?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Conventional polymers are made from fossil resources and consume fossil energy in their production, yielding CO2 emissions. Production of large-volume compounds from CO2, either from point sources or from the air, could reduce anthropogenic global warming. Poly(3-hydroxybutyrate) (PHB) is a biodegradable and biobased thermoplastic polymer which can be synthesized sustainably using CO2. PHB exhibits a high crystallinity and shows properties similar to synthetic polyesters and also to polyolefins such as polypropylene. However, the low elongation at break and the brittleness of PHB are limitations, which, however, can be overcome by using other polyhydroxyalkanoates (PHA) as blends or copolymers. In this study, PHB produced by cyanobacteria from CO2 was extracted and characterized using various techniques. The Fourier Transform Infrared spectroscopy (FTIR) showed the characteristic signals expected for the phototrophic PHB. Differential Scanning Calorimetry (DSC) for thermal characterization of PHB showed a melting temperature of 151.42°C and 73.11°C for the crystallization process. From the Gel Permeation Chromatography (GPC) the molecular weight of cyanobacterial PHB was determined to be 1,051,900 g mol−1. The analytics results show that the photosynthetically produced PHB ressembles conventional PHB from sugar fermentation, which is an established biopolymer today. It can be concluded that photosynthetically produced PHB has a strong market potential, backed by its sustainable production technology.
Introduction
Our world’s dependency on limited fossil resources for the production of energy and materials such as commodity products, amongst them plastics materials, puts a strong burden on the environment. The huge economies of scale and ample market proliferation of conventional polymer materials, with annual production in excess of 350 million tons, makes the commercialization of renewable and (more) sustainable polymers extremely demanding. Biopolymers not only have a narrower processing window than conventional polymers, they also often have a different set of properties – and costs are higher. Also, recycling of bioplastics [Citation1] is an issue due to low volumes and a broad variety. Yet, bioplastics have seen a growing interest in recent years, due to various material improvements, legal frameworks and increased environmental awareness, so that today, approx. 1-2% of all polymers are biobased and/or biodegradable. The full potential of biopolymers is estimated at 90% of all conventional polymers, and in order to achieve this potential, material properties and costs need to be worked on. Today there is a broad mix of biopolymers, ranging from established materials such as TPS (thermoplastic starch) or polylactic acid (PLA) to novel, promising candidates such as polyethylenefuranoate (PEF) [Citation2].
Naturally produced polyesters, so-called polyhydroxyalkanoates (PHAs), are a nice biopolymer material, with a particularly strong potential. PHA are a versatile group of biodegradable polyesters produced by microorganisms accumulated intracellularly to levels as high as 90 % dry cell weight (DCW) under stressed growth conditions acting as carbon and energy storage material [Citation3,Citation4]. The most important member of the PHA family is polyhydroxybutyrate (PHB) [Citation5].
PHA are nontoxic, biocompatible and biodegradable thermoplastics that can be produced from renewable resources, typically sugar or starch (which is hydrolyzed to sugar). They have a high degree of polymerization, are highly crystalline, are optically active and isotactic (stereochemical regularity in repeating units), piezoelectric and insoluble in water [Citation3]. These features make them highly competitive with polypropylene, the petrochemical-derived plastic with a huge production volume on the order of 50 million tons/year and applications in short-lived products like packaging, medium-lived applications such as automotive components, and also durable goods such as pipes.
More than 100 different monomer units have been identified as constituents of the intracellular energy storage compound class PHA. This creates a possibility for producing different types of biodegradable polymers with an extensive range of properties [Citation3].
The copolymer PHBV (3-hydroxybutyrate-co-3-hydroxyvalerate) is a very prominent example, where a few % of valerate reduce the brittleness of pure PHB significantly [Citation6].
In general, PHAs are classified into two groups according to the carbon atoms that comprise their monomeric unit. Short-chain-length PHAs (scl-PHAs) consist of 3–5 carbon atoms, whereas medium-chain-length PHAs (mcl-PHAs) contain 6–14 carbon atoms in the monomers [Citation7]. Poly(3-hydroxybutyrate) (PHB), the most well-known scl-PHA member, possesses extensive interesting functions and can replace fossil-based plastics in many applications [Citation8]. PHB is biobased and shows biocompatibility and biodegradability, also in the marine environment. Degradation rates of PHB are up to 20 µm/day in fresh compost and up to 1 µm/day in sea water.
