical Manuel of Mental Disorders) Axis I Disorders (SCID-I) (a semi-structured evaluation scale) [Citation8,Citation9]. The BD group was assessed with the Hamilton Depression Rating Scale (HDRS) and the Young Mania Rating Scale (YMRS) [Citation10–13]. HAMD consists of 17 items. Each item is scored between 0 and 4. The severity of depression is measured by the score range (0–7 no depression, 8–15 mild depression, 16–28 moderate depression, ≥29 severe depression) [Citation14]. YMRS measures the severity of manic episode with 11 items. Each item is scored between 0 and 5. YMRS > 5 is accepted as mania [Citation12,Citation13]. The SZ group was assessed with the Scale for the Assessment of Positive Syndrome (SAPS) and the Scale for the Assessment of Negative Syndrome (SANS). SAPS consists subscales of hallucinations, delusions, bizarre behaviour, positive formal thought disorder, and inappropriate affect [Citation15,Citation16]. SANS consists subscales of affective flattening or blunting, alogia, avolition-apathy, anhedonia-asociality and attention [Citation17,Citation18]. Exclusion criteria were substance and alcohol use disorders, psychiatric comorbidity, cerebrovascular, cardiovascular, respiratory, metabolic (diabetes mellitus, metabolic syndrome, body mass index-BMI >35, renal failure, cirrhosis, infections, allergies, autoimmune diseases, and malignancy), and neurologic disorders. Patients taking mineral or vitamin supplements, medications such as anticonvulsants, contraceptives, xanthine oxidase inhibitors- i.e. allopurinol, folic acid, glucocorticoids were also excluded from the study due to their effects on the trace element metabolism. Subjects with a history of major physical trauma, surgery, minimal acidosis, or slight dehydration and heavy exercise in the last three months and other physiologic conditions were excluded from the study. Abnormal levels of serum thyroid, liver, renal tests, B12, folic acid were excluded from the study. Manic patients (YMRS > 5) were clinically excluded in the BD group. Mild to moderate depression (HDRS < 29) were allowed in the BD and MD groups [Citation14]. No clinical exclusion was performed in the SZ group. The patients’ weight and height were measured. BMI was calculated with kg/m2 formula. The flow-chart diagram in represents the study enrolment protocol.
A written informed consent was also provided by each participant after a full explanation of the study. The study was approved by the local Ethical Committee (27.04.2016, 80576354-050-99/47) and was conducted according to the declaration of Helsinki.
Biochemical analyses
After overnight fasting, the venous blood samples were left thirty minutes for coagulation after withdrawal and then centrifuged at 1500 g for fifteen minutes. All centrifuged serum was stored in Eppendorf tubes and kept frozen at −80°C until the biochemical analyses have been performed. All tubes were made of polypropylene instead of glass materials for preventing metal contamination. Blood collection and separation were both carried out in a dust-free room. Serum copper level was measured by an automated flame atomic absorption spectrophotometer (hermoScientific, USA). In this assay coefficient of variation was 4%. Serum ceruloplasmin ferroxidase activity was measured [Citation19] by an automated clinical chemistry analyzer (Cobas c 501, Roche, Switzerland). In this assay coefficient of variation was 1.6%. Ceruloplasmin mass amount was measured by a commercial immunometric method (Cobas, Roche, Switzerland). In this assay coefficient of variation was 3.6%. Serum non-ceruloplasmin bound copper level was determined by calculation [Citation20].
Statistical analysis
Statistical Package for the Social Sciences (SPSS, version 18.0; Chicago, IL) statistics programme was used for evaluating the data. Whether the data distribution was normal or not was evaluated through the Kolmogorov–Smirnov test. Student’s t-test was performed on normally distributed variables and was reported as the mean ± standard deviation (SD). Mann–Whitney U-test was performed on non-normally distributed data and reflected as median (quartiles). Kruskal–Wallis Test was performed for group comparisons, chi-square and p values are reported. Pearson or Spearman Rank Correlation Analysis was performed between clinical and biochemical parameters in groups and p ≤ .05 was accepted as statistically significant between groups. All test results were two-tailed. Lineer regression analyses were performed to examine the determinants of ferroxidase activity in the groups.
Results
Sociodemographic and clinical variables of the groups are presented in . There was no significant difference between the groups regarding age, gender, socioeconomic status, and education. The groups had similar socioeconomic status characteristics. There were differences between the groups in age at onset, duration of the disease, number of hospitalizations (p < 0.05, ).
