ABSTRACT
INTRODUCTION: while around half of the patients with obsessive-compulsive disorder do not respond efficiently to current serotonin-reuptake inhibitors, the objective of the present study was to compare the effectiveness and safety of quetiapine versus aripiprazole in patients with obsessive-compulsive disorder, who had not responded effectively to fluvoxamine.
METHOD: Forty-four patients with obsessive-compulsive disorder, who had not responded effectively to fluvoxamine at maximum dose (300 milligram per day) and duration (twelve weeks), were allocated randomly, in a double-blind trial, to receive quetiapine (n = 22) or aripiprazole (n = 22), in addition to their serotonin-reuptake inhibitor, for twelve weeks. While treatment response was evaluated by the Yale-Brown Obsessive-Compulsive Scale (YBOCS), as primary outcome scale, Clinical Global Impressions-Severity Scale (CGI-S), as well, had been used as an ancillary measure.
RESULTS: 54.54% of patients in the quetiapione group (n = 12) and 27.27% of cases in the aripiprazole group (n = 6) responded partially to the abovementioned augmentation. According to the findings, the mean +/− SD baseline YBOCS’ score, dropped from 31.18+/−4.93 to 27.97+/−3.71 (p < 0.01), and 33.27 +/− 3.90 to 30.72+/−4.67 (p < 0.06), by quetiapine and aripiprazole, respectively. In this regard, no significant alteration with respect to CGI-S was evident in either of the aforementioned groups.
CONCLUSION: This study indicated that patients with treatment-resistant obsessive-compulsive disorder could benefit more from adding quetiapine, in comparison with aripiprazole, to their ongoing serotonergic medication.
Introduction
Obsessive-compulsive disorder (OCD) is characterized by the presence of “obsessions” and/or “compulsions.” “Obsessions” are recurring and insistent thoughts, impulses, or imageries that are experienced as intrusive and undesirable, whereas “compulsions” are repetitive mental acts or behaviours that an individual feels compelled to perform in response to an obsession or according to rules that should be applied rigidly [Citation1,Citation2]. While dysfunction in the orbitofrontal cortex, anterior cingulate cortex, and striatum, has been strongly implicated in OCD [Citation3], neuroimaging has shown functional abnormalities in the frontal cortex and basal ganglia, too [Citation4]. According to biological models, OCD results from pathology in the caudate nucleus, which fails to suppress signals from the orbitofrontal cortex [Citation5,Citation6]. The effectiveness of pharmacotherapy in OCD has been verified in many clinical trials and is heightened by the remark that the studies had found a placebo response rate of only about 5 percent [Citation7]. The typical approach is to start management with an SSRI or clomipramine, and then move to other pharmacological approaches if the serotonin-specific drugs are not successful [Citation7]. Each of the specific serotonin-reuptake inhibitors (SSRIs) available in the United States – fluoxetine (Prozac), paroxetine (Paxil), fluvoxamine (Luvox), citalopram (Celexa), sertraline (Zoloft) – has been approved by the US Food and Drug Administration (FDA) for the treatment of OCD [Citation8]. On the other hand, as many as half of patients with OCD, who have been treated with an adequate trial of serotonin-reuptake inhibitors (SRIs), fail to react meaningfully to treatment and continue to show remarkable symptoms [Citation9]. So, in 2006, the National Institute of Clinical and Health Excellence (NICE) guidelines for Obsessive Compulsive Disorder recommended anti-psychotics as an adjuvant for SSRI treatment-resistant OCD [Citation10]. There was also evidence suggesting that OCD patients should be treated with at least 3 months of maximal-tolerated therapy of an SRI before initiating antipsychotic augmentation due to the high rate of treatment response to continued SRI monotherapy [Citation11]. But unfortunately, only one-third of treatment-refractory OCD patients show a meaningful response to antipsychotic augmentation [Citation11]. In this regard, while there is adequate proof in the available literature, which demonstrates the effectiveness of haloperidol and risperidone, evidence regarding the efficiency of quetiapine and olanzapine is insufficient [Citation12,Citation13]. Though there is a quantity of clinical trials, with diverse conclusions, as regards the effectiveness of quetiapine [Citation14–17] and aripiprazole [Citation18–23], in the present study, the efficacy and safety of aripiprazole have been compared with quetiapine in a group of the non-western patient population with treatment-resistant OCD.