However, the low elongation at break and the brittleness of PHB are limitations, but they can be overcome using other PHA, as blends or copolymers such as polyhydroxyvalerate (PHV) and poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (PHBV). The copolymer can either be directly biosynthesized under varying cultivation conditions or be chemically produced in vitro. Apart from short-chain length PHA, there are medium- and long-chain-length polymers which can help to tailor the material properties [Citation5].
Current industrial PHA production processes rely mostly on the availability of agricultural resources, which are unsustainable (compare the food vs. fuel discussion with first-generation biofuels) and leaving sometimes an ecological footprint [Citation9]. PHB is commercially produced by heterotrophic bacteria such as Cupriavidus necator [Citation4], and recombinant Escherichia coli [Citation10]. Despite relatively high yields of PHB, production from bacterial fermentation requires sugar supplementation and continuous oxygen supply which results in high substrate and operation costs [Citation11,Citation12]. PHAs can be produced through sustainable bioprocess engineering [Citation13].
As an alternative method, PHB has been produced in cyanobacteria using sustainable resources CO2 and sunlight. The use of photosynthetically derived PHB is not economically feasible yet due to the low productivity of the polymer. The price of cyanobacterial PHB needs to be and can be reduced to make the material more competitive in the market either by increasing the productivities or reducing the cultivation costs.
In this study the polymer produced from the high PHB producing mutant of cyanobacterial strain Synechocystis sp. PCC 6714, MT_a24 [Citation14] was extracted using liquid-liquid extraction from the whole cells. The characterization of the polymer was done using various methods such as Fourier Transform Infrared spectroscopy (FTIR) for the chemical characterization whereas Differential Scanning Calorimetry (DSC) was used to determine the thermal properties. Also, Gel Permeation Chromatography (GPC) was performed for the analysis of the molecular weight of the polymer.
Material and methods
Strain and inoculum preparation
An axenic culture of wild-type strain Synechocystis sp. PCC 6714 was purchased from Pasteur Culture Collection of Cyanobacteria (Pasteur Institute, Paris, France). Unless stated otherwise, Synechocystis sp. PCC 6714 was grown in BG-11 medium [Citation15] supplemented with 10 mM HEPES buffer pH 8.2 with the addition of 15 g L−1 of Kobe agar for plates and 5 mM NaHCO3 as carbon source prior to inoculation. In order to induce nitrogen deficiency, cells were cultured in BG-11 media without nitrate and ammonia. Ferrous ammonium citrate and Co (NO3)2.6H2O were substituted with equimolar concentrations of Ferric citrate and CoCl2.6H2O in terms of iron and copper content. For phosphorus limitation, KH2PO4 was replaced with an equimolar concentration of KCl in terms of potassium content
Bioreactor cultivations
Lab-scale bioreactor experiments were carried out under sterile conditions and light/dark cycles of 16:8 in a 1.5 L jacketed glass reactor with a working volume of 1 L (Applikon B.V, the Netherlands) as previously described by Kamravamanesh et al. [Citation16]. The temperature was maintained at 28 °C and pH was measured with a pH-electrode (Mettler Toledo GmbH, Vienna, Austria) and was automatically maintained at 8.5 by the addition of 0.5 M HCl or 0.5 M NaOH, respectively. Agitation speed was at 300 rpm. Gas flow was controlled by mass flow controllers for air and CO2 (Brooks Instrument, Matfiels, USA). The reactor was bubbled with a mixture of sterile filtered air and 2 % CO2 at a flow rate of 0.02 vvm (vessel volumes per minute, 20 mL min−1). The illumination was done using LED strips wrapped around the reactor vessel providing a light intensity of 50 µmol m−2 s−1 photons in PAR (photosynthetically active radiation).
The pilot-scale cultivations were performed in a 40-liter glass reactor, the tubular hanging garden system at ecoduna AG in Bruck an der Leitha, Austria, under non-sterile conditions. The pH was controlled at 8.5 using CO2 sparging. The circulation was done using sterile filtered air.
All fermentation parameters and variable pump set-points were controlled using the process information management system Lucullus online monitoring system 3.2 (Securecell AG, Schlieren, Switzerland).
Photobioreactor experiments were performed in duplicates and one is shown as an example. Samples were taken in triplicates at 24-hour intervals and were analyzed for biomass and PHB content.
