ABSTRACT
Most carbonatites were emplaced in continental extensional settings and range in age from Archean to recent. They commonly coexist with alkaline silicate igneous rocks, forming alkaline-carbonatite complexes, but some occur as isolated pipes, sills, dikes, plugs, lava flows, and pyroclastic blankets. Incorporating cone sheets, ring dikes, radial dikes, and fenitisation-type halos into an exploration model and recognising associated alkaline silicate igneous rocks increases the footprint of the target. Undeformed complexes have circular, ring, or crescent-shaped aeromagnetic and radiometric signatures. Carbonatites can be effectively detected by soil, till, and stream-sediment geochemical surveys, as well as biogeochemical and indicator mineral surveys Carbonatites and alkaline-carbonatite complexes are the main sources of rare earth elements (REE) and Nb, and host significant deposits of apatite, vermiculite, Cu, Ti, fluorite, Th, U, natural zirconia, and Fe. Nine per cent of carbonatites and alkaline-carbonatite complexes contain active or historic mines, making them outstanding multi-commodity exploration targets.
Introduction
Mineralised carbonatites and alkaline-carbonatite complexes are highly sought after, multi-commodity, but poorly understood exploration targets (Verwoerd Citation1986; Mariano Citation1989a, Citation1989b; Richardson and Birkett Citation1996a, Citation1996b; Pell Citation1996; Birkett and Simandl Citation1999). They are the main source of niobium (Nb; Mackay and Simandl Citation2014a; Simandl et al. Citation2018) and rare earth elements (REE; Simandl Citation2014; Verplank et al. Citation2016), which are considered critical metals for key economic sectors in industrialised countries (European Commission Citation2017), and have become popular exploration targets for mining companies worldwide. Alkaline-carbonatite complexes are significant sources of Cu, apatite, fluorite, vermiculite, and other commodities. Most modern studies on carbonatites address their origin and aim to improve our understanding of the Earth's mantle (Bell and Tilton Citation2001, Citation2002; Bell and Simonetti Citation2010; Rukhlov et al. Citation2015).
Carbonatites and alkaline-carbonatite complexes were considered controversial ever since A.G. Högbom began detailed geological work at the Alnö Island alkaline-carbonatite complex in 1889. The history of carbonatite research up to 1966 is well summarised in the 1st and 2nd editions of the widely available benchmark publication entitled ‘The Geology of Carbonatites’ by Heinrich (Citation1980). A number of additional reviews have been performed since that time, including the book edited by K. Bell (Citation1989) entitled ‘Carbonatites: Genesis and Evolution’. However, the most important review from an exploration geologist's point of view is probably the compilation of Wooley and Kjarsgaard (Citation2008a) entitled ‘Carbonatite occurrences of the word; map and database’, available free of charge for download from the Geological Survey of Canada website. The highly descriptive and factual nature of this publication avoids traps and controversies associated with the genesis of carbonatites, and takes into consideration some of the key suggestions of Mitchell (Citation2005) who attempts to improve currently accepted classification of Le Maître (Citation2002) by making it more relevant to geological mappers and exploration geologists. The descriptive approach, in combination with the availability of data as spreadsheets, will make the Wooley and Kjarsgaard (Citation2008a) compilation a valuable source of information for years to come, since spreadsheets can be easily updated and customised by the user.
Herein, we review the definition and classification of carbonatites and summarize information pertinent for carbonatite exploration such as tectonic setting, morphology, carbonatite-related alkali silicate rock associations, accompanying alteration, and temporal distributions. We also discuss carbonatites and alkaline-carbonatite complexes in terms of resources, exploration methods, and prospectivity. The four main objectives of this paper are to (i) alert the exploration community to the multi-commodity exploration potential of carbonatites and alkaline-carbonatite complexes, (ii) highlight the relevance of tectonic setting, carbonatite-related alkali metasomatism (fenitisation-type metasomatism), and alkaline-carbonatite complex morphology to the exploration geologist, (iii) present relevant advances in exploration methods, and (iv) bridge the gap separating carbonatite-related academic researchers and mineral explorers.
Definitions and classifications
Carbonatites are defined by the International Union of Geological Sciences (IUGS) as igneous rocks containing more than 50% modal primary carbonates (Le Maitre Citation2002). Depending on the predominant carbonate mineral, a carbonatite is referred to as a ‘calcite carbonatite’, ‘dolomite carbonatite’ (), or ‘ferrocarbonatite’, where the main carbonate is iron-rich. If more than one carbonate mineral is present, the carbonates are named in order of increasing modal concentrations. For example, a ‘calcite-dolomite carbonatite’ is composed predominately of dolomite. If non-essential minerals (e.g. biotite) are present, this can be reflected in the name as ‘biotite-calcite carbonatite’.
Figure 1. Upper Fir dolomite carbonatite, British Columbia, Canada; slightly weathered surface; dolomite-brown, Na-amphibole – green, apatite – white. Coin for scale is 2 cm in diameter.

Where the modal classification cannot be applied, the IUGS chemical classification may be used ((a)). This classification, based on wt.% ratios, subdivides carbonatites into calciocarbonatites, magnesiocarbonatites, and ferrocarbonatites. For calciocarbonatites, the ratio of CaO/(CaO + MgO + FeO + Fe2O3 + MnO) is greater than 0.8. The remaining carbonatites are subdivided into magnesiocarbonatite [MgO > (FeO + Fe2O3 + MnO)] and ferrocarbonatite [MgO < (FeO + Fe2O3 + MnO)] (Woolley and Kempe Citation1989; Le Maitre Citation2002). If the SiO2 content of the rock exceeds 20%, it is referred to as silicocarbonatite. When the IUGS chemical classification is used, iron content in sulphides and oxides should be excluded from consideration, otherwise, magnetite and hematite-rich calciocarbonates or magnesiocarbonatites may be erroneously classified as ferrocarbonatites (Gittins and Harmer Citation1997). A natrocarbonatite is a special variety of carbonatite consisting mainly of Na–K–Ca carbonates, such as nyerereite [(Na,K)2Ca(CO3)2] and gregoryite [(Na,K,Cax)2–x(CO3)], known from Ol Doinyo Lengai volcano (Tanzania).
Figure 2. Carbonatite classifications according to (a) IUGS based on wt.% (Le Maitre Citation2002) and (b) Gittins and Harmer (Citation1997) based on molar proportions. C/CMF is the molar ratio of CaO/[CaO + MgO + FeO* + MnO]; FeO* expressed as molar FeO if both FeO and Fe2O3 are determined.
![Figure 2. Carbonatite classifications according to (a) IUGS based on wt.% (Le Maitre Citation2002) and (b) Gittins and Harmer (Citation1997) based on molar proportions. C/CMF is the molar ratio of CaO/[CaO + MgO + FeO* + MnO]; FeO* expressed as molar FeO if both FeO and Fe2O3 are determined.](/cms/asset/e1efca2a-9cd7-4f0f-afd9-e61669ada042/yaes_a_1516935_f0002_oc.jpg)
A refinement to the IUGS chemical classification based on molar proportions, proposed by Gittins and Harmer (Citation1997), introduces the term ‘ferruginous calciocarbonatites’ ((b)). The boundary separating calciocarbonatites from magnesiocarbonatites and ‘ferruginous calciocarbonatites’ is set at 0.75, above which carbonatites contain more than 50% calcite on a molar basis. Although not universally accepted, Gittins and Harmer's classification is commonly used in studies of carbonatite-hosted ore deposits (e.g. Trofanenko et al. Citation2016).
A mineralogical-genetic classification of carbonatites was proposed by Mitchell (Citation2005). His benchmark paper points to pitfalls of the IUGS classification, and subdivides carbonatites into ‘primary carbonatites’ and ‘carbothermal residua’. The introduction of the term ‘carbothermal residua’ is significant as it alerts mantle specialists to the fact that not all rocks currently called ‘carbonatites’ by academicians satisfy IUGS definition. In their well-known and widely referenced compilation, Woolley and Kjarsgaard (Citation2008a) have taken into consideration suggestions proposed by Mitchell (Citation2005), but instead of using ‘primary’ and ‘carbothermal residua’ they adopted the terms ‘magmatic’ and ‘carbohydrothermal’. The recognition of ‘carbohydrothermal carbonatites’ by academia is beneficial to the exploration community as it eliminates a deep historic divide between researchers with an interest in the Earth's mantle studying igneous carbonatites sensu stricto (as defined by IUGS), and those that study or explore for carbonatite-related ore deposits. The term ‘carbohydrothermal carbonatite’ is defined by Woolley and Kjarsgaard (Citation2008b) as carbonatite which precipitated at subsolidus temperatures from a mixed CO2–H2O fluid that can be either CO2-rich (i.e. carbothermal), or H2O-rich (i.e. hydrothermal). The carbohydrothermal aspect of some carbonatite phases is in line with number of recent studies highlighting the role of fluids in formation of carbonatite-related REE deposits, such as at Bayan Obo Fe-REE-Nb deposit, Inner Mongolia (Smith et al. Citation2015; Lai et al. Citation2016) and Wicheeda Lake carbonatite-related REE mineralisation, Canada (Trofanenko et al. Citation2016), and with studies that provide constraints on transportation of REE by hydrothermal fluids and their deposition (e.g. Migdisov et al. Citation2016). Similarly, it may play an important role in the genesis of other carbonatite-related deposit types (e.g. apatite ores at Seligdar apatite deposit, Russia; Prokopyev et al. Citation2017).
For the benefit of exploration geologists and field mappers, in this paper, we use the term carbonatite to broadly include both magmatic carbonatites and related carbohydrothermal phases, as it may be extremely difficult to distinguish between them on the outcrop or hand specimen scale without follow-up laboratory studies. We use the term alkaline-carbonatite complex to denote occurrences where magmatic and or carbohydrothermal carbonatites are spatially associated with alkaline silicate rocks of igneous or metasomatic origin.
In this review paper, the term ‘carbonatite-related ore deposit’ is used in the broadest sense. It refers to deposits that are genetically or spatially associated with carbonatites or alkaline-carbonatite complexes. A carbonatite-related deposit could consist of unaltered magmatic minerals; minerals that crystalised from carbohydrothermal, hydrothermal, or metamorphic fluids transporting metals; or minerals concentrated by weathering or supergene enrichment, which commonly overly carbonatite protore. Consequently, a ‘carbonatite-related ore deposit’ could be hosted by a carbonatite or alkaline-carbonatite complex, be situated at the contact between the intrusion and host rock, or be distal to the intrusion.