Table 1. Sociodemographic and clinical variables of the groups with nicotine comsumption and psychotropic drug usage.
Comparison of the biochemical parameters is presented in . There were no significant differences between the groups in lipid levels and white blood cell counts (p > 0.05). BMI (F = 4.48, p = 0.005), and waist circumference (F = 7.45, p < 0.005) differed between the groups. Post-hoc comparisons showed that BMI of the BD group was significantly higher than the healthy control group (p = 0.006) and than the MD group (p = 0.026). Post-hoc comparisons showed that waist circumference of the BD group was significantly higher than the healthy control group (p < 0.001) and the MD group (p = 0.013). Post-hoc comparisons showed that waist circumference of the SZ group was significantly higher than the healthy control group (p = 0.048). Neutrophil (F = 3.05, p = 0.031) counts differed between the groups. Post-hoc comparisons showed that neutrophil counts of the SZ group were significantly higher than the BD group (p = 0.034).
Table 2. Comparison of the metabolic and biochemical parameters between groups.
In comparison with the control group, there was a statistically significant difference between groups in serum ceruloplasmin ferroxidase activity (χ2 = 9.11, p = 0.028) (). Post-hoc comparisons showed that serum ceruloplasmin ferroxidase activity of the BD group was significantly higher than the healthy control group (p = 0.012) and than the MD group (p = 0.027) and than the SZ group (p = 0.019). BMI, waist circumference and neutrophil counts were the covariates in the ANCOVA analysis. Waist circumference was the only significant covariate (F = 1.10, p = 0.350). Comparison of Cp-ferroxidase activity between nicotine consumers and non-consumers did not show any significant difference between the groups (results are not reported).
Table 3. Comparison of copper and ceruloplasmin parameters between the groups.
Correlations between Cp-ferroxidase activity and biochemical variables are displayed in . Correlation analysis showed that there was statistically significant correlation between number of consumed cigarettes per day and serum copper levels in the SZ group (r = 0.65, p = 0.012). Correlation analyses showed that Cp-ferroxidase activity was positively correlated with BMI (0.58), waist circumference (0.49), ESR (0.61), and (CRP 0.66) in the BD group. In the MD group, Cp-ferroxidase activity was positively correlated with ESR (0.43). In the SZ group, Cp-ferroxidase activity was positively correlated with CRP (0.64). No other significant correlation was detected (). Correlations between Cp-ferroxidase activity and biochemical variables are displayed in .
Table 4. Correlations between ceruloplasmin ferroxidase activity and other biochemical parameters.
Predictors of Cp-ferroxidase activity were analyzed with multiple linear regression analyses in the groups (). Dependent variable was ceruloplasmin ferroxidase activity in all regression models. Independent variables were BMI, waist circumference, LDL, HDL, cholesterol, triglyceride, ESR, CRP, WBC, lymphocyte, neutrophil, neutrophil/lymphocyte ratio. Waist circumference (p = 0.005) and ESR (p = 0.028) in the BD group, CRP in the MD group (p = 0.008), CRP (p < 0.001) and cholesterol (p = 0.043) in the SZ group and CRP (p = 0.002) and HDL (p = 0.021) in the HC group were the determinants of Cp-ferroxidase activity.
Table 5. Determinants of ceruloplasmin ferroxidase activity in groups.
Discussion
In this study, Cu levels, non-Cp bound Cu proportion, weight of Cp and Cp-ferroxidase activity were examined in patients with SZ, BD, and MD in comparison to a HC group. Cp-ferroxidase activity was significantly increased in BD compared to the MD, SZ, and HC groups. No other difference was observed between the groups. According to the regression analysis, Cp-ferroxidase activity was found to be correlated with CRP and ESR levels as well as obesity in all groups. The ESR levels and waist circumferences were higher in the BD group in comparison to the SZ, MD, and HC groups. Therefore, waist circumference and the ESR levels were found to be the determinants of Cp-ferroxidase activity in the BD group. Serum cholesterol and CRP levels were associated with higher Cp-ferroxidase activity in the SZ group. The HDL levels were inversely associated with Cp-ferroxidase activity in the HC group. These results indicate that the inflammation, obesity and metabolic syndrome are associated with a higher Cp-ferroxidase activity.