Determination of the PHB content
PHB quantification was done using the procedure described by Schlebusch and Forchhammer [Citation17]. Pre-weighed dried cells (2–5 mg) were boiled with 1 mL conc. H2SO4 at 100°C on a heating block (AccublockTM, Labnet, USA) for one hour to convert PHB to crotonic acid. Samples were allowed to cool down and subsequently diluted 20 times using 0.014 M H2SO4. Crotonic acid was determined using a high-performance liquid chromatography system (Thermo-Fischer Scientific, USA) with a Nucleosil C8 column (Macherey-Nagel, Germany) using an isocratic method. The mobile phase used was 20 mM NaH2PO4 buffer; pH 2.5 and acetonitrile (70:30 v/v) with a flow rate of 0.85 mL min−1 and a column temperature of 30°C. Detection of crotonic acid was done using a diode array detector (DAD) detector (Thermo-Fischer Scientific, USA) at 210 nm. For calibration, pure PHB (Sigma- Aldrich, USA) was treated accordingly and analyzed in parallel with samples. Instrument control and peak evaluation were done with Chromeleon 7.2 (Thermo-Fischer Scientific, USA). The percentage PHB per dry cell weight (DCW) was determined by the amount of PHB obtained from HPLC analysis and the cell dry weight of biomass used for the analysis using Equation (3):
PHB extraction
The PHB was extracted using a method previously described by Hahn et al. [Citation18] with some modifications. The biomass was lyophilized and suspended in chloroform 30 ml g−1 of biomass. The suspension was put on a heating block and allowed to boil for one hour under continuous shaking at 300 rpm. The hot suspension was filtered through a Whatman filter paper (Merck, Austria). The PHB was extracted using 10 times volume of ice-cold methanol. The polymer was separated using centrifugation at 30,000 rpm, and the pellet was air-dried. The PHB was finally washed using cold acetone.
Dynamic Scanning Calorimetry (DSC)
The DSC was done using the extracted PHB with a TA instrument DSC Q20 in a heat-cool-heat cycle using the following parameters:
Equilibration at −40°C
Isothermal for 5 minutes
Ramp at 10°C min−1 to 200°C
Isothermal for 3 minutes
Ramp at 10°C min−1 to −40°C
Isothermal for 5 minutes
Ramp at 10°C min−1 to 200°C
And for the analysis, the second cycle was used.
Infrared spectroscopy
Attenuated Total Reflection Infrared Spectroscopy was done using a Nicolet 5700 FTIR spectrometer.
Gel Permeation Chromatography (GPC)
The GPC was performed using an Agilent 1100 chromatography system equipped with a refractive index (RI) response detector. The polymer was dissolved in chloroform with the concentration of 2 mg mL−1 overnight. The analysis was carried out at 40 °C in chloroform, at the flow rate of 1 ml min−1 and injection volume of 100 µL. The GPC system was calibrated prior to analysis using narrow polystyrene standards ranging from 1470 g mol−1 to 6035000 g mol−1. The weight average molar mass Mw, number average molar mass Mn, and molar mass dispersity D for the measurements were determined from the peaks corresponding to the polymer fraction and are therefore determined as “polystyrene-relative” molecular weights.
Results and discussions
PHB extraction
Once PHB was extracted from the cells the purity was determined using HPLC. The analysis of extracted PHB from cyanobacteria using chloroform: methanol extraction showed a purity of more than 95 % (DCW).
Dynamic scanning calorimetry
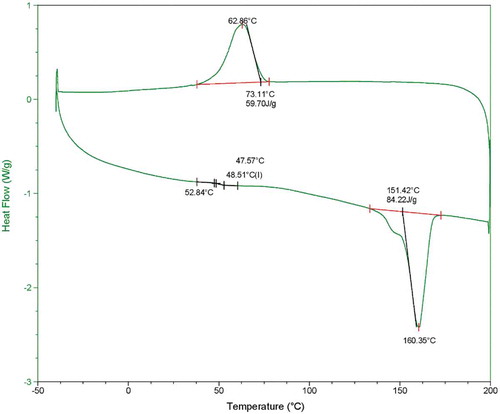
The melting temperature and enthalpies of fusion of the PHB extracted from cyanobacteria was determined using DSC. The provided thermogram in shows the distinct characteristic signals for melting, crystallization and the glass transition. The onset temperature amounts to 151.42 °C for the melting temperature (Tm) of the phototrophically synthesized PHB and 73.11 °C for the crystallization process (Tc). The glass-transition temperature (Tg) can be seen in the enlargement of the thermogram. The temperature at the inflection point amounts to 48.51 °C. The values obtained here suggest a high crystallinity of the extracted polymer.