Origin of carbonatites
There are currently three main hypotheses explaining the origin of carbonatite melts: (1) immiscible separation of parental carbonated silicate magmas at crustal or mantle pressures (Kjarsgaard and Hamilton Citation1989; Lee and Wyllie Citation1998; Wyllie and Lee Citation1998); (2) crystal fractionation of parental carbonated silicate magmas such as olivine melilitites or kamafugites (Veksler and Lentz Citation2006); and (3) low-degree partial melting of carbonated mantle peridotite below 70 km depth (Wyllie and Huang Citation1976a, Citation1976b; Eggler Citation1978; Bell Citation1989; Dalton and Presnall Citation1998; Harmer and Gittins Citation1998; Bell and Rukhlov Citation2004; Rukhlov et al. Citation2015). Hypotheses invoking or supporting a possible derivation of carbonatites from the Earth's crust (Lentz Citation1999; Ferrero et al. Citation2016), or from the Earth's mantle with some crustal contribution (Cheng et al. Citation2017; Song et al. Citation2017), have also been proposed. Furthermore, a recent study based on boron isotopes of carbonatites worldwide suggests that, although most carbonatites may originate in the upper mantle, younger carbonatites (<300 My) probably involve at least partial subducted crustal component (Hulett et al. Citation2016). It is likely that not all carbonatite-forming melts are of the same origin. However, regardless of their mode of formation, most researchers agree that alkalis (Na and K) play an important role in the genesis of calcite and dolomite carbonatites, and ferrocarbonatite intrusions.
The importance of alkalis in the genesis of carbonatites is consistent with studies of low-temperature (<600°C) natrocarbonatitic lavas from Ol Doinyo Lengai, Tanzania that contain 38–40 wt.% combined Na2O and K2O, 4.5 wt.% F, 5.7 wt.% Cl, approximately 15 wt.% Ca, and less than 1 wt.% combined Mg and Fe (Keller and Krafft Citation1990; Keller and Zaitsev Citation2012). Petrographic and geochemical evidence from extrusive carbonatites, such as Homa Mountain, Kenya (Clarke and Roberts Citation1986); Tinderet, Kenya (Deans and Roberts Citation1984; Zaitsev et al. Citation2013); Kerimasi, Tanzania (Hay Citation1983; Zaitsev Citation2010; Guzmics et al. Citation2011); and Kaluwe, Zambia (Ngwenya and Bailey Citation1990), as well as evidence from intrusive carbonatites such as Bol'shaya Tagna, Russia (Andreeva et al. Citation2006); Oka, Canada (Chen et al. Citation2013); Guli, Russia (Kogarko et al. Citation1991); and Palabora, South Africa (Sharygin et al. Citation2011), suggests that calcite- and dolomite-rich carbonatites are residues or cumulates derived from alkali-bearing (moderately alkaline) melts. Recently, ‘halite intergrown with, and included in, dolomite, calcite, apatite, REE- fluorocarbonates, pyrochlore, fluorite, and phlogopite of a dolomitic carbonatite’ was reported at the St.-Honoré alkaline-carbonatite complex (Canada) by Kamenetsky et al. (Citation2015), further supporting the argument that parental magmas of primary carbonatites are enriched in Na and Cl. A comprehensive review of fluid and melt inclusion studies of intrusive carbonatites by Veksler and Lentz (Citation2006) further documents the alkaline nature of melts to which intrusive carbonatites are related or from which they originated. A hypothesis suggesting that calcite carbonatites evolve from natrocarbonatite parental magmas by loss of alkali due to fenitisation was advanced by Le Bas (Citation1981) and Woolley (Citation1982); however, this hypothesis was challenged, if not discredited, by Twyman and Gittins (Citation1987), Mitchell and Belton (Citation2004), and Mitchell (Citation2005).
A thermal barrier, as established by Cooper et al. (Citation1975), was a major obstacle to most hypotheses proposing that natrocarbonatites evolved from moderately alkaline calcite carbonatites. However, recent experimental work suggests that natrocarbonatite lavas may evolve from a moderately alkaline calcite carbonatite precursor (which is unmixed from nephelinites) by crystal fractionation of calcite and apatite (Weidendorfer et al. Citation2017). This appears possible because of the ability of halogens to suppress the calcite liquidus, thereby eliminating the thermal barrier (Weidendorfer et al. Citation2017). There is no consensus regarding the origin of magnesium- and Fe-rich carbonatites. They are believed to either evolve from early calcite carbonatites by fractional crystallisation or be of hydrothermal origin (Thompson et al. Citation2002), essentially ‘carbohydrothermal’.
Exploring for carbonatites: where to look and what to look for
Tectonic setting
Most carbonatites and alkaline-carbonatite complexes are emplaced in continental (88% cratonic, 10.5% non-cratonic) settings () in Archean and Proterozoic rocks, or in Phanerozoic rocks underlain by a Precambrian basement (Sage and Watkinson Citation1991; Woolley and Kjarsgaard Citation2008a; Pirajno Citation2015). They form in extensional tectonic settings (Bailey Citation1974, Citation1977, Citation1992), along major linear trends related to large-scale intra-plate fracture zones, in association with doming features (crustal arching), or in relation to slab windows in subducting plates (Duke Citation2009; Duke et al. Citation2014). The link between these tectonic features and intense magmatic activity means that many carbonatites are also temporally and spatially related to ‘large igneous provinces’ (Pirajno Citation2000; Ernst and Bell Citation2010). Some researchers (e.g. Nelson et al. Citation1988; Bell Citation2001, Citation2005; Pirajno Citation2015) consider mantle plumes essential to carbonatite genesis.
Figure 3. Tectonic setting of carbonatites and carbonatite complexes. Based on data from 527 carbonatites from Woolley and Bailey (Citation2012).
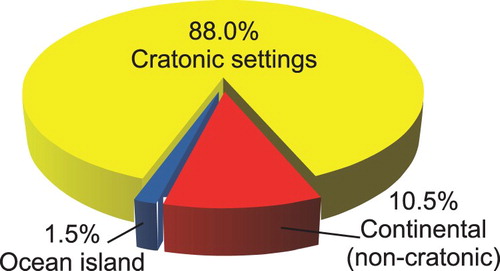
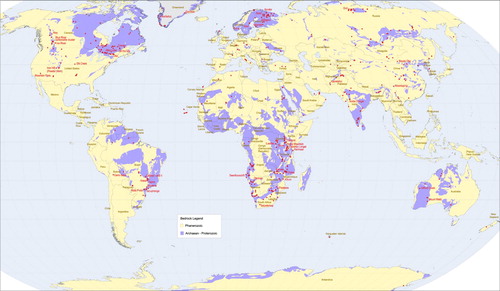
Figure 4. World map of carbonatite occurrences and their spatial relationship to Proterozoic and Archean rocks. Areas underlain by unsubdivided Proterozoic and Phanerozoic rock are not shown. Modified from Woolley and Kjarsgaard (Citation2008a).
Carbonatites in orogenic settings are sometimes referred to as ‘post-collisional’ (Chakhmouradian et al. Citation2008; Woodard and Hetherington Citation2014). This is an unfortunate term because carbonatites that are found in orogenic settings may have been emplaced before a transition from extensional to compressional tectonic regimes, or during post-orogenic extensional relaxation and collapse prior to dynamothermal metamorphic climax. Examples of specific orogenic settings containing carbonatites are: (1) British Columbia Alkaline Province, Canada (Pell Citation1994, Millonig et al. Citation2012); (2) The Himalayan collision zone in western Sichuan, China (Hou et al. Citation2006, Citation2015); (3) Northwest Pakistan (Tilton et al. Citation1998); and (4) The Great Indian Proterozoic Belt, India (Leelanandam et al. Citation2006). The Naantali carbonatite in southwest Finland is also located in orogenic setting (Woodard and Hetherington Citation2014).
Carbonatites are identified in three oceanic island regions: (1) The Canary Islands; (2) The Cape Verde Islands, and (3) The Kerguelen Islands; all of which are located off the African continent (; Woolley and Kjarsgaard Citation2008a). However, it is possible that these islands are underlain by remnants of continental lithosphere stranded during drifting of the African plate (Bonadiman et al. Citation2005; Woolley and Bailey Citation2012).
Temporal patterns of carbonatite emplacement: can age be used to screen exploration targets?
Ages of carbonatite emplacement on a global scale
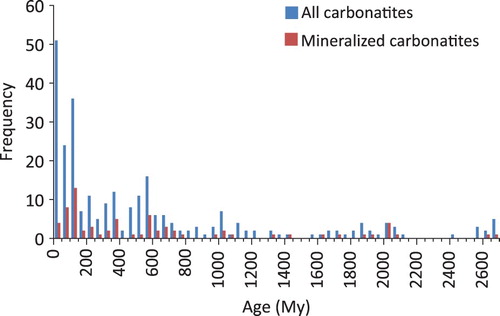
Radiometric ages of cabonatites vary from Archean (e.g. Short Lake, Canada, 2684±170 Ma; Rukhlov and Bell Citation2010) to very recent (e.g. Ol Doinyo Lengai, Tanzania, which is considered an active volcano). Frequency plots of carbonatites with well-established ages indicate that younger carbonatites are more abundant than older ones (). This trend may be due to changes in tectonic activity during the Neoarchean Era that permitted emplacement of carbonatites. As favourable conditions became more widespread, carbonatites became more common (Woolley and Kjarsgaard Citation2008a). The difficulty to distinguish carbonatites from carbonate-rich metasediments in areas affected by a post-carbonatite dynamothermal metamorphism may be another reason (Woolley and Kjarsgaard Citation2008a). Veizer (Citation1992) proposed that the overall exponential decrease in the probability of carbonatite preservation with increasing age of crustal segments is the main reason for this trend. Variations in boron (B) concentrations in young (<300–400 Ma) carbonatites and their isotopic composition (δ11B) suggest that greater quantity of subducted material may have reached plumes, and indirectly contributed increase in carbonatite activity during that time period (Hulett et al. Citation2016).
Figure 5. The frequency of all known carbonatite occurrences with isotopic ages decreases with time. With the exception of carbonatites younger than 150 Ma, the frequency of mineralised carbonatite occurrences shows a similar decrease. Figure based on data from Woolley and Kjarsgaard (Citation2008a), with minor updates.
Carbonatites with mineralogy similar to Ol Doinyo Lengai natrocarbonatite may have been relatively common in Earth's history; however, because they convert rapidly into calcite carbonatites in the near surface environment (Zaitsev and Keller Citation2006), they are not preserved in the geological record. Destruction of nyerereite (the main natrocarbonatite-forming mineral) in surface environments takes place over a few months or less according to the following alteration sequence: nyerereite → pirssonite → calcite → shortite (Zaitsev et al. Citation2008).