Method
Forty-four female in-patients, as available sample in the chronic ward of the hospital, after full description of the procedure and attaining signed informed consent, were entered, into a twelve-week parallel-group, double-blind study, for random allocation to adjunctive aripiprazole (n = 22 patients) or quetiapine (n = 22 patients), in addition to their regular medication. “CONSORT 2010 Statement” has been taken into consideration as guideline for organizing the present parallel-group randomized trial [Citation24]. While the study had been approved by College’s Medical Ethics Comity, patients had been diagnosed as Obsessive-Compulsive Disorder, according to specified criteria in Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision [Citation25]. Moreover, while comorbidity has been defined as the presence of one or more additional diseases or disorders co-occurring with (that is, concomitant or concurrent with) obsessive-compulsive disorder, patients with known co-morbid complications, based on documented past psychiatric and medical history, in Axis I or III, respectively, had been excluded from the assessment. Inclusion criteria, as well, involved: (1) Obsessive-compulsive symptoms resistant to current SSRI (Fluvoxamine), at maximum dosage (300 milligrams per day) and adequate duration (twelve weeks). (2) Score of at least 18, on the Yale-Brown Obsessive Compulsive Scale (YBOCS). Once more, throughout the evaluation, while the assessor, staff and patients were unaware of the recommended augmentations, which were packed into undistinguishable capsules, all cases continued to take fluvoxamine, at the maximum dosage, in the course of assessment. Quetiapine (immediate release formulation) was started by 25 milligram two times per day, and then increased by 75 mg in weekly meetings, to a maximum of 300 milligram by week 4, and that dose was held constant up to the end of the trial. Aripiprazole, as well, was initiated by 2.5 milligram per day, and then increased by 2.5 mg increments in weekly visits, to a maximum of 10 mg by week 4, and then this dosage was held constant up to the end of the evaluation. Meanwhile, in the course of trial, no other psychosocial intervention or psychotropic drug could be administrated. The primary outcome measure in the current evaluation was YBOCS [Citation26]. Clinical Global Impressions-Severity Scale (CGI-S), as well, had been used as the supplementary scale [Citation27]. While the full response to treatment was demarcated of at least 50% decrease in YBOCS’ score [Citation28], partial response was defined as equal to or >25% - <50% decrease in YBOCS’ score in comparison with the starting point [Citation29].
Duration of the assessment was twelve weeks, and the patients had been evaluated by YBOCS at baseline (week 0), and weeks two, four, eight, and twelve. On the other hand, CGI-S had been scored only at baseline and end of the assessment. Adverse effects had been measured and listed at each visit by means of the same associate psychiatrist, based on patients’ reports and medical checkup, and in comparison with the starting point. Treatment-emergent adverse events, as well, have been defined as undesirable events not present prior to add-on treatment, or an already present event that worsens either in intensity or frequency following the aforesaid augmentative treatments.
Statistical analysis
While patients had been compared on baseline characteristics by means of “t tests,” treatment efficacy was analysed by “t tests” and “repeated –measures analysis of variance (ANOVA),” comparing both groups over twelve weeks. Statistical significance, also, was defined as a “two-sided p value < or = to 0.05.” “MedCalc,” version 9.4.1.0, had been used as statistical software instrument for analysis of data [Citation30].