Many studies have shown that dysregulation of Cu adversely influences biological processes of several psychiatric disorders including SZ [Citation21–30], BD [Citation25,Citation31–33], and MD [Citation25,Citation28,Citation34]. The reduced [Citation28,Citation30,Citation35,Citation36], elevated [Citation21–23,Citation26–29,Citation37,Citation38], and unaltered [Citation25,Citation28,Citation39,Citation40] Cu levels in SZ patients have been mentioned in different studies. Higher Cu concentrations were reported in the course of the BD [Citation41]. Cu concentrations were elevated in the scalp hair of male and female BD patients [Citation42]. Interestingly, Cu levels and Cu/Zn ratios were remarkably higher in women with a past psychiatric history of post-partum depression [Citation28]. Total Cu intake may be oppositely related to depression [Citation43]. No differences in Cu concentrations were demonstrated between the BD and MD [Citation44]. The Cu levels were found similar in depressive patients (both with a current depressive episode and in remission) and HC group [Citation45]. Elevated levels of blood Cu were reported in depressive disorder with a probable role of Cu as a biomarker of depression by a recent review [Citation46]. Serum Cu levels did not differ between the groups in this study. These conflicting findings might result from methodological differences, ethnic factors, heterogeneity of the patients’ biochemical states, and relatively small sample size of the patients.
Cp is also named as iron-oxidase as it has a ferroxidase activity. Cp-ferroxidase activity thus plays an important role as an antioxidant [Citation2,Citation4,Citation47]. The increased Cp-ferroxidase activity demonstrates antioxidant protection, which may influence the pathogenesis of BD and SZ. Previous studies have shown that increased Cp may also be related to clinical properties. These properties may include, the stage of the disease [Citation21,Citation23], the duration of the disease [Citation23,Citation47], the effects of accompanying drug treatment [Citation22,Citation23], the gender [Citation47] and other physical conditions [Citation23]. In this study, Cu and Cp levels were not significantly associated with the smoking status, stage or duration of disease as well as the effects of accompanying treatment. An association between Cp levels and SZ has been reported by several studies [Citation47]. Although higher levels of Cp were reported by the majority of studies, lower levels of Cp were also found in some studies. In contrary, the normal levels of Cp were shown by other studies in SZ patients than HC group [Citation23,Citation24,Citation28,Citation47]. Higher Cp levels were reported in first episode depression both previously and later antidepressant drug use [Citation48]. Cp was indicated as a serum biomarker for drug-free MD patients [Citation49]. Unchanged levels of Cp were reported in BD and MD [Citation25]. Lower Cp levels were shown in MD [Citation50]. Higher levels of Cp as an APR were reported in MD and BD patients [Citation51]. We also found normal levels of Cp in SZ, BD, and MD groups. These differences may be due to small sample size, application of different methods, clinical features, and ethnobiological variations of the patients. Effects of other medical conditions on Cp levels have been shown in such as liver function deteriorations, pregnancy, oral contraceptive use, lymphoma, infections, angina and other disease-states [Citation5,Citation23]. A similar physical disease or conditions have not been present in any of our patients. The nature of association between higher levels of Cp and SZ has not been explained so far. The immunology-inflammatory hypothesis of SZ has been recommended as an explanation for the relationship of Cp and SZ by some authors. These authors claimed that the higher Cp levels serve as an APR indicating the Cp as a potent antioxidant. Moreover, Cp was found more potent antioxidant than albumin and even superoxide dismutase (SOD) [Citation47].
CRP is an APR that rapidly reacts to inflammatory processes as well as the major protein carrier for serum Cu [Citation51–53]. Most psychiatric disorders, especially SZ and BD are related to an inflammatory reaction by the activation of the glia in the neural tissue [Citation54,Citation55]. On the other hand, obesity/overweight is associated with increased inflammation in SZ and BD patients [Citation56,Citation57]. Higher plasma Cu concentrations were also associated with metabolic risk factors such as plasma glucose and diastolic blood pressure in SZ patients [Citation26]. In this study, Cp-ferroxidase activity was increased in all groups in comparison to the HC group. The significantly higher Cp-ferroxidase activity was shown in BD in comparison to the MD and SZ group. The Cp-ferroxidase activity was correlated with ESR in the BD and with CRP in the SZ group. These results were significant because they demonstrated that Cp was also up-regulated by inflammation with the other APRs such as ESR and CRP. The SZ and BD patients have a potent inflammatory response with an extreme elevation of APRs such as CRP and ESR respectively.