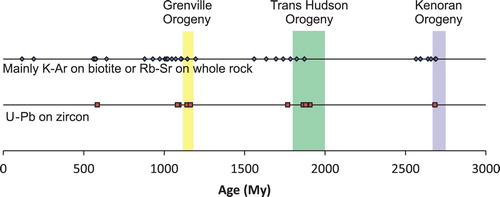
Woolley and Bailey (Citation2012) covered the importance of tectonic controls on carbonatite emplacement. Their study focused on six well documented carbonatite provinces: (a) East Africa (Kenya, Uganda, Tanzania, and one occurrence in Zambia); (b) Namibia and Angola; (c) Eastern Russia (East Tuva, Enisei, East Sayan, Baikal, and Aldan); (d) Greenland; (e) Ontario and southwest Quebec; and (f) Northern Europe, including the Kola Peninsula, northern Norway, Sweden, and Finland. They recognised up to five episodes of carbonatite emplacement separated by hundreds of millions of years in some of these provinces. They pointed out that within at least three of these carbonatite provinces, including one comprising Ontario and southwest Quebec (), plumes can be eliminated as a mode of carbonatite emplacement because of the lack of relationship between the direction of the plate movement and the age of intrusions. Instead, carbonatite magmatism appears to coincide with periods of reactivation of faults and fissures associated with changes in tectonic regimes (extensional period). Consider as an example the Ontario and Southwest Quebec carbonatite province covered by Woolley and Bailey (Citation2012). Although age dates from K-Ar on biotite and Rb-Sr on whole rock may have been reset by medium grade metamorphic events, the U-Pb age dates on zircon and baddeleyite corroborate the original data and confirm that the timing of each carbonatite emplacement episode coincides closely with a period of relaxation linked to a major orogeny ().
Figure 6. Temporal relationship between intensive carbonatite magmatism and main orogenic events in Ontario, Canada and southwest Quebec, Canada. Potassium–Ar dates on biotite and Sr–Rb dates on whole rock from Woolley and Bailey (Citation2012) and U–Pb dates on zircon and baddeleyite from Rukhlov and Bell (Citation2010). Age estimates for Grenville, Trans Hudson, and Kenoran orogenies from Tohver et al. (Citation2006), Corrigan et al. (Citation2009), and Moser et al. (Citation2008), respectively.
The frequency of mineralised carbonatite occurrences follows a similar pattern to the frequency of all carbonatites, except for the interval between 150 and 0 Ma (). Relatively shallow erosional levels may explain this divergence, with few exceptions, as most carbonatite-related mineralisation is spatially related to intrusive carbonatite phases at depth, whereas their volcanic (near surface) equivalents are commonly low grade or barren (unless subjected to supergene enrichment). In view of the broad temporal range of carbonatites on the world scale and the divergence of trends between non-mineralised and mineralised carbonatites within the 150 to 0 Ma time interval, the age alone is of limited use for screening carbonatite exploration targets.
Age relationships between individual barren and mineralised carbonatite pulses on the complex-scale
Within the same alkaline-carbonatite complex, calcite carbonatites are generally the oldest (Richardson and Birkett Citation1996a). Radiometric ages are seldom used systematically to age date discrete intrusive phases of the same alkaline-carbonatite complex; however, where field relationships are observable, calcite carbonatites are cut by magnesian (dolomite carbonatite) pulses, which in turn predate more Fe±Mn-rich (ferrocarbonatite) pulses as illustrated by the Chilva Island Complex (Garson and Smith Citation1958; Woolley Citation1982). However, in many field studies of cabonatite complexes, the cross-cutting relationships are either not discernable or only dolomite carbonatite or ferrocarbonatite pulses were observed. Magmatic mineralisation (e.g. Nb, apatite, magnetite, and baddeleyite) is commonly hosted by early to intermediate magmatic pulses (Richardson and Birkett Citation1996a). Such mineralisation may be remobilised at later stages, possibly as a result of post-magmatic overprint (e.g. Aley carbonatite, British Columbia; Chakhmouradian et al. Citation2015). The REE mineralisation commonly post-dates Nb mineralisation (Richardson and Birkett Citation1996a), as exemplified by the dolomite carbonatite-hosted REE mineralisation at Wicheeda Lake deposit, British Columbia (Trofanenko et al. Citation2016), where the third generation dolomite and REE mineralisation is interpreted as hydrothermal. Another example of where Nb mineralisation is post-dated by REE mineralisation is the St. Honoré alkaline-carbonatite complex, Quebec. Here, the principal host to Nb mineralisation is a crescent-shaped, dolomite carbonatite cone sheet, surrounded in part by barren calcite carbonatite; the late core of this complex is strongly enriched in REE (Lafleur and Ayad Citation2012).
Although in most cases REE mineralisation is paragenetically late and appears hosted by carbonatites that could be considered carbohydrothermal, frequently cited research of Mariano (Citation1989a) suggests that, at the Mountain Pass REE deposit (California), carbonatite-hosted REE mineralisation is late magmatic based largely on textures. However, the lack of reported pyrochlore and columbite or their alteration products, which are common in typical magmatic carbonatites, and local enrichment in Ba suggests that by today's terminology, the host to REE (bastnaesite-parisite) mineralisation may be ‘carbohydrothermal’ in origin. A follow-up study may be justified to confirm Mariano's (Citation1989a) original findings and eliminate incertitude.
Carbonatite-associated igneous rocks
Most intrusive carbonatite occurrences are part of alkaline-carbonatite complexes and are spatially tied to one or more intrusive silicate rock groups including melilitolites, ijolites, alkali gabbros, feldspathoid syenites, syenites, kimberlites, and lamprophyres or their volcanic equivalents (Woolley and Kjarsgaard Citation2008a, Citation2008b). Worldwide, only 24% of carbonatite rocks are not part of alkaline-carbonatite complexes (Woolley and Kjarsgaard Citation2008a).
Peridotites and pyroxenites, commonly found near ijolites and melilitolites, are not considered a distinct association because they are interpreted as cumulates (Woolley and Kjarsgaard Citation2008a). Nevertheless, these rocks are important from the exploration point of view because they are commonly observed in deeply eroded alkaline-carbonatite complexes, providing indirect information about the depth of erosion and, by extension, the mineralisation potential of the complex (Frolov Citation1971; Epshteyn and Kaban’kov Citation1984).
Phoscorites are magnetite, olivine, apatite rocks usually associated with carbonatites (Le Maitre Citation2002) and ultramafic rocks of alkaline-carbonatite complexes (Woolley and Kjarsgaard Citation2008b). In some cases, there is gradation between ultramafic rocks and phoscorite (e.g. Yonghwa phoscorite-carbonatite pipe; Seo et al. Citation2016). The definition presented by Le Maitre (Citation2002), is very restrictive because olivine commonly retrogrades into pyroxene, amphibole, and serpentine. A much broader definition and classification of phoscorites are anchored in Russian literature (e.g. Yegorov Citation1993; Krasnova et al. Citation2004) and proposes that phoscorite should be redefined as a ‘plutonic ultramafic rock comprising magnetite, apatite, and one of the silicates, forsterite, diopside, or phlogopite’. According to Krasnova et al. (Citation2004), most of the 21 phoscorite (sensu lato) occurrences that they are aware of are mineralised. Should this broader definition be applied worldwide, the number of phoscorite occurrences would increase substantially. For example, in British Columbia, the Aley carbonatite and several carbonatites in the Blue River area would be considered to contain lenses or fragments of phoscorite (). This has important implications from an exploration point of view because, within several alkaline-carbonatite complexes, phoscorites are host to Nb, baddeleyite (natural zirconia), apatite and iron, with a potential to recover scandium as a co-product (Rudashevsky et al. Citation2004; Ivanyuk et al. Citation2016; Kalashnikov et al. Citation2016).
Figure 7. Phoscorite lenses and irregular pods (sensu Krasnova et al. Citation2004) consisting of magnetite with minor apatite, dolomite, and serpentine (possibly replacement of olivine) are enclosed in dolomite carbonatite, Aley carbonatite, British Columbia, Canada. Marker for scale measures 14 cm in length.

Recognition of carbonatite-related intrusive silicate rocks is also important. In some cases, carbonatite represents only a minor part of an alkaline-carbonatite complex. Consequently, recognising the spatial association of carbonatites to phoscorite and commonly associated intrusive silicate rocks substantially increases the size of an exploration target (; ).
Figure 8. The average surface area of carbonatites is approximately 3 km2. In most cases, the carbonatites are surrounded by alkali silicate rocks, which are surrounded by a zone of fenitisation. If an explorationist recognizes the carbonatite, related silicate rocks (∼2 km2), and associated fenitised zone (∼5 km2), the target area increases to 10 km2. This figure is based on data from 26 carbonatite complexes, with surface areas varying from 0.01 to 78.5 km2, listed in .
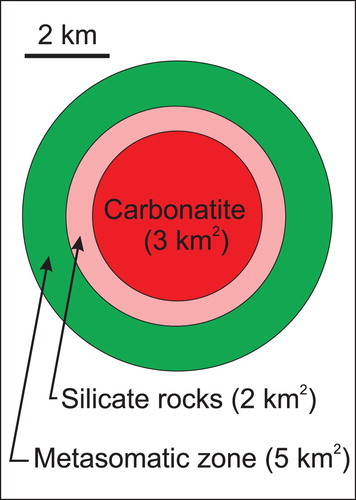
Table 1. Surface areas of selected carbonatite complexes in terms of carbonatite rocks, alkaline silicate rocks, and fenites.