Results
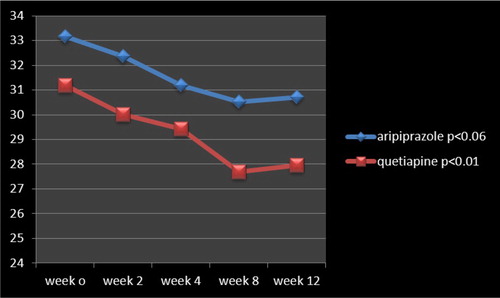
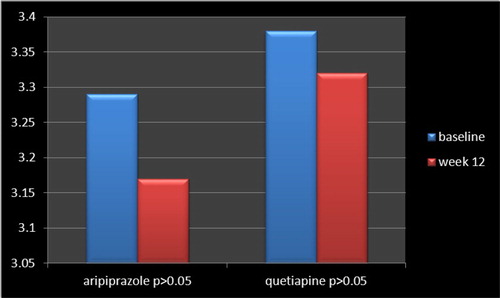
Analysis for efficacy was based on information from an equivalent number of patients in both groups, which were comparable in the beginning, with analogous demographic and diagnostic variables (). While, in keeping with some scholars, for small sample sizes, normality tests have little power to reject the null hypothesis and therefore small samples most often pass normality tests, calculations based on “Skewness and Kurtosis” method came across the assumption of normality with respect to present assessment. In accordance with the findings, no full response was evident in any of samples in either group. Six patients in the aripiprazole group and twelve patients in the quetiapine group showed partial response, with a mean total decrease of YBOCS from “33.17 +/− 3.90” to “30.72+/−4.67” and “31.18+/− 4.93” to “27.97+/−3.71” by aripiprazole and quetiapine, respectively ( and ). Besides, within-group analysis indicated that while the enhancement was significant in the quetiapine group (t = 2.44, p < 0.01, CI: 0.56, 5.86) at the end of the assessment, it was not so with respect to aripiprazole (t = 1.889, p < 0.06, CI: −0.17, 5.07) (). In addition, “repeated-measures analysis of variance (ANOVA)” regarding YBOCS, did not show any significant enhancement by aripiprazole [F(4,84) = 0.636 p < 0.63 SS = 1058.00 MSe = 416.07], though a significant improvement was palpable by means of quetiapine [F(4,84) = 10.8 p < 0.04 SS = 286.64 MSe = 8.45 ] at the end of the assessment, and, similarly, in comparison with aripiprazole [F(1,105) = 3.71 p < 0.05 SS = 4.09 MSe = 1.10 ], in a “Split-plot (Mixed) design ANOVA.” Moreover, though head to head analysis could not demonstrate any significant dissimilarity between quetiapine and aripiprazole in weeks 2, 4 and 8, it was significant in week 12 ( and ). Similarly, regarding CGI-S, no significant alteration was apparent by aripiprazole (3.29+/−0.33 to 3.17+/−1.02; t = 0.52, p < 0. 60) or quetiapine (3.38+/−0.08–3.32+/−0.14, t = 1.74, p < 0.08), although maybe a better response was noticeable with the later one (). Since the sample size was small, the Effect size (ES) was analysed for changes on the YBOCS and CGI-S at the end of the evaluation. The results indicated a medium enhancement of YBOCS by both of aripiprazole (d = 0.5, r = 0.2) and quetiapine (d = 0.7, r = 0.3). Also, there were medium and small improvements of CGI-S by quetiapine (d = 0.5, r = 0.2) and aripiprazole (d = 0.15, r = 0.07), respectively.
Table 1. Demographic and clinical characteristics of participants.
Table 2. Percentage of positive response on the YBOCS, in the aripiprazole and quetiapine groups.
Table 3. Between-group analysis of primary outcome measure at baseline and weeks 2, 4, 8 and 12.