One of the most important limitations of this study was that groups were not similar in terms of waist circumference and waist circumference was the determinant of Cp-ferroxidase activity. Psychotropic medications and nicotine consumption could not be controlled thoroughly and these factors may have altered metabolic states in the groups. Cross-sectional design, sample size of the study, and psychotropic medications were the clinical limitations. Sample size is relatively small to generalize these findings. Examining only the serum levels of Cu, Cp and ferroxidase activity forms a significant limitation to this study. Cu, Cp and Cp-ferroxidase activity in peripheral blood serum should also be tested whether such alterations express relevant changes in the brain tissue. Serum iron (Fe) levels were not tested. All patients were under drug treatment and in remission at the time of the study. Therefore we could not compare our results with the results of some previous studies in which the patients were on drug-free or acute exacerbation of these disorders. This study also has some critical advantages. We highlighted the role of Cp-ferroxidase in BD and SZ through the relationship of inflammation. Additionally, the exclusion of comorbidities, mental retardation, in concurrence with the combined analysis of ESR and CRP to copper metabolism (Cu, Cp, and Cp-ferroxidase activity) are methodologically robust factors of our study. These strict exclusion criteria helped us to enrol a disease-free sample. The match on age, sex, and residential areas of the patients and HCs was the other methodologically robust factor of this study.
Future studies investigating this subject should be performed with larger and homogeneous patient groups [Citation58]. On the other hand, normal concentrations of specific molecules do not reject molecular dysfunction, and thus further studies on functions of the proteins and enzymes may help to clarify specific biological mechanisms or abnormalities of the specific cascades. Cp-Ferroxidase activity is increased in BD group. Cp-Ferroxidase activity is also associated with waist circumference and the ESR levels in the BD group as well as the cholesterol levels and CRP in the SZ group. These results are indicating that metabolic syndrome may cause inflammatory activation that may enhance the activity of Cp.
As a conclusion, these results also implicates that the Cp-ferroxidase activity has a capacity to bind trace elements and to act as an APR during the inflammation processes. This study may establish a reliable clinical tool for demonstrating the strong association of inflammation in these disorders. Cp-ferroxidase activity thus may be an encouraging candidate in the evaluation of neuro-immune interaction while studying the complex pathophysiology of SZ and BD.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Serhat Tunç http://orcid.org/0000-0002-2057-4074
Murat İlhan Atagün http://orcid.org/0000-0002-8514-0576
Hamit Serdar Başbuğ http://orcid.org/0000-0002-1363-6783
Özcan Erel http://orcid.org/0000-0002-2996-3236
References
- Carmona F, Palacios Ò, Gálvez N, et al. Ferritin iron uptake and release in the presence of metals and metalloproteins: chemical implications in the brain. Coord Chem Rev. 2013;257(19–20):2752–2764.
- Manto M. Abnormal copper homeostasis: mechanisms and roles in neurodegeneration. Toxics. 2014;2(2):327–345.
- Modabbernia A, Velthorst E, Gennings C, et al. Early-life metal exposure and schizophrenia: a proof-of-concept study using novel tooth-matrix biomarkers. Eur Psychiatry. 2016;36:1–6.
- Vassiliev V, Harris ZL, Zatta P. Ceruloplasmin in neurodegenerative diseases. Brain Res Brain Res Rev. 2005;49(3):633–640.
- Gaware V, Kotade K, Dhamak K, et al. Ceruloplasmin its role and significance: a review. Int J Biol Res. 2010;1:153–162.
- Heidari M, Johnstone DM, Bassett B, et al. Brain iron accumulation affects myelin-related molecular systems implicated in a rare neurogenetic disease family with neuropsychiatric features. Mol Psychiatry. 2016;21(11):1599–1607.
- Dean B, Tsatsanis A, Lam LQ, et al. Changes in cortical protein markers of iron transport with gender, major depressive disorder and suicide. World J Biol Psychiatry. 2018. doi:10.1080/15622975.
- First MB, Spitzer RL, Gibbon M, et al. American Psychiatric Press, Washington DC, 1997.
- Özkürkçügil A, Aydemir Ö, Yıldız M, et al. DSM-IV Eksen I bozuklukları için yapılandırılmış klinik görüşmenin Türkçe’ye uyarlanması ve güvenilirlik çalışması. İlaç ve Tedavi Derg. 1999;12(4):233–236.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62.