Alkali metasomatism
Most intrusive carbonatites, alkaline-carbonatite complexes, and many agpaitic and miaskitic alkaline intrusions are surrounded by country rock affected by intrusion-related metasomatism. Metasomatism is defined as: ‘a solid state process by which the chemical composition of a rock is altered in a pervasive manner and which involves the introduction and/or removal of chemical components as a result of the interaction of the rock with fluids’ (Zharikov et al. Citation2007). Fenitisation is defined as: ‘a metasomatic process leading to the formation of fenites’; where fenite is described as: ‘a high-temperature metasomatic rock characterised by the presence of alkali feldspar, sodic amphibole and sodic pyroxene; nepheline, calcite and biotite/phlogopite may also be present and typical accessories are titanite and apatite’ (Zharikov et al. Citation2007). However, over the years, geological vocabulary has evolved and today, most of carbonatite and alkaline-carbonatite complex-related alkali metasomatism is broadly referred to as fenitisation or fenitisation-type metasomatism (Eckermann Citation1948; Le Bas Citation1981; Woolley Citation1982; Morogan Citation1994; Martin Citation2006, Liu et al. Citation2018). Fenitisation-type metasomatism is used here because it represents the compromise between those accepting the broad use of the term fenitisation (sensu lato) and purists insisting on the use of the term fenitisation (sensu stricto) as advocated by Zharikov et al. (Citation2007). Fenitisation-type metasomatism commonly consists of desilication accompanied by the addition of Na, K, Fe3+, ± Ca, ± Al to the host rock that surrounds carbonatites or carbonatite-alkaline complexes (; Morogan Citation1994; Williams-Jones and Palmer Citation2002; Smith Citation2007; Le Bas Citation2008). Other elements that may be introduced into country rock by fenitisation-type metasomatism are Ba, Nb, Sr, Sc, Rb, Zn, V (Le Bas Citation2008) and in some cases REE, and Nb. Such metasomatism may manifest itself by the development of Na- and K-amphiboles, aegirine-augite, K-feldspar, albite, perthite, mesoperthite, antiperthite, nepheline, and pale brown mica, and albite (). Minor and trace constituents commonly observed in rocks affected by fenitisation-type metasomatism are carbonates, apatite, barite, rutile, magnetite, titanite, pyrochlore, monazite-(Ce), bastnaesite-(Ce), parisite-(Ce), and goyazite. Most of the silica removed from the country rock during desilication migrates towards the intrusive () and from that point of view, the process may be considered as bimetasomatic (forming endocontact and exocontact zones), as defined by Zharikov et al. (Citation2007). Since 1921, a large number of alkaline-carbonatite complex related zones affected by fenitisation-type metasomatism have been studied and reviewed in terms of their morphology, extent, texture, mineralogy (Garson Citation1962; Woolley Citation1969, Citation1982; Le Bas Citation2008), major and trace element analysis of bulk rock (Kresten Citation1988; Morogan Citation1989, Citation1994), and mineral chemistry (Mian and Le Bas Citation1986). Similar to metasomatic zones related to better-documented mineralising systems such as porphyry copper (e.g. Sillitoe Citation2010; Hedenquist and Taran Citation2013) and massive sulphide deposits (e.g. Hannington, Citation2014), the extent and intensity of metasomatism related to carbonatites and alkaline-carbonatite complexes depends on large number of parameters including (1) Chemical composition, temperature, and pH of the fluids; (2) Chemical and mineralogical composition of country rock (protolith); (3) Permeability and porosity of the country rock; (4) Temperature gradient between the source of fluids and the country rock, (5) Fluid/rock ratio; (6) Duration of fluid movement, and (7) Others parameters (Korzhinskii, Citation1970; Zharikov et al. Citation2007; Elliot et al. Citation2018). From the geological mapper's and exploration geologist's point of view, the simplest and most efficient method to vector towards the source of fenitizing fluids is based on mineralogical observations. Under ideal conditions, fenitisation-type metasomatism and its intensity can be observed with the naked eye ( and ), using a hand lens or polarising microscope ().
Figure 9. Schematic representation of bi-metasomatic fenitisation-type interaction between carbonatite melt and related fluids with country rock. Direction of migration of elements is indicated by arrows. Minerals commonly observed in country rock affected by fenitisation-type metasomatism are listed.
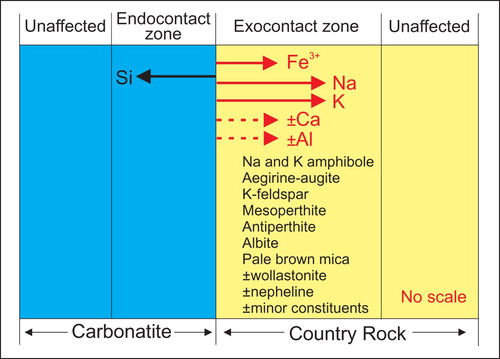
Figure 10. Nearly monomineralic, massive, K-feldspar-rich fenite locally observed at the contact of Lonnie carbonatite with metapelite country rock, British Columbia, Canada.

Figure 11. Green-blue rock consisting of Na-amphibole, Na-clinopyroxene, and feldspar produced by fenitisation-type metasomatism observed in contact with carbonatite (pale to medium brown, left upper corner of picture). The late centimeter-thick white vein, cutting green-blue rock, consists of calcite; Aley Nb deposit, British Columbia, Canada.

Figure 12. Variation in intensity of fenitisation-type metasomatism perpendicular to the strike of a 30 m thick carbonatite lens. Intensity of metasomatism decreases with increasing distance from calcite carbonatite contact, as recorded by disappearance of aegirine and Na-amphibole over the distance of approximately 25 m. Lonnie carbonatite, British Columbia, Canada.
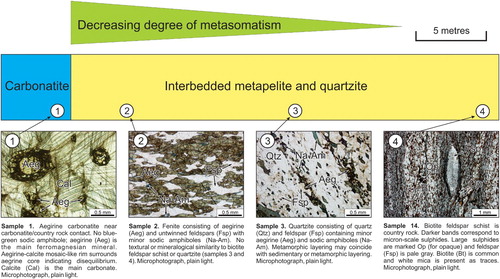
As with carbonatite-associated silicate rocks, recognising fenitisation-type metasomatic halos during exploration significantly increases the footprint of the carbonatite or alkaline-carbonatite complex (). The documented thickness of fenitisation-type metasomatc zones varies from a few centimetres to several kilometres and their shape is influenced to a large extent by the geometry of permeable zones open to metasomatic fluids.
Estimates of surface areas affected by fenitisation related to carbonatites or alkaline-carbonatite complexes based on maps in scientific and government publications are contained in . This data demonstrates that in some cases the fenitisation-type metasomatic aureole substantially increases the footprint of the carbonatite or alkaline-carbonatite complex. For example, at Panda Hill, Naantali, Callander Bay, Kangankkunde, and Ipanema, fenitisation represents more than 80% of the complex in terms of surface area.
For many alkaline-carbonatite complexes, it is difficult or impossible to determine if fenitisation-type metasomatism is related directly to the carbonatite or to the spatially associated ijolite rocks (Morogan Citation1994). Where more than one source of magmatic or carbohydrothermal fluids is involved, several fenitisation-type metasomatic patterns may overlap, complicating the vectoring procedure. For the purpose of this paper, we adopt the classification developed by Heinrich (Citation1985) and popularised by Le Bas (Citation2008), which divides fenites into ‘sodic’, ‘potassic’, and ‘sodic-potassic’ (an intermediate between the two end-members). Sodic fenites are characterised by the presence of Na-rich amphiboles and pyroxenes, and K- and Na-feldspars. In extreme cases of Na fenitisation, albitite rock will develop (Denaeyer Citation1966; Le Bas Citation2008). Within carbonatite complexes, sodic fenites are believed to form earlier, at higher temperatures, and at deeper levels than potassic fenites (Le Bas Citation2008). Potassic fenites consist mainly of K-rich feldspars (orthoclase or microcline), and in some cases may contain low-alumina phlogopite or biotite. Potassic fenites most commonly develop adjacent to or above the upper levels of intrusive calcite and dolomite carbonatites. These fenites plot in the leucite solidus field on the quartz-nepheline-kalsilite diagram (Le Bas Citation2008). Historically, it has been suggested that within alkaline-carbonatite complexes, early calcite carbonatites are accompanied by strong Na-K fenitisation-type metasomatism, and that there is no fenitisation-type metasomatism related to ferrocarbonatites (Le Bas Citation1987); however, there are a number of cases where ferrocarbonatite-related fenitisation-type metasomatism has been recognised, such as the Swartbooisdrift complex, Namibia (Drüppel et al. Citation2005) and the Gifford Creek ferrocarbonatite complex, Western Australia (Pirajno et al. Citation2014).
Morphology and geometry of alkaline-carbonatite complexes
This section contrasts the morphologies of undeformed carbonatites in unmetamorphosed intracratonic settings to those that are strongly deformed (pre-metamorphic) in high grade metamorphic terranes. In the latter case, there may be a need to use microscopy, geochemistry, stable isotopes, and rock associations to distinguish between carbonatites and carbonate metasediments.
Undeformed carbonatites in extensional settings
The classic carbonatite model ((a)) proposed by Garson and Smith (Citation1958) was popularised by Heinrich (Citation1980) and Bowden (Citation1985), and is still in use (e.g. Pirajno Citation2015). This model, based largely on field observations from Chilva alkaline province in southern Malawi, fits many complexes from Eastern Africa and elsewhere. More recent models ((b, c)) have been proposed by Le Bas (Citation1977, Citation1987), and Sage and Watkinson (Citation1991). The model of Le Bas (Citation1987) displays well the age relationships between lithological units and highlights fenitisation-type overprints (). The model produced by Sage and Watkinson (Citation1991) displays a limited number of lithologies () relative to the Garson and Smith (Citation1958) model; however, it better depicts the relationship between the volcanic edifice and crater facies. The erosional level shown in this model ((c)) corresponds to the current level of exposure displayed by carbonatites in southwest Quebec and Ontario, Canada.
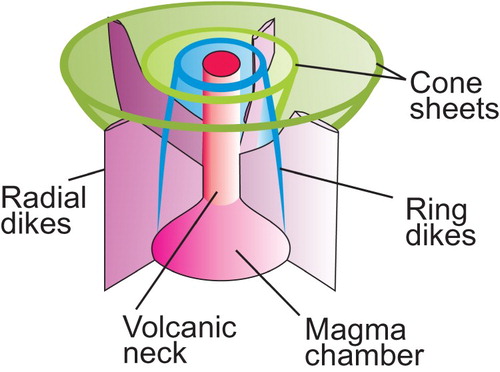
Figure 13. Morphology of carbonatite complexes as proposed by: (a) Garson and Smith (Citation1958); (b) Le Bas (Citation1987); and (c) Slightly modified from Sage and Watkinson (Citation1991) to show convex and concave nature of ring dikes and cone sheets, respectively. None of these models provides a complete picture; however, in combination these models provide a useful summary for exploration geologists.
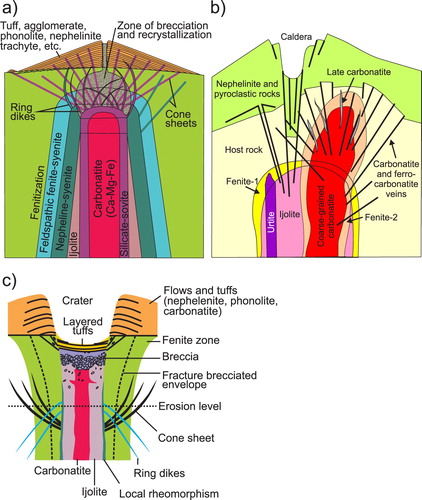
No model depicts all of the possible rock associations encountered in alkaline-carbonatite complexes, or is universally applicable. At deep erosion levels, carbonatites are commonly spatially associated with ultramafic rocks. At moderate levels, they are spatially associated with pyroxenites and jacupirangites, and with ijolites and nepheline syenites at progressively shallower levels (Garson and Smith Citation1958).