Post-hoc analysis exhibited an intermediary power equal to 0.49 on behalf of the current assessment, which turned to a power equal to 0.79 in compromise power analysis. The most common side effects of quetiapine and aripiprazole among the present samples, during assessment, included somnolence (45.55% and 27.77%, respectively), dizziness (54.54% and 18.18, respectively), inner unrest (18.18% in the aripiprazole group) and finally, weight gain (27.27% in the quetiapine group, with a mean increase of 0.36+/−0.11 kg). Since the side effects were insignificant and bearable, thus no one dropped out due to drug intolerance.
Discussion
In keeping with the end-results of the present study, while the addition of quetiapine and aripiprazole, as add-on medications, to current SSRI treatment, could be useful for treatment-resistant OCD patients, it was mostly significant in the first group. Generally, the course of OCD, particularly if untreated, is usually chronic with waxing and waning symptoms. While some patients have an episodic sequence, and a minority has a deteriorating course, without treatment, remission rates in adults are low [Citation31]. Thus, OCD is related with reduced quality of life as well as high levels of occupational and social impairment [Citation32]. Though SSRIs are recommended as the first-line pharmacologic treatment for OCD, SSRIs’ response is thought to be delayed in OCD, even more so than in major depression [Citation33]. On the other hand, while data guiding treatment for adults with refractory OCD are often suboptimal or incomplete, many frequently used interventions for treatment-refractory OCD do not have proven efficacy in double-blind, placebo-controlled trials (e.g mavoglurant augmentation, N-acetylcysteine augmentation and riluzole augmentation) [Citation34–37]. Moreover, many interventions for treatment-refractory OCD have only shown efficacy in placebo-controlled trials in single-site, small pilot trials [Citation38]. While meta-analysis has confirmed a significant benefit of antipsychotic augmentation, compared to continuing SSRI monotherapy, in randomized, placebo-controlled trials in adults with OCD [Citation39], within the nine trials included in the meta-analysis with respect to risperidone, haloperidol, quetiapine, and olanzapine, there was no evidence that one particular antipsychotic was more effective than the other ones. Likewise, though randomized, placebo-controlled trials of aripiprazole, suggest that this antipsychotic agent may be useful as a treatment strategy in refractory OCD [Citation40], small, underpowered clinical trials involving head-to-head comparisons between antipsychotic agents have similarly been unable to detect significant differences in efficacy between treatments [Citation28,Citation41]. Back to our study, perhaps, a longer period of assessment or higher doses could result in momentous outcomes in the aripipazole group, and better results by quetiapine. By the way, though the baseline characteristics were in general and statistically analogous, the severity of baseline measurements in the aripiprazole group was greater than the quetiapine group. Hence, perhaps this could have influenced the end-result of the present assessment. The same question also is applicable to dosages of these medications, which could be roughly considered as equivalent. Anyway, in a rather similar approach, Selvi Y et al. as well, in a single-blind, randomized study, had found risperidone more operative than aripiprazole for enhancement of YBOCS [Citation41]. On the whole, and with regard to quetiapine, in opposite to Kordon A et al. [Citation14] and Carey PD et al. [Citation15], the outcome of the current study was comparable with the results of Denys D et al. [Citation16] and Fineberg NA et al. [Citation17]. Then again, with respect to aripiprazole, the present outcome was not in harmony with the findings of Pessina E et al. [Citation18], Muscatello MR et al. [Citation19], Delle Chiaie R et al. [Citation20], Ak M et al. [Citation21], Akyol Ardic et al. [Citation22] and Hegde et al.