- Akdemir A, Önsel S, Dağ İ, et al. Hamilton depresyon derecelendirme ölçeği (HDDÖ)’nin geçerliği, güvenirliği ve klinik kullanımı. Psikiyatri Psikol Psikofarmakol Derg. 1996;4:251–259.
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435.
- Karadağ F, Oral ET, Aran Yalçın F, et al. Young mani derecelendirme ölçeğinin Türkiye’de geçerlik ve güvenilirliği. Türk Psikiyatri Derg. 2001;13:107–114.
- Williams JB. A structured interview guide for the Hamilton depression rating scale. Arch Gen Psychiatry. 1988;45(8):742–747.
- Andreasen NC. The scale for the assesment of positive symtoms (SAPS). Iowacity (IA): University of Iowa Press; 1984.
- Erkoç Ş, Arkonaç O, Ataklı C, et al. Pozitif semptomları değerlendirme ölçeğinin güvenirliği ve geçerliliği. Düşünen Adam. 1991;4:20–24.
- Andreasen NC. The scale for the assesment of negative symptoms (SANS). Iowacity (IA): University of Iowa Press; 1983.
- Erkoç Ş, Arkonaç O, Ataklı C, et al. Negatif semptomları değerlendirme ölçeğinin güvenirliği ve geçerliliği. Düşünen Adam. 1991;4:16–19.
- Erel O. Automated measurement of serum ferroxidase activity. Clin Chem. 1998;44(11):2313–2319.
- Twomey PJ, Viljoen A, Reynolds TM, et al. Non-ceruloplasmin-bound copper in routine clinical practice in different laboratories. J Trace Elem Med Biol. 2008;22(1):50–53.
- Sharma S, Sood S, Sharma A, et al. Estimation of serum zinc and copper levels patients with schizophrenia: a preliminary study. SL J Psychiatry. 2014;5(1):14–17.
- Nechifor M, Vaideanu C, Palamaru I, et al. The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia. J Am Coll Nutr. 2004;23(5):549S–551S.
- Wolf TL, Kotun J, Meador Woodruff JH. Plasma copper, iron, ceruloplasmin and ferroxidase activity in schizophrenia. Schizophr Res. 2006;86(1–3):167–171.
- Yanik M, Kocyigit A, Tutkun H, et al. Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol Trace Elem Res. 2004;98(2):109–117.
- Demily C, Parant F, Cheillan D, et al. Screening of Wilson’s disease in a psychiatric population: difficulties and pitfalls. A preliminary study. Ann Gen Psychiatry. 2017;16:19.
- Vidović B, Dorđević B, Milovanović S, et al. Selenium, zinc, and copper plasma levels in patients with schizophrenia: relationship with metabolic risk factors. Biol Trace Elem Res. 2013;156(1–3):22–28.
- Rahman A, Azad MA, Hossain I, et al. Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol Trace Elem Res. 2009;127(2):102–108.
- Ghanem AEA, Ali EM, El-Bakary AA, et al. Copper and Zinc levels in hair of both schizophrenic and depressed patients. Journal of Forensic Medicine and Clinical Toxicology. 2009;17:89–102.
- Zimbrean PC, Schilsky ML. Psychiatric aspects of Wilson disease: a review. Gen Hosp Psychiatry. 2014;36(1):53–62.
- Liu T, Lu QB, Yan L, et al. Comparative study on serum levels of 10 trace elements in schizophrenia. PLoS One. 2015;10(7):e0133622.
- Virit O, Selek S, Bulut M, et al. High ceruloplasmin levels are associated with obsessive compulsive disorder: a case control study. Behav Brain Funct. 2008;4(1):52.
- González-Estecha M, Trasobares EM, Tajima K, et al. Trace elements in bipolar disorder. J Trace Elem Med Biol. 2011;25(Suppl 1):S78–S83.
- Carta M, Mura G, Sorbello O, et al. Quality of life and psychiatric symptoms in Wilson’s disease: the relevance of bipolar disorders. Clin Pract Epidemiol Ment Health. 2012;8:102–109.
- Stelzhammer V, Haenisch F, Chan MK, et al. Proteomic changes in serum of first onset, antidepressant drug-naïve major depression patients. Int J Neuropsychopharmacol. 2014;17(10):1599–1608.
- Chen X, Li Y, Zhang T, et al. Association of serum trace elements with schizophrenia and effects of antipsychotic treatment. Biol Trace Elem Res. 2018;181(1):22–30.