Infrared spectroscopy
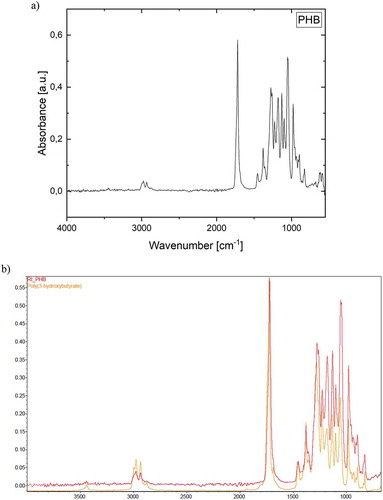
The FTIR spectrum for the photosynthetically produced PHB is presented in . The spectrum visibly shows the expected characteristic bands for PHB. In order to validate this observation the obtained spectrum is aligned with the PHB spectrum from databases. The stacked signal illustrated in ) suggests the similarity of the measured PHB sample from cyanobacteria and the specimen from the database.
Gel Permeation Chromatography (GPC)
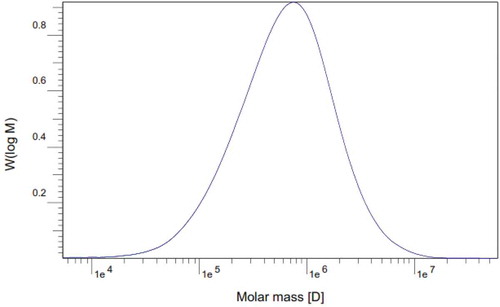
The GPC analysis () of PHB produced from CO2 using cyanobacteria showed that the molecular weight was (Mw = 1,051,900 g mol-1) and the number average of the molecular weight of the PHB was (Mn = 316,060 g mol−1). The polydispersity index (Mw/Mn) of the cyanobacterial PHB was determined to be (D = 3.328).
Depending on various factors such as the production strain, the nutrient supply, and the cultivation parameters biosynthesized PHA polymer chains may have 102 to 105 3HA monomers. PHB consists merely of 3HB subunits, featuring rather high crystallinity and restricted processability. The low difference between the decomposition temperature and the high melting point provides a window of processability which is too narrow for many processing techniques [Citation19]. These obstacles can be overcome by interrupting the crystalline PHB matrix by additional building blocks like 3HV and 4HB, resulting in improved material properties and a broader range of applications [Citation19].
Besides the raw material used and the process specifications, downstream processing for polymer extraction and recovery is a rather cost-determining factor in the PHA production process. Research needs more focus on the downstream processing of PHA making the process more sustainable and economically feasible.
PHA, poly(lactic acid) (PLA), poly(trimethylene terephthalate) (PTT) and poly(p-phenylene) (PPP) are among the best-studied polymers containing at least one monomer synthesized via bacterial fermentation [Citation20]. From these, PHA and PLA are substantial for their biodegradability, and PHA is the only polymer which is completely synthesized in vivo. represents various bacterially synthesized polymers and their thermal properties and molecular weights. PHA has the most versatile structure, resulting in most variable Mw, Tm, Tg, and thermo-degradation temperature [Citation20,Citation21]. In comparison, the low-cost PLA is brittle with an elongation at break of 5.2–2.4%, yet its tensile strength is the highest among these bacterial plastics, ranging from 49.6 to 61.6 MPa; Tg of 60°C has been a weak point for PLA application as articles made from PLA change shape at this temperature [Citation20,Citation22].
Table 1. Comparison of various bacterial polymers in thermal and molecular properties.