[Citation23]. It deserves to be mentioned that the primary outcome measure of all of the abovementioned studies was YBOCS, except than two of them [Citation20,Citation21], who had used YBOCS plus CGI-S. But, this variety could be the result of differences between a number of intervening factors, like the patient’s gender, kind of medication, length of study, and essentially, the norms of response. The main problem is that some contradiction exists in the definition of treatment refractoriness, and there is not yet any general agreement on the meaning of responsiveness or refractoriness with respect to OCD. For instance, while Weiss et al. [Citation28] had used a cutoff of 50% reduction in YBOCS as responders, Francobanderia [Citation29] had selected a cutoff of 25% for an analogous survey. Then again, McDougle et al. [Citation42] had used a more limiting norm, with a cutoff of 35% for YBOCS reduction, and a final score of sixteen on the YBOCS, together with a final Clinical Global Impressions scale rating of “much improved” or “very much improved.” Thus, such dissimilarities undermine the comparison of effect sizes among evaluations. With respect to quetiapine, also, Komossa et al. had found that though quetiapine plus antidepressants were not any more effective than placebo plus antidepressants in treatment-resistant OCD, but it had revealed a meaningful superiority, in comparison with risperidone and olanzapine, as regards enhancement of YBOCS. Moreover, some valuable effects of quetiapine, regarding anxiety or depressive symptoms were discernible [Citation43]. In addition, as stated by Denys et al., not only quetiapine was better than placebo as regards enhancement of YBOCS in treatment-resistant OCD, but the best outcome was observable by its combination with fluvoxamine, fluoxetine and clomipramine, in company with the lowest SRI dosages [Citation16]. It deserves to be mentioned that as said by some scholars, when faced with a P value that has failed to reach some specific threshold (generally P < 0.05, and in the current study p < 0.06 on behalf of aripiprazole), this may imply a “trend towards statistical significance” or otherwise suggest that the failure to achieve statistical significance was due to insufficient data [Citation44]. But in keeping with Wood et al. [Citation45] such descriptions give a misleading impression and undermine the principle of accurate reporting. According to Wood et al., the clearest context in which to consider the correct interpretation of a P value is within a randomized trial. Fisher described how the “simple precaution of randomization will suffice to guarantee the validity of the test of significance.” Random allocation of participants to groups ensures that only the play of chance or a real effect of treatment can explain any difference seen in outcome between the groups. A P value tells us how far chance alone can explain the observed difference and acts as a “snapshot” measure of the strength of evidence at the end of the trial. According to them, Describing near significant P values as “trends towards significance” (or similar) is not just inappropriate but actively misleading, as such P values would be quite likely to become less significant if extra data were collected. P values in the region of 0.05 represent quite modest degrees of evidence, whichever side of the divide they lie on [Citation45]. Unlike other studies by olanzapine and respiridone, in the present study, weight gain and extra-pyramidal symptoms were not prominent common side effects, and complaints like dizziness and somnolence were generally slight and did not produce severe problems for the cases. Nonetheless, according to some scholars, though atypical antipsychotic agents have been found effective in the augmentation of SRIs for treatment-resistant OCD in short-term studies, there are few data on the efficacy and safety of these agents in clinical settings over the long-term [Citation46]. The short duration of study, gender-based sampling, and small sample size were among the weak points of the present study. Besides, lack of placebo arm, which may have a significant impact on the assay sensitivity of the study and may perhaps artificially inflate results in an active comparator trial, could be considered as another confounding variable. Additional parallel and larger methodical studies in the future may well improve our knowledge regarding the management of treatment-resistant OCD.
Conclusion
This study indicated that patients with treatment-resistant obsessive-compulsive disorder could benefit more from adding quetiapine, in comparison with aripiprazole, to their ongoing serotonergic medication.
Acknowledgments
The authors gratefully acknowledge dear colleagues, Sadeghi P (M.S), Akbari S (M.D), and the department of research for their practical and financial support of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sasson Y, Zohar J, Chopra M, et al. Epidemiology of obsessive compulsive disorder: a world view. J Clin Psychiatry. 1997;58(12):7–10.
- Katona C, Cooper C, Robertson M. Psychiatry at a glance. 5th ed. Oxford: Blackwell; 2012. p. 30–31.
- Piallat B, Polosan M, Fraix V, et al. Subthalamic neuronal firing in obsessive-compulsive disorder and Parkinson disease. Ann Neurol. 2011;69:793–802.