Regardless of the model that we adopt, subvolcanic (hypabyssal) rocks in carbonatite complexes commonly form radial dikes, cone sheets, and ring dikes (). Examples include Salpeterkop structure, South Africa (Verwoerd Citation1990); Alnö, Sweden (Eckermann Citation1942, Citation1948, Citation1966; Kresten Citation1980); Gardiner complex, Greenland (Nielsen Citation1980); and Oka, Canada (Gold et al. Citation1967). These dikes and cone sheets may consist of nephelinites, alkali-rich mafic rocks, melilitolites, phonolites, trachytes, lamprophyres, ijolites, or carbonatites. The emplacement of a magma chamber (carbonatite or otherwise) and its related radial dikes, cone sheets, ring dikes, and doming is fundamentally understood based on a combination of geological field observations, laboratory experiments, and numerical modelling (Koide and Bhattacharji Citation1975; Kresten Citation1980; Walter and Troll Citation2001; Klausen Citation2004; Bistacchi et al. Citation2012; Andersson et al. Citation2013). Where anisotropic regional (tectonic) stress exceeds intrusion-related stresses, steeply dipping or subvertical dike swarms are likely to form. Where local stresses related to a shallow magma chamber predominate, subvertical radial dikes; subvertical to outward dipping, semicircular to crescent shaped, nearly concentric ring dikes; and inward dipping, circular, oval, or crescent shaped, concentric cone sheets are expected to form (). These intrusion-related features can be used to vector towards mineralisation. Radial dikes are expected to intersect at the vertical projection of the intrusive centre. Because ring dikes are subvertical to steeply dipping, the outermost of these roughly coincide with the horizontal extent of the magma chamber. Cone sheets dip toward the intrusive centre. In most older models, the projections of the visible portions of cone sheets are interpreted to meet near a single point coinciding with an explosion focus, such as at Chilwa, Malawi (Garson and Smith Citation1958); Alnö, Sweden (Eckermann Citation1966); and Homa Mountain, Kenya (Le Bas Citation1977). In such models, the cone sheets farthest away from the magma chamber will have the shallowest dip; however, modelling by Bistacchi et al. (Citation2012), shows that at least in some cases cone sheets may be coaxial with the same apical angle. At the levels where magma pressure exceeds lithostratigraphic pressure, zones of brecciation develop and doming takes place. The volcanic edifices of carbonatites and related intrusions commonly consist of tuffs, agglomerates, and lava flows consisting of nephelinites, mellilite-bearing rocks, and, in some cases, phonolites and trachytes (Garson and Smith Citation1958).
Distinction between carbonatites and sedimentary carbonate rocks affected by high grade metamorphism and tectonic activity
Distinction between well exposed intrusive carbonatites and carbonate rocks of sedimentary origin does not present a problem in unmetamorphosed intracontinental extensional settings because the morphology of lithological units, cross-cutting relationships ((a, b, c); ), primary magmatic and carbohydrothermal textures, and mineralogical compositions are preserved (e.g. Barker Citation1993). Post-emplacement deformation significantly changes the shape of a carbonatite and related mineralised zones, and commonly associated metamorphism modifies the mineralogical, chemical, and textural characteristics of the ore and gangue minerals. This is especially true where dynamothermal metamorphism reaches upper amphibolite or granulite facies, at which point many distinguishing features are partially or completely obliterated. Examples include Dahomeyide suture zone, West Africa (Attoh et al. Citation2007); carbonatite-marble dikes of Abyan Province, Yemen Republic (Le Bas et al. Citation2004); metacarbonates from Paleoproterozoic collision zone of the Borborema province, NE Brazil (EJ dos Santos et al. Citation2013; RV Santos et al. Citation2013); and some carbonatites of the British Columbia alkaline province, Canada (a, b; Kulla and Hardy Citation2015). Both marbles derived from carbonatites and those from sedimentary carbonates may appear concordant with country rocks, be repeated by folding () or faulting, display metamorphic layering, and develop identical granoblastic, porphyroblastic, lepidoblastic, and mylonitic textures.
Figure 15. Morphology of the Upper Fir carbonatite, part of the Blue River carbonatite cluster, British Columbia, Canada shown in (a) vertical longitudinal section and (b) vertical cross-section perpendicular to longitudinal section [blue vertical line in (a)]. Metamorphosed carbonatites commonly appear concordant with lithological contacts and metamorphic layering within the country rock and are repeated by folding (simplified from Kulla and Hardy Citation2015).
![Figure 15. Morphology of the Upper Fir carbonatite, part of the Blue River carbonatite cluster, British Columbia, Canada shown in (a) vertical longitudinal section and (b) vertical cross-section perpendicular to longitudinal section [blue vertical line in (a)]. Metamorphosed carbonatites commonly appear concordant with lithological contacts and metamorphic layering within the country rock and are repeated by folding (simplified from Kulla and Hardy Citation2015).](/cms/asset/f381ba8e-ac01-4278-9e6e-349664a5c140/yaes_a_1516935_f0015_oc.jpg)
Figure 16. Folds within dolomite carbonatite highlighted by white apatite-rich layers; Aley Carbonatite, British Columbia, Canada.

Metacarbonates (marbles) of uncertain origin that are spatially related to alkaline silicate gneiss, blue–green amphibolite (preserved fenitisation zone), or pyroxenite should be suspected to have originated from a carbonatite protolith. Shoshonitic igneous rocks or their metamorphic equivalents commonly indicate a transition in tectonic regimes involving block faulting and uplift within island arcs (Morrison Citation1980) and have been reported in association with post-collisional carbonatites by Hou et al. (Citation2006), Chakhmouradian et al. (Citation2008), and Woodard and Hetherington (Citation2014). The presence of blue–green Na- and K- amphiboles, perovskite, pyrochlore, columbite-(Fe), and fersmite in marble is evidence of a carbonatite origin. Le Bas et al. (Citation2004) note that the presence of aluminous minerals such as anorthite, scapolite, and ‘fassaitic’ pyroxene are (subsilicic aluminium ferrian diopside; Ca(Mg,Fe3+,Al)(Si,Al)2O6) indicative of a sedimentary origin; however, relying on these minerals to be diagnostic of a non-carbonatite origin in metamorphosed rocks may be misleading, as carbonatites can incorporate Al-rich country rock xenoliths or xenocrysts, and volcanic carbonatites may have been in contact with an Al-rich regolith or interbedded with Al-rich sediments prior to metamorphism.
If diagnostic textural and mineralogical criteria are missing, geochemical analysis of REE, Nb, Sr, and Ba concentrations is recommended. Carbonatites are strongly enriched in light rare earth elements (LREE) and are characterised by steep chondrite-normalised REE patterns, without a Eu anomaly (). The [Sr + Ba]-[REE + Y] discrimination diagram () proposed by Samoilov (Citation1991) can also be used to discriminate between marble of sedimentary and carbonatitic origins.
Figure 17. Chondrite-normalised REE plot of representative samples from Aley cabonatite (Nb-deposit) and Wicheeda carbonatite (REE-deposit), British Columbia, Canada, and field of unmineralised carbonatites from Hornig-Kjarsgaard (Citation1998).
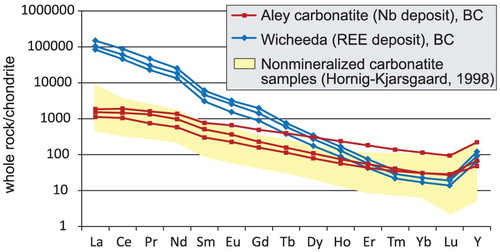
Figure 18. The [Sr + Ba] to [REE + Y] discriminative bivariate plot is one of the criteria recommended to distinguish carbonatites from marbles derived from carbonate sedimentary rocks (Samoilov Citation1991).
![Figure 18. The [Sr + Ba] to [REE + Y] discriminative bivariate plot is one of the criteria recommended to distinguish carbonatites from marbles derived from carbonate sedimentary rocks (Samoilov Citation1991).](/cms/asset/f938b0d9-69bf-489c-a6db-c5f676b87fe8/yaes_a_1516935_f0018_oc.jpg)
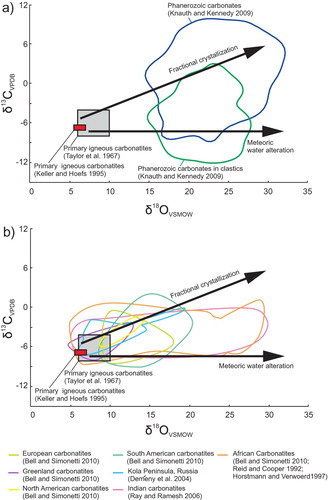
Isotopic composition can also be used to differentiate carbonatites from marbles of sedimentary origins. Ideally, magmatic carbonatites have well constrained isotopic compositions and on δ18O and δ13C diagrams plot within the primary carbonatite fields ((a)) defined by Taylor et al. (Citation1967) and Keller and Hoefs (Citation1995), easily distinguishing them from carbonates of sedimentary origin. However, carbonatites that are interpreted as primarily based on mineralogical criteria may plot outside of these fields, as illustrated on (b) by the data from Indian carbonatites from Ray and Ramesh (Citation2006). The horizontal trend (no change in δ13C), indicates the effects of alteration by meteoric water, whereas an increase in both δ18O and δ13C is commonly attributed to fractional crystallisation ((a)). If a marble plots within or near the primary carbonatite fields ((a, b)) or if the data form a trend originating from them, then this marble is likely derived from a carbonatite protolith, as is the case of several carbonatites metamorphosed to upper amphibolite facies in the British Columbia Alkaline province, Canada. If a marble has higher than expected δ18O and plots along the fractional crystallisation trend, it could have been derived from (1) a carbonatite protolith that interacted with late carbohydrothermal or metamorphic fluids; (2) metamorphism of carbonate-rich sedimentary rocks; or (3) metamorphism of hydrothermal carbonate bodies. Distinguishing metamorphosed carbonatites and carbohydrothermal carbonates from metacarbonates of sedimentary origin may be impossible if they plot within the Phanerozoic carbonates fields (e.g. African and Indian carbonatites; (a, b)).
Figure 19. δ18O and δ13C plots showing: (a) Composition of primary igneous carbonatites, fractional crystallisation and meteoric water alteration trends, and composition of Phanerozoic sedimentary carbonates and carbonates in clastic sedimentary rocks; and (b) Compositional fields of European, Greenland, South American, Indian, African, and Kola peninsula carbonatites. Compositional fields corresponding to African and Indian carbonatites are elongated along the trend representing alteration by interaction with meteoric water. The Kola Peninsula carbonatite field follows the trend representing fractional crystallisation. Chondrite value from McDonough and Sun (Citation1995Citation Citation Citation Citation Citation Citation Citation Citation).