- Schoonover KE, Queern SL, Lapi SE, et al. Impaired copper transport in schizophrenia results in a copper-deficient brain state: a new side to the dysbindin story. World J Biol Psychiatry. 2018. doi:10.1080/15622975.2018.1523562.
- Devanarayanan S, Nandeesha H, Kattimani S, et al. Elevated copper, hs C-reactive protein and dyslipidemia in drug free schizophrenia: Relation with psychopathology score. Asian J Psychiatr. 2016;24:99–102.
- Li Z, Liu Y, Li X, et al. Association of elements with schizophrenia and intervention of selenium supplements. Biol Trace Elem Res. 2018;183(1):16–21.
- Cao B, Yan L, Ma J, et al. Comparison of serum essential trace metals between patients with schizophrenia and healthy controls. J Trace Elem Med Biol. 2019;51:79–85.
- Firth J, Carney R, Stubbs B, et al. Nutritional deficiencies and clinical correlates in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. 2018;44(6):1275–1292.
- Siwek M, Styczeń K, Sowa-Kućma M, et al. The serum concentration of copper in bipolar disorder. Psychiatr Pol. 2017;51(3):469–481.
- Pradeep AS, Naga Raju GJ, Sattar SA, et al. Trace elemental distribution in the scalp hair of bipolars using PIXE technique. Med Hypotheses. 2014;82(4):470–477.
- Li Z, Wang W, Xin X, et al. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J Affect Disord. 2018;228:68–74.
- Styczeń K, Sowa-Kućma M, Dudek D, et al. Zinc and copper concentration do not differentiate the bipolar disorder from major depressive disorder. Psychiatr Pol. 2018;52(3):449–457.
- Styczeń K, Sowa-Kućma M, Siwek M, et al. Study of the serum copper levels in patients with major depressive disorder. Biol Trace Elem Res. 2016;174(2):287–293.
- Ni M, You Y, Chen J, et al. Copper in depressive disorder: a systematic review and meta-analysis of observational studies. Psychiatry Res. 2018;267:506–515.
- Virit O, Altindag A, Selek S, et al. Increased plasma ceruloplasmin levels in schizophrenia. Klinik Psikofarmakoloji Bulteni. 2008;18(4):282–287.
- Kaya MC, Bez Y, Selek S, et al. No effect of antidepressant treatment on elevated serum ceruloplasmin level in patients with first-episode depression: a longitidunal study. Arch Med Res. 2012;43(4):294–297.
- Lee MY, Kim EY, Kim SH, et al. Discovery of serum protein biomarkers in drug-free patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;69:60–68.
- Bajpai A, Verma AK, Srivastava M, et al. Oxidative stress and major depression. J Clin Diagn Res. 2014;8(12):4–7.
- Maes M, Carvalho AF. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol. 2018;55(12):8885–8903.
- Arnal N, Cristalli DO, de Alaniz MJ, et al. Clinical utility of copper, ceruloplasmin, and metallothionein plasma determinations in human neurodegenerative patients and their first-degree relatives. Brain Res. 2010;1319:118–130.
- Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–744.
- Baumann PS, Griffa A, Fournier M, et al. Impaired fornix-hippocampus integrity is linked to peripheral glutathione peroxidase in early psychosis. Transl Psychiatry. 2016;6(7):e859. doi:10.1038/tp.2016.117.
- Tunc S, Atagun MI, Neselioglu S, et al. Ischemia-modified albumin: a unique marker of global metabolic risk in schizophrenia and mood disorders. Psychiatry and Clinical Psychopharmacology. 2018. doi:10.1080/24750573.2018.1517466.
- Boozalis T, Devaraj S, Okusaga OO. Correlations between body mass index, plasma high-sensitivity C-reactive protein and lipids in patients with schizophrenia. Psychiatr Q. 2018 Oct 12. doi:10.1007/s11126-018-9606-3.
- Bond DJ, Andreazza AC, Hughes J, et al. Association of peripheral inflammation with body mass index and depressive relapse in bipolar disorder. Psychoneuroendocrinology. 2016;65:76–83.
- Kul M, Kara M, Unal F, et al. Serum copper and ceruloplasmin levels in children and adolescents with attention deficit hyperactivity disorder. Klinik Psikofarmakoloji Bülteni-Bulletin of Clinical Psychopharmacology. 2014;24(2):139–145.