The polymer obtained in this study represents an adequately high molecular weight and polydispersity index. In order to offset the relatively high production cost of the polymer and to further improve the physical properties of the material, it can be blended with other polymers or be processed to compounds. For instance, blends of PHA with natural fibers were found to have improved mechanical properties [Citation23]
As shows, the photosynthetically produced PHB ressembles traditionally produced PHB samples. Today, the market share of bioplastics is approx. 1 % and price-driven mass markets such as packaging are difficult to enter (compare film for food packaging, which is often a multilayer material). Applications for PHA range from new fields such as additive manufacturing (filament) to high end (and less price sensitive) therapeutic use. PHA show both advantages of biobased carbon content and full biodegradability, also in the marine environment, and compared to PHA from carbohydrate fermentation, cyanobacterial PHA can be more sustainable and more cost effective. It can be a carbon-neutral or even carbon-negative material, making the process not only attractive for PHA converters and users, but also for CO2 emitters like power stations. There are plenty of medium-size CO2 point sources, e.g. biogas production facilities, where the CO2 could be used in an adjacent cyanobacterial PHA factory erected on non-arable land. Best would be a biorefinery approach, where valuable compounds such as phytohormones and pigments are extracted from the cyanobacteria, then PHA and finally the biomass is anaerobically digested in a biogas plant, yielding a cost-effective, fully integrated process.
In a next step, a larger amount of photosynthetically produced PHB should be obtained and tested in compounded formulations and/or applications. A life cycle analysis of the novel production process could be made.
Conclusions
PHA and in particular PHB production from CO2 has the potential of reducing the production cost of this biodegradable polymer class and material, also making the material more sustainable and competitive in the market with other bioplastics. Here, the PHB produced from CO2 using cyanobacteria as cell factory was characterized, in light of the potential of the polymer PHB for the commercial production. PHB has been envisaged as a replacement for PP for a considerable time, and now a process to avoid making PHB from feed and food raw had been developed. In this very study, that photosynthetically produced PHB by Kamravamanesh et al. [Citation14], was characterized, and the material was found to exhibit comparable properties to “traditional” PHB samples. Due to limited amounts of sample, no mechanical characterization could be performed, which could be the basis for further tests toward compound formulations and/or application testing. Since PHB is already an established biopolymer in the market place, with strong push toward broader commercialization, e.g. by a novel initiative GO!PHA [Citation27], it can be expected that photosynthetically produced PHB is apt for several applications already today, with yet more of them to be developed. For instance, Gruenbichler [Citation28], has suggested the use of PHB as hydrocarbon adsorber for oil spill containment and contaminated soil remediation, since a positive co-metabolism for persistent pollutant degradation was observed in the presence of PHB.
Whether or not a 90% replacement rate of conventional plastics by bioplastics within a foreseeable time scale, on the order of a decade from now, is feasible technically, commercially and socially, cannot be told today, but it is for sure that PHB and PHA in general would play a pivotal role in such a paradigm shift.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Maximilian Lackner
Maximilian Lackner is a scholar and entrepreneur with experience in the polymer industry and in bioplastics in particular.
Donya Kamravamanesh
Donya Kamravamanesh has obtained her PhD from Vienna University of Technology doing research on PHB production from CO2 using cyanobacteria.
Margit Krampl
Margit Krampl is a student of chemical Engineering at Vienna University of Technology.
Regina Itzinger
Regina Itzinger is a PhD student at the Institute for Chemical Technology of Organic Materials at the Johannes Kepler University Linz.
Christian Paulik
Christian Paulik is Professor at the Johannes Kepler University Linz and Head of the Institute for Chemical Technology of Organic Materials with expertise in conventional and biobased plastics and biotechnololgy.
Ivan Chodak
Prof. Ivan Chodak is a senior scientist at Polymer Institute of the Slovak Academy of Sciences dealing with development and applications of multiphase systems with polymeric matrix, especially blends of biodegradable polymers.
Christoph Herwig
Christoph Herwig, bioprocess engineer from RWTH Aachen, worked in industry in the design and commissioning of large chemical facilities prior to enter his interdisciplinary PhD studies at EPFL, Switzerland in bioprocess identification. Subsequently he positioned himself at the interface between bioprocess development and facility design in biopharmaceutical industry. Since 2008, he is full professor for biochemical engineering at the Vienna University of Technology.
References
- Gulitah V, Liew KC. Three different recycle codes of plastic/Acacia fibre composites: physical and morphological properties. Int J Biobased Plast. 2019;1(1):1–7.
- Lackner M. Biokunststoffe [bioplastics]. 2019 April 8–9, St. Poelten/ Austria,ecoplus, Seminar.
- Reddy CSK, Ghai R, Kalia VC. Polyhydroxyalkanoates: an overview. Bioresour Technol. 2003;87(2):137–146.
- Madison LL, Huisman GW. Metabolic engineering of Poly(3-Hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev. 1999;63(1):21–53.