Carbonatite-related ore deposits
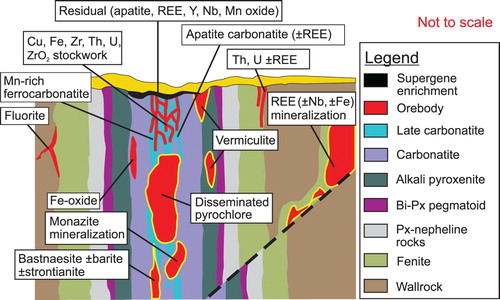
Carbonatites and alkaline-carbonatite complexes and, in some cases, associated fenitisation-type metasomatic zones and overlying regolith (including zones of supergene enrichment) are favourable hosts of metallic and industrial mineral deposits (). Reviews and compilations of carbonatite-related mineralisation are provided by Deans (Citation1966), Heinrich (Citation1980), Mariano (Citation1989a, b), Pell (Citation1996), Richardson and Birkett (Citation1996a, Citation1996b), Birkett and Simandl (Citation1999), Woolley and Kjarsgaard (Citation2008a), Berger et al. (Citation2009), Simandl (Citation2014), and Mackay and Simandl (Citation2014a, Citation2015).
Figure 20. Vertical section of a hypothetical carbonatite mineralising system displaying the relationship between metallic and industrial mineral deposits relative to lithological units and geological contacts. The ‘distal’ carbo-hydrothermal fluid-related mineralisation or hydrothermally remobilised mineralisation (away from alkaline-carbonatite complex) and residual deposits within weathered crust above the carbonatite complex are also highlighted. Bi – biotite, Px- pyroxene. Modified from Laznicka (Citation2006).
Alkaline-carbonatite complex-related ore deposits represent large resources in terms of REE ((a, b) and (a), e.g. Bayan Obo, China; Maoniuping, China; Mountain Pass, USA; and Mount Weld, Australia). Most of these deposits are strongly enriched in LREE and have a high LREE/REE(total) ratio relative to peralkaline intrusion-related, and ion adsorption clay deposits (Mariano Citation1989a, Simandl Citation2014; Verplank et al. Citation2016); however, they also contain significant resources of heavy rare earth elements (HREE; (b)). Under current market conditions, concentrates of REE-bearing minerals from typical alkaline-carbonatite complex related deposits are the main source of LREE. The ion adsorption clay deposits are low grade relative to alkaline–carbonatite intrusion-related- and peralkaline intrusion-related deposits and can't compete effectively against high-grade-carbonatite-related deposits as a source of LREE ((a, b)). However, because peralkaline complex-related REE deposits are metallurgically challenging (Verbaan et al. Citation2015), and carbonatite concentrates have a high LREE/REE(total) ratio, the REE-bearing ion adsorption deposits which benefit from simple and cost-effective metallurgy are currently the main source of HREE (Simandl Citation2014). With the exception of the LREE-enriched, loparite and eudialyte–bearing, nepheline-feldspar-aegirine pegmatite at Lovozero (Russia), there is currently no production of REE from peralkaline intrusion-hosted deposits.
Figure 21. Examples of main REE and Nb minerals and their concentrates; (a) Mixture of monazite [(Ce,La,Nd,Th)PO4] and bastnaesite [(Ce, La)(CO3)F] forms orange-brown fracture fillings in dolomite carbonatite; pen for scale; Wicheeda Lake prospect, British Columbia, Canada. (b) Bastnaesite concentrate containing 60–65 wt.% rare earth element oxides (REO), Mountain Pass carbonatite, California, USA; scale in millimetres. (c) Pyrochlore crystal [(Na,Ca)2Nb2O6(OH,F)] from the Upper Fir carbonatite, British Columbia, Canada; scale in millimetres (d) Pyrochlore concentrate, Niobec Mine, Quebec, Canada; scale in millimetres.
![Figure 21. Examples of main REE and Nb minerals and their concentrates; (a) Mixture of monazite [(Ce,La,Nd,Th)PO4] and bastnaesite [(Ce, La)(CO3)F] forms orange-brown fracture fillings in dolomite carbonatite; pen for scale; Wicheeda Lake prospect, British Columbia, Canada. (b) Bastnaesite concentrate containing 60–65 wt.% rare earth element oxides (REO), Mountain Pass carbonatite, California, USA; scale in millimetres. (c) Pyrochlore crystal [(Na,Ca)2Nb2O6(OH,F)] from the Upper Fir carbonatite, British Columbia, Canada; scale in millimetres (d) Pyrochlore concentrate, Niobec Mine, Quebec, Canada; scale in millimetres.](/cms/asset/2f0e7e77-34c3-4614-9c1f-c8c132cee0b4/yaes_a_1516935_f0021_oc.jpg)
Figure 22. Grade and tonnage of REE-bearing deposits associated with carbonatite complexes in terms of (a) Total REO (TREO) and (b) Heavy REO (HREO) relative to pegmatites, peralkaline complexes, and rare element granites. Updated from Simandl (Citation2014).
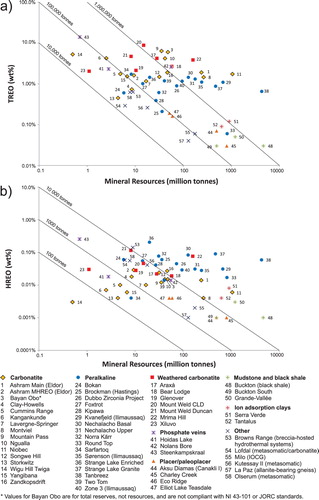
Alkaline-carbonatite complex-related REE mineralisation may consist of unweathered carbonatite rock such as at Mountain Pass, USA, (Castor Citation2008) and St-Honoré, Canada, (Lafleur and Ayad Citation2012) or corresponds to overlying regolith such as at Mount Weld, Australia (Lottermoser Citation1990) and Araxá, Brazil (Neumann and Medeiros Citation2015). The REE mineralisation tends to be concentrated in late carbonatite pulses forming central breccia zones, ring dikes, or cone sheets, and fracture and open space fillings within these features. The REE are probably concentrated in carbohydrothermal fluids and melts produced by fractional crystallisation from carbonatitic melts (Richardson and Birkett Citation1996a; Song et al. Citation2016). In several cases, the REE mineralisation is interpreted as distal to carbonatite or alkaline-carbonatite complex; however, in most of these cases the relation of REE mineralisation to the ultimate source of fluids from which it formed is not clear or is controversial (e.g. the famous Bayan Obo REE-Nb-Fe deposit in Central Mongolia; Smith et al. Citation2015; Lai et al. Citation2016; Liu et al. Citation2018). Based on the close spatial relationship of many REE deposits to faults, fractures, suture zones, breccias, and other paleo-permeable zones, in combination with paragenetic studies of ore minerals, and in agreement with supporting experimental evidence indicating that REE, Nb, and Ta can be transported by hydrothermal or carbo-hydrothermal fluids (e.g. Williams-Jones et al. Citation2012; Timofiev et al. Citation2015, Citation2017, Migdisov et al. Citation2016; Trofanenko et al. Citation2016), it is reasonable to expect the presence of REE (± Nb) deposits exploration targets distal to the alkaline-carbonatite complexes ().
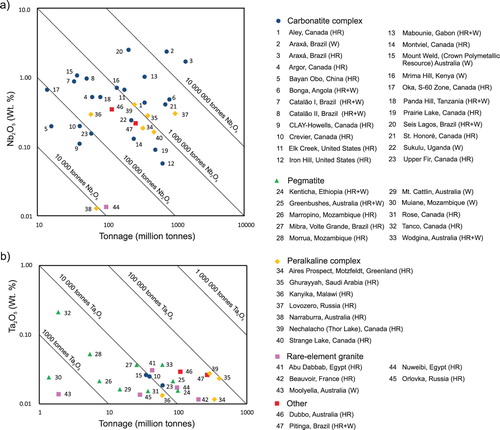
Alkaline-carbonatite complex-related deposits are also the main source of Nb ((c, d) and (a); e.g. Catalão, Brazil; Lueshe, Democratic Republic of Congo; and St. Honoré, Oka, and Aley, Canada) as summarised by Verwoerd (Citation1986), Mariano (Citation1989b), Berger et al. (Citation2009), Mackay and Simandl (Citation2014a), and Simandl et al. (Citation2018). The Nb mineralisation may be part of the carbonatite rock unit (e.g. St-Honoré, Canada; Tremblay et al. Citation2015), carbonatite-associated alkaline rock (e.g. Crevier deposit, Canada; Solgadi et al. Citation2015), fenitised zone, or overlying regolith (e.g. Catalão I, Brazil; Cordeiro et al. Citation2011; and Araxá, Brazil; Issa Filho et al. Citation2015). Although high Ta concentrations are expected mainly in pegmatites and peralkaline intrusion-related deposits (Mackay and Simandl Citation2014a), some deposits related to alkaline-carbonatite complexes have comparable Ta concentrations to pegmatites and contain significant resources of tantalum (e.g. Upper Fir carbonatite and Crevier alkaline-carbonatite complex, Canada; (b)). Unfortunately, under current market conditions, low Ta2O5/[Ta2O5 + Nb2O5] ratio in concentrates makes carbonatites unattractive for Ta extraction using conventional technology (Simandl et al. Citation2018).
Figure 23. Grade and tonnage of (a) Nb and (b) Ta deposits associated with carbonatite complexes relative to pegmatites, peralkaline complexes, and rare element granites. Diagonal lines indicate tonnage of contained Nb2O5 and Ta2O5. From Simandl et al. (Citation2018). Most grade and tonnage references are available in Mackay and Simandl (Citation2014a). Abbreviations: (HR) hard rock ore, (W) weathered ore, (HR+W) hard rock and weathered ore combined.
Vermiculite and phlogopite deposits are predominantly hosted by mafic or ultramafic rocks of the alkaline-carbonatite complex (e.g. Northern pyroxenite at Palabora, South Africa; Heinrich Citation1970; Fourie and De Jager Citation1986) near the contacts of carbonatites with these rocks, or within mafic country rocks (e.g. Upper Fir carbonatite, Canada; Simandl et al. Citation2010). Most carbonatite-related apatite deposits currently in production, such as Tapira, Brazil (Capponi et al. Citation2009); Ipanema, Brazil (Born Citation1986); Catalão I, Brazil (Carvalho and Bressan Citation1986); and Matongo, Burundi (Decree et al. Citation2015) were enriched by weathering. Examples of exceptions are the Siilinjärvi mine, Finland (O’Brien et al. Citation2015), and Cajati mine, Jacupiranaga Complex, Brazil (Alves Citation2008). Copper, U, Th, and baddeleyite (natural zirconia) were produced for decades from the Palabora carbonatite-phoscorite complex in South Africa (Heinrich Citation1970; Clarke Citation1981; Milani et al. Citation2017), but baddeleyite is currently produced only from the Kovdor deposit in Russia (Dickson Citation2015).