- Via E, Cardoner N, Pujol J, et al. Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Brit J Psychiatry. 2014;204(1):61–68.
- Burton N. Neurotic, stress-related, and somatoform disorders (anxiety disorders), in Psychiatry. 2nd ed. Oxford: Blackwell; 2010. p.125–127.
- Cicek E, Cicek IE, Kayhan F, et al. Quality of life, family burden and associated factors in relatives with obsessive-compulsive disorder. Gen Hosp Psychiatry. 2013;35(3):253–258.
- Brakoulias V, Starcevic V, Albert U, et al. Treatments used for obsessive-compulsive disorder – an international perspective. Hum Psychopharmacol. 2019;34(1):e2686.
- Albert U, Marazziti D, Di Salvo G, et al. A systematic review of evidence-based treatment strategies for obsessive-compulsive disorder resistant to first-line pharmacotherapy. Curr Med Chem. 2018;25(41):5647–5661.
- Zhou DD, Zhou XX, Li Y, et al. Augmentation agents to serotonin reuptake inhibitors for treatment-resistant obsessive-compulsive disorder: a network meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;2(90):277–287.
- Pignon B, Tezenas du Montcel C, Carton L, et al. The place of antipsychotics in the therapy of anxiety disorders and obsessive-compulsive disorders. Curr Psychiatry Rep. 2017;7(12):103.
- Dolda M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015 Jul;18(9):47.
- Zhou DD, Zhou XX, Lv Z, et al. Comparative efficacy and tolerability of antipsychotics as augmentations in adults with treatment-resistant obsessive-compulsive disorder: a network meta-analysis. J Psychiatr Res. 2019;14(111):51–58.
- Brakoulias V, Stockings E. A systematic review of the use of risperidone, paliperidone and aripiprazole as augmenting agents for obsessive-compulsive disorder. Expert Opin Pharmacother. 2018;26:1–7.
- Kordon A, Wahl K, Koch N, et al. Quetiapine addition to serotonin reuptake inhibitors in patients with severe obsessive-compulsive disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2008;28(5):550–554.
- Carey PD, Vythilingum B, Seedat S, et al. Quetiapine augmentation of SRIs in treatment refractory obsessive-compulsive disorder: a double-blind, randomised, placebo-controlled study. BMC Psychiatry. 2005;24(5):5.
- Denys D, Geus F, Megen HJ, et al. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors. J Clin Psychiatry. 2004;65(8):1040–1048.
- Fineberg NA, Stein DJ, Premkumar P, et al. Adjunctive quetiapine for serotonin reuptake inhibitor-resistant obsessive-compulsive disorder: a meta-analysis of randomized controlled treatment trials. Int Clin Psychopharmacol. 2006;21(6):337–343.
- Pessina E, Albert U, Bogetto F, et al. Aripiprazole augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a 12-week open-label preliminary study. Int Clin Psychopharmacol. 2009;24(5):265–269.
- Muscatello MR, Bruno A, Pandolfo G, et al. Effect of aripiprazole augmentation of serotonin reuptake inhibitors or clomipramine in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2011;31(2):174–179.
- Delle Chiaie R, Scarciglia P, Pasquini M, et al. Aripiprazole augmentation in patients with resistant obsessive compulsive disorder: a pilot study. Clin Pract Epidemiol Ment Health. 2011;7:107–111.
- Ak M, Bulut SD, Bozkurt A, et al. Aripiprazole augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a 10-week open-label study. Adv Ther. 2011;28(4):341–348.
- Ardic U A, Ercan ES, Kutlu A, et al. Successful treatment response with aripiprazole augmentation of SSRIs in refractory obsessive-compulsive disorder in childhood. Child Psychiatry Hum Dev. 2017;48(5):699–704.
- Hegde D, Kalyani G, Arumugham SS, et al. Aripiprazole augmentation in highly treatment-resistant obsessive-compulsive disorder – experience from a specialty clinic in India. Int J Psychiatry Clin Pract. 2017;21(1):67–69.