- Lackner M, Markl E, Grünbichler H. Cyanobacteria for PHB bioplastics production: a review. Novel Tech Nutrition Food Sci. 2018;2(4):4.
- Langford A, Chan CM, Pratt S, et al. The morphology of crystallisation of PHBV/PHBV copolymer blends. Eur Polym J. 2019 March;112:104–119.
- Kourmentza C, Plácido J, Venetsaneas N, et al. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering. 2017;4(2):55.
- Ten E, Jiang L, Zhang J, et al. 3 - Mechanical performance of polyhydroxyalkanoate (PHA)-based biocomposites. In: Misra M, Pandey JK, Mohanty AK, editors. Biocomposites. Cambridge, UK: Woodhead Publishing; 2015. p. 39–52.
- Behler J, Vijay D, Hess WR, et al. CRISPR-based technologies for metabolic engineering in cyanobacteria. Trends Biotechnol. 2018;36(10):996–1010.
- Schubert P, Steinbüchel A, Schlegel HG. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in. Escherichia Coli J Bacteriol. 1988;170(12):5837–5847.
- Wu G, Wu Q, Shen Z. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour Technol. 2001;76(2):85–90.
- Steinbüchel A. PHB and other polhydroxyalkanoic acids. In: Biotechnology set. 2nd ed. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 2008. p. 403–464.
- Koller M. Recent advances in biotechnology volume, 1: microbial biopolyester production, performance and processing microbiology, feedstocks, and metabolism. Sharjah, U.A.E: Bentham Science Publishers; 2016.
- Kamravamanesh D, Kovacs T, Pflügl S, et al. Increased poly-β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: mutant generation and characterization. Bioresour Technol. 2018;266:34–44.
- Rippka R, Deruelles J, Waterbury JB, et al. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111(1):1–61.
- Kamravamanesh D, Pflügl S, Nischkauer W, et al. Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. AMB Express. 2017;7(1):143.
- Schlebusch M, Forchhammer K. Requirement of the nitrogen starvation-induced protein Sll0783 for polyhydroxybutyrate accumulation in Synechocystis sp. strain PCC 6803. Appl Environ Microbiol. 2010;76(18):6101–6107.
- Hahn SK, Chang YK, Lee SY. Recovery and characterization of poly(3-hydroxybutyric acid) synthesized in Alcaligenes eutrophus and recombinant Escherichia coli. Appl Environ Microbiol. 1995;61(1):34–39.
- Koller M, Salerno A, Braunegg G. Polyhydroxyalkanoates: basics, production and applications of microbial biopolyesters. Bio‐Based Plast Mater Appl. 2013;137–170.
- Chen G-Q. Introduction of bacterial plastics PHA, PLA, PBS, PE, PTT, and PPP. In Chen GG (Ed.). Plastics from bacteria. Berlin Heidelberg, Germany: Springer; 2010. p. 1–16.
- Wang -H-H, Li X-T, Chen G-Q. Production and characterization of homopolymer polyhydroxyheptanoate (P3HHp) by a fadBA knockout mutant Pseudomonas putida KTOY06 derived from P. putida KT2442. Process Biochem. 2009;44(1):106–111.
- Żenkiewicz M, Richert J, Rytlewski P, et al. Characterisation of multi-extruded poly (lactic acid). Polym Test. 2009;28(4):412–418.
- Avella M, La Rota G, Martuscelli E, et al. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and wheat straw fibre composites: thermal, mechanical properties and biodegradation behaviour. J Mater Sci. 2000;35(4):829–836.
- Lee W-H, Loo C-Y, Nomura CT, et al. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour Technol. 2008;99(15):6844–6851.
- Bhubalan K, Rathi D-N, Abe H, et al. Improved synthesis of P (3HB-co-3HV-co-3HHx) terpolymers by mutant Cupriavidus necator using the PHA synthase gene of Chromobacterium sp. USM2 with high affinity towards 3HV. Polym Degradation Stab. 2010;95(8):1436–1442.
- Chiu F-C, Ting M-H. Thermal properties and phase morphology of melt-mixed poly (trimethylene terephthalate)/polycarbonate blends—mixing time effect. Polym Test. 2007;26(3):338–350.
- GO!PHA. 2019. [cited 2019 Nov 17] Available from: https://www.gopha.org/
- Gruenbichler H. 2019. Co-metabolism of hydrocarbons in the presence of biopolymer PHB, private communication.