Other materials produced from carbonatites or related rocks are: iron (e.g. Kovdor, Russia; Dickson Citation2015; Bayan Obo, China; Smith et al. Citation2015; and Palabora, South Africa; Heinrich Citation1970); fluorite (e.g. Mato Preto, Brazil; Okorusu, Namibia; and Amba Dongar, India; Hagni Citation1999); carbonates for lime and cement production (e.g. Tororo, Uganda and Xiluvo, Mozambique; van Straaten Citation2002; and Jacupiranga, Brazil; Alves Citation2008); and sodalite for use as dimension, ornamental, and semi-precious stone (e.g. Swartboosdrift, Namibia; Menge Citation1986; and Cerro Sapo, Bolivia; Schultz et al. Citation2004). Some alkaline-carbonatite complexes, such as Tapira, Brazil (Swanepoel Citation2014) and Powder Horn, USA (resource of 350 million tonnes with an average grade of 11.5% TiO2; Van Gosen and Lowers Citation2007; Van Gosen Citation2009), contain titanium minerals, and were considered for future development. However, both projects stalled due to difficulties in producing market-acceptable TiO2 feedstock using conventional or alternative technologies.
In summary, carbonatites and alkaline-carbonatite complexes should be considered exceptional multi-commodity targets. Several of them host large deposits containing metals (Nb, Fe, Cu and a variety of noble metals as by-products), industrial minerals (e.g. apatite and vermiculite), construction materials required to build and maintain local infrastructure (crushed aggregate and dimension stone), and a variety of niche products such as badelleyite and sodalite, which is used as ornamental and semi-precious stone. Projects involving niche products (e.g. badelleyite and sodalite) are not ideal for large exploration and mining companies; however, such projects can sustain smaller communities and offer value-adding opportunities and related employment.
Exploration methods
Copper and Fe deposits within the Palabora alkaline-carbonatite complex were discovered and mined for copper and iron by African tribes about 1300 A.D. (Heinrich Citation1970), long before Bose (Citation1884) first described a carbonatite occurrence and before A.G. Högbom recognised the magmatic origin of carbonate rocks at the Alnö carbonatite complex in Sweden near the end of the nineteenth century (Heinrich Citation1980) using traditional prospecting. Worldwide, a significant number of carbonatites and related deposits were discovered after the Second World War by traditional prospecting and during regional geological mapping. The rate of discovery increased significantly between 1956 and 1966 due to technical improvements in airborne radiometric and magnetic survey technology in combination with aerial photointerpretation. This mid-century surge in the rate of discovery is apparent from the numbers of known carbonatites reported in a series of consecutive review studies: Pecora (Citation1956), Heinrich (Citation1958), Heinrich (Citation1966) and Woolley and Kjarsgaard (Citation2008a), listed 32, 60, 320, and 527 known carbonatite occurrences, respectively. Selected examples of the use of well-established methods (radiometric and magnetic) and new promising exploration methods are described below.
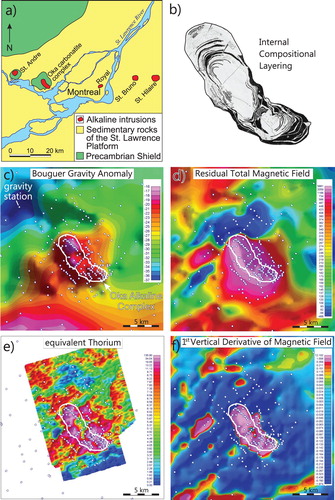
Alkaline-carbonatite complexes which have not been severely deformed by post-intrusive tectonic activity have oval, circular, ring-shaped, and crescent-shaped configurations of diagnostic rock units with contrasting geophysical properties. These characteristic morphological features are commonly detected by ground and airborne magnetic and radiometric surveys, and in some cases gravity surveys (Satterly Citation1970; Thomas et al. Citation2011, Citation2016; Shives Citation2015). The Oka alkaline-carbonatite complex, located approximately 15 km west of Montreal, Canada ((a)), is a particularly good example of applying these geophysical methods (Thomas et al. Citation2011). It is of Cretaceous age and is emplaced in the gneisses of the Canadian Shield exposed within an erosional window through flat lying Paleozoic sedimentary rock of the St. Lawrence Platform ((a)). The complex aligns roughly with Cretaceous Monteregian intrusions (e.g. St. Bruno). The complex is oval-shaped, approximately 10 km in length, and consists of two distinct lobes and numerous concentric ring dikes ((b); Gold et al. Citation1967). It hosted the St. Lawrence Columbium mine which operated from 1961 to 1977, and consisted of two open pits and underground workings. Significant Nb resources remain in the ground. The Bouguer gravity anomaly ((c)), residual total magnetic field ((d)), equivalent Th ((e)), and the first vertical derivative of the magnetic field ((f)) coincide closely with the outline of the complex.
Figure 24. Oka carbonatite complex, Quebec, Canada (a) Geological setting, (b) Internal compositional rings, (c) Bouger gravity anomaly, (d) Residual total magnetic field, (e) Equivalent thorium, and (f) First derivative of magnetic field. Modified from Thomas et al. (Citation2016).
In some cases, glacial dispersal of ore minerals down-ice from the deposit enhances the size of both the geophysical and geochemical exploration target. For example, the Allan Lake carbonatite in Ontario, Canada, which was discovered by airborne gamma-ray spectrometry (5 km line spacing), is a small unexposed carbonatite body approximately 0.4 km2 in area, not including the fenite zone. The discovery of such a small intrusion was possible because carbonatite-derived material was incorporated into till, increasing concentrations of Ba, Nb, Th, Ce, La, Zn, Mn, and Fe 10 to 20 times, and Y, P, Cu, Pb, Mo, Co, and U 5 to 10 times those of background levels. The initial Th anomaly detected by an airborne gamma-ray survey coincided with the Th-rich till and large Th-bearing boulders that were dispersed down-ice for nearly 5 km from the deposit (Ford et al. Citation1988).
Examples of other carbonatites with distinctive geophysical signatures are the Elk Creek (Nebraska, USA) and Catalão I (Brazil) intrusive alkaline-carbonatite complexes. Elk Creek is a semi-circular (6×8 km) intrusive complex covered by a 200 m thick sequence of sedimentary rocks. It hosts the largest known Nb resource in the United States and significant REE mineralisation (Drenth Citation2014). This complex coincides with a roughly annular vertical gravity gradient high, a subdued central low, and a magnetic high surrounded by magnetic lows (Drenth Citation2014). The highest Nb concentrations are encountered within a dolomitic carbonatite, and REE are concentrated in a barite-dolomite carbonatite. Catalão I alkaline-carbonatite complex hosts important Nb and phosphate deposits, and has been covered by radiometric, magnetic, and gravimetric surveys. Inversions of gravity and magnetic data reveal very similar models in terms of volume and shape of the complex (Mantovani et al. Citation2016), and highlight the value of integrated studies.
Remote sensing involves acquiring, processing, and interpreting spectral and spatial data from space and airborne platforms resulting from the interaction between matter and electromagnetic energy (Sabins Citation1997). In general, multi- and hyperspectral instruments are able to map host rock mineralogy based on material specific absorptions by vibrational processes in the Short-Wave Infrared (SWIR) region and are promising techniques for delineating carbonatites and associated REE mineralisation (e.g. Rowan et al. Citation1986; Boesche et al. Citation2015).
Limitations of using ASTER and similar multispectral instruments over the Mountain Pass REE deposit, California, are provided by Rowan and Mars (Citation2003). Due to limited spectral resolution, major mineralogy can be mapped, but minor differences (type of carbonate, accessory minerals) cannot. Hyperspectral remote sensing methods proved useful in outlining alkaline-carbonatite complexes that are not heavily covered by vegetation or glacial overburden such as the Sarfartoq carbonatite complex, southern West Greenland (Bedini Citation2009); Epembe, Namibia (Zimmermann et al. Citation2016); and the Khanneshin carbonatite, Afghanistan (Mars and Rowan Citation2011).
A review of the application of visible and shortwave infrared spectroscopy for the analysis of REE-bearing minerals is provided by Turner et al. (Citation2015). The REE minerals exhibit characteristic absorptions, primarily in the Visible to Near Infrared (VNIR) region. However, as these absorptions are caused by transitional processes in the atom, they are sharp and a high spectral resolution is required. Several laboratory studies (e.g. Rowan et al. Citation1986; Turner et al. Citation2015; Neave et al. Citation2016) suggest that Nd is the key pathfinder element when exploring directly for REE deposits. Neave et al. (Citation2016) indicate that hyperspectral instruments such as the Airborne Visible-Imaging Spectrometer (AVIRIS), HyMap, or Environmental Mapping and Analysis (EnMap) may provide the spectral resolution needed to detect targets containing more than 30,000 ppm Nd if datasets are collected at metre-scale spatial resolution. However, since most carbonatite-hosted mineralisation is rich in La and Ce relative to Nd (), and grades of carbonatite-related deposits do not normally exceed 10% REO(total) (), future technological refinements are required to make this approach adequate in exploration for REE deposits.
Limitations due to high detection limits of the above remote sensing methods in terms of total REE and Nd may be at least partially circumvented by taking advantage of recent advances in drone-borne technology. Booysen et al. (Citation2017) were able to detect Nd absorptions and differentiate REE enriched dikes at Lofdal Farm, Namibia, with a small snapshot hyperspectral camera (attached to drone) from 40 m altitude.
Soil, till, and stream-sediment geochemical surveys are among geochemical methods used in exploration for carbonatite and related deposits. Soil geochemistry, where applicable, is favoured for its ability to delineate or extend mineralised zones prior to extensive trenching or drilling programmes, as shown by the results of surveys over the Upper Fir Carbonatite, British Columbia, Canada (Kulla and Hardy Citation2015). Typically, conventional sampling of the B soil horizon followed by an aggressive sample dissolution method (such as fusion with sodium peroxide, lithium meta-borate, or lithium tetra-borate) prior to inductively coupled plasma mass or emission spectroscopy (ICP-MS/ES) is recommended for analysis, especially if Nb, Ta, REE, and Zr are of interest, because fusion-ICP-MS/ES measures the near total concentration of an element in the sample. Fusion-ICP-MS/ES analyses ensure that Nb, Ta, REE, and Zr present in weathering-resistant minerals are detected. Without total dissolution, anomalies corresponding to weathering-resistant minerals dispersed by mechanical processes such as hydraulic and glacial transport (Winterburn Citation2015) are unlikely to be detected. Additionally, ‘Mobile Metal Ion’ (MMI), designed for detecting mineral deposits below glacial overburden (e.g. clay, till, and sand), is a promising carbonatite exploration technique as illustrated by an orientation survey aiming to detect mineralisation over the Fen alkaline-carbonatite complex, Norway (Lie and Østergaard Citation2011). This method is based on the premise that metal ions released from blind oxidising mineralisation rise vertically due to capillary rise and evaporation, and are adsorbed into clay particles in the top 10–25 cm of the soil cover, allowing detection in soil samples by MMI technology (Mann Citation2007; Turner et al. Citation2007).