- Schulz KF, Altman DG, Moher D, CONSORT. Statement: updated guidelines for reporting parallel group randomized trials. Lancet. 2010;2010:1–6.
- American psychiatric association. Diagnostic and statistical manual of mental disorders. 4th ed. Text rev. Washington (DC): American Psychiatric Association; 2000. p. 262–270.
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown obsessive compulsive Scale, 1: development, use, and reliability. Arch Gen Psychiatry. 1998;46:1006–1011.
- Clinical Global Impressions. ECDEU assessment manual for psychopharmacology. In: Guy W, Rockville: U.S Department of Health, Education, and Welfare; 1976. DHEW Publication NO .(ADM) 76-338.
- Weiss E, Potenza MN, McDougle CJ, et al. Olanzapine addition in obsessive-compulsive disorder refractory to selective serotonin reuptake inhibitors; an open-label case series. J Clin Psychiatry. 1999;60:524–527.
- Francobandiera G. Olanzapine augmentation of serotonin uptake inhibitors in obsessive-compulsive disorder: an open study. Can J Psychiatry. 2001;46:356–358.
- http://www.medcalc.be/30-
- Markarian Y, Larson MJ, Aldea MA, et al. Multiple pathways to functional impairment in obsessive-compulsive disorder. Clin Psychol Rev. 2010;30:78–88.
- Bloch MH, Craiglow BG, Landeros-Weisenberger A, et al. Predictors of early adult outcomes in pediatric-onset obsessive-compulsive disorder. Pediatrics. 2009;124(4):1085–1093.
- Jakubovski E, Bartley CA, Pittenger C, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016;77(5):e605–e611.
- Stein DJ, Subramanian G, Smith B, et al. Mavoglurant augmentation in OCD patients resistant to Selective serotonin reuptake inhibitors: a proof-of-Concept, randomized, placebo-controlled, Phase 2 study. Adv Ther. 2017;34(2):524–541.
- Diniz JB, Requena G, Joaquim MA, et al. Randomized, double-blind, placebo-controlled trial of N-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017;78(7):e766–e773.
- Paydary K, Akamaloo A, Ahmadipour A, et al. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. 2016;41(2):214–219.
- Bloch MH, Storch EA. Assessment and management of treatment-refractory obsessive-compulsive disorder in Children. J Am Acad Child Adolesc Psychiatry. 2015;54(4):251–262.
- Bloch MH, Landeros-Weisenberger A, Kelmendi B, et al. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11(7):622–632.
- Li X, May RS, Tolbert LC, et al. Risperidone and haloperidol augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder: a crossover study. J Clin Psychiatry. 2005;66(6):736–743.
- Maina G, Pessina E, Albert U, et al. 8-week, single-blind, randomized trial comparing risperidone versus olanzapine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2008;18(5):364–372.
- Selvi Y, Atli A, Aydin A, et al. The comparison of aripiprazole and risperidone augmentation in selective serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a single-blind, randomized study. Hum Psychopharmacol. 2011;26(1):51–57.
- McDougle CJ, Epperson CN, Pelton GH, et al. A double-blind, placebo-controlled study of resperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:794–801.
- Komossa K, Depping AM, Meyer M, et al. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. 2010;8(12):CD008141.
- Bangalore S, Messerli FH. Of statistical significance: “trends” toward significance and optimism bias. J Am College Cardiol. 2006;48(7):66–74.
- Wood J, Freemantle N, King M, et al. Trap of trends to statistical significance: likelihood of near significant P value becoming more significant with extra data. Br Med J. 2014;348:2215.
- Matsunaga H, Nagata T, Hayashida K, et al. A long-term trial of the effectiveness and safety of atypical antipsychotic agents in augmenting SSRI-refractory obsessive-compulsive disorder. J Clin Psychiatry. 2009;70(6):863–868.