Biogeochemical exploration is another promising approach; however, carbonatite-related cases employing this method are exceedingly rare in western scientific and government reports. Two examples that the authors are familiar with are the orientation surveys over the Allan Lake carbonatite, Ontario, Canada (Ford et al. Citation1988) and the Upper Fir carbonatite, British Columbia, Canada (Fajber et al. Citation2015). Barium, P, Sr, Co, and Cu in leaf tissue of sugar maple trees were analysed for pathfinder minerals at Allan Lake. Vegetation over the carbonatite showed concentrations of 13 times the background concentrations for Ba, and 21 times for Sr (Ford et al. Citation1988). Lanthanum, Ce, Pr, Nd, Sm, Dy, Fe, Nb, Ta, P, and Y were identified by Fajber et al. (Citation2015) as preferred pathfinder elements in spruce and fir twigs and needles at the Upper Fir carbonatite. This study also showed that, in contrast to the absence of Eu anomalies in REE profiles of carbonatite rocks, the chondrite normalised REE profiles of vegetation and soil over the carbonatite may display strong negative Eu anomalies (Fajber et al. Citation2015). Although Nb is considered relatively immobile in surface environments, Nb and U anomalies in humus corresponding to major structural features within the Sokli alkaline complex, Finland, were documented by Vuotovesi et al. (Citation1980).
Carbonatites and related mineralisation are known to have detectable signatures in till (e.g. Allan Lake, Ontario; Ford et al. Citation1988), glaciofluvial sediments (e.g. Sokli carbonatite complex, Finland; Perttunen and Vartiainen Citation1992), and stream-sediments (e.g. Aley carbonatite, British Columbia, Canada; Mackay and Simandl Citation2014b). An important development in recent years was the commercialisation of hand held XRF instruments and analytical protocols permitting the analysis of many key pathfinder elements such as La, Ce, Nd, Pr, Y, Nb, Th, Sr, and Ba in concentrations required for carbonatite exploration (Simandl et al. Citation2014a, Citation2014b, Citation2014c). Hand held XRF significantly reduces the costs of lithogeochemical, soil, and stream-sediment sample analyses, and increases exploration efficiency by providing real-time data in the field.
Recent studies demonstrated that high density (ϱ > 3.9 g/cm3) Nb- and REE-bearing minerals commonly present in carbonatites, such as pyrochlore, columbite-(Fe), fersmite, monazite, and REE-bearing fluorocarbonates, are easy to concentrate using shaking tables or other gravity-based separators. Most of these minerals are not ideal for visual identification, and in many cases occur in grain size fractions too small for hand-picking; however, they can be identified by one of several automated measurement techniques based on Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS). Orientation surveys over three known carbonatite deposits that combine stream-sediment geochemistry and automated quantitative scanning electron microscopy of stream sediments suggest that carbonatite-related Nb mineralisation can be detected more than 11 km downstream (Mackay et al. Citation2015, Citation2016). This approach, in combination with newly developed pyrochlore and columbite-Fe discrimination diagrams (Mackay and Simandl Citation2015) and use of the ‘direct indicator mineral concept’ (Simandl et al. Citation2017) may popularize the use of indicator minerals for detection of carbonatites and exploration for related Nb and REE deposits. Apatite may become particularly useful indicator mineral (Mao et al. Citation2016; Chakhmouradian et al. Citation2017).
The ‘direct indicator mineral’ concept combines low cost stream-sediment surveys and automated scanning electron microscopy to replace traditionally expensive indicator mineral surveys, eliminating the need for large samples and mineral hand-picking. When this approach is applied, only sediment samples anomalous in carbonatite pathfinder elements (detected using pXRF), with or without gravity pre-concentrating, need to be analysed by automated quantitative scanning electron microscopy to determine from which deposit-type direct indicator minerals were derived.
Successful exploration and development of carbonatite-related deposits: what are the odds?
The exploration industry estimates that less than one in 1000 discoveries become a profitable mine (Marshall Citation2012; Preston Citation2015). According to the Ontario Mining Association (Citation2015), the odds are even lower: ‘Only about one mineral exploration project in ten is taken to the drill stage, and one drill programme in 1000 finds a viable mineral deposit’. Data compiled by Woolley and Kjarsgaard (Citation2008a) indicates that approximately 6% of the 527 reported carbonatites and alkaline-carbonatite complexes host active mines, 3% hold historic mines, and 11% contain an established mineral resource. These numbers suggest that carbonatites have exceptional potential, and that a newly discovered alkaline-carbonatite complex has a 9 out of 100 probability of hosting a mine.
Conclusion
Carbonatite-related deposits are the main sources of Nb and LREE; significant sources of baddeleyite, Fe, Cu, vermiculite, phlogopite, fluorite, apatite, calcium carbonate, and sodalite; and have historically produced U and Th. Given that 9 out of 100 carbonatites and alkaline-carbonatite complexes contain currently producing or historic mines, carbonatite-related mineralising systems represent outstanding multi-commodity exploration targets.
Knowledge of tectonic setting, typical rock associations, and deposit morphology can assist explorationists seeking carbonatites and related deposits. Statistically, the most favourable exploration areas for carbonatites and related deposits are intracratonic rift (extensional) settings; however, most outcropping, or near-surface intrusive carbonatites and alkaline-carbonatite complexes in these settings are likely already discovered. The search for carbonatites in orogenic settings is not favoured by current statistics, but Bayan Obo, the world's largest REE-Fe-Nb deposit, is found in such a setting. Strongly metamorphosed and deformed carbonatites were historically difficult to identify; consequently, most new intrusive carbonatite discoveries will probably be in collisional settings. Identifying and incorporating carbonatite-related silicate igneous rocks and fenitisation-type metasomatic zones into carbonatite models significantly increase the size of exploration targets. Under ideal conditions, the intensity of fenitisation-type metasomatism may also be used to vector toward the source of metasomatising fluids. Knowledge of carbonatite morphology (spatial relationships between ring dikes, radial dikes, and cone sheets) permits vectoring toward the intrusive centre in undeformed geological settings; however, applications of these relationships in deformed terrains can be problematic at best. Age alone is not a valid parameter of prospectivity; however, within the same complex, the latest carbonatite pulses are commonly enriched in REE.
Although there is no universally applicable method used in exploration for carbonatites and related mineral deposits, on the regional or reconnaissance-scale, airborne geophysical methods (mainly radiometric and magnetic) are proven to be able to locate and delineate carbonatites. Ground radiometric and magnetic methods are useful for follow-up surveys. A variety of geochemical (e.g. stream-sediment, soil, and till chemistry; ‘direct indicator minerals’) and biochemical methods have been successfully used, tested, or customised for exploration and detection of carbonatites and related deposits.
Acknowledgements
This project was supported by the Targeted Geoscience Initiatives 4 (2010–2015) and 5 (2016–2020), a Natural Resources Canada programme. The Specialty Metal components of these initiatives are collaborative efforts between the Geological Survey of Canada and the British Columbia Geological Survey, Victoria, BC. Reviews and drafting by Pearce Luck, Michaela Neetz, and Carlee Akam from the British Columbia Geological Survey were essential for completion of this manuscript. Sections covering geochemical, geophysical and remote sensing exploration methods were reviewed for completeness by Ray Lett, Geochemical Consultant (Victoria), Michael Thomas from the Geological Survey of Canada (Ottawa), and Robert Zimmermann from Helmholtz Institute Freiberg for Resource Technology (Freiberg, Germany). The section on genesis benefited from constructive comments by Alexei Rukhlov, British Columbia Geological Survey, Victoria, BC, Canada. Encouragement from Alan Galley, Malleus Consulting Corp., Ottawa, ON., Mike Villeneuve, Geological Survey of Canada (Ottawa, ON), and David Lefebure formerly of the British Columbia Geological Survey and currently with Geologic Ltd. (Salt Spring Island, BC, Canada) were essential for initiating and completing this document. The final version of this manuscript benefited from suggestions and comments of three anonymous reviewers and handling by Simon Jowitt, co-editor of Applied Earth Science.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alves PR. 2008. The carbonatite-hosted apatite deposit of Jacupiranga, SE Brazil: styles of mineralization, ore characterization and association with mineral processing [unpublished master’s thesis]. Rolla (MO): Missouri University of Science and Technology. [accessed 2017 Apr 20]. http://scholarsmine.mst.edu/cgi/viewcontent.cgi?article=7722&context=masters_theses.
- Andersson M, Malehmir A, Troll VR, Dehghannejad M, Juhlin C, Ask M. 2013. Carbonatite ring-complexes explained by caldera-style volcanism. Sci Rep. 3(1677):1–9.
- Andrade FRD, Möller P, Lüders V, Dulski P, Gilg HA. 1999. Hydrothermal rare earth elements mineralization in the Barra do Itapirapuã carbonatite, southern Brazil: behaviour of selected trace elements and stable isotopes (C, O). Chem Geol. 155:91–113. doi: 10.1016/S0009-2541(98)00143-0
- Andreeva IA, Kovalenko VI, Kononkova NN. 2006. Natrocarbonatitic melts of the Bol’shaya Tagna Massif, the eastern Sayan region. Dokl Earth Sci. 408:542–546. doi: 10.1134/S1028334X06040088
- Attoh K, Corfu F, Nude P. 2007. U–Pb zircon age of deformed carbonatite and alkaline rocks in the Pan-African Dahomeyide suture zone, West Africa. Precambrian Res. 155:251–260. doi: 10.1016/j.precamres.2007.02.003
- Bailey DK. 1974. Continental rifting and alkaline magmatism. In: Sorensen H, editor. The alkaline rocks. New York (NY): Wiley; p. 148–159.
- Bailey DK. 1977. Lithosphere control of continental rift magmatism. J Geol Soc Lond. 133:103–106. doi: 10.1144/gsjgs.133.1.0103
- Bailey DK. 1992. Episodic alkaline activity across Africa: implications for the causes of continental break-up. Geol Soc Lond Spec Publ. 68:91–98. doi: 10.1144/GSL.SP.1992.068.01.06
- Barbosa ESR, Brod JA, Junqueira-Brod TC, Dantas EL, Cordeiro PFO, Gomide CS. 2012. Bebedourite from its type area (Salitre I complex): a key petrogenetic series in the Late-Cretaceous Alto Paranaíba kamafugite-carbonatite-phoscorite association, Central Brazil. Lithos. 144–145:56–72. doi: 10.1016/j.lithos.2012.04.013
- Barker DS. 1993. Diagnostic magmatic features in carbonatites: implications for the origins of dolomite- and ankerite-rich carbonatites. South Afr J Geol. 96:131–138.
- Basu NK, Mayila A. 1986. Petrographic and chemical characteristics of the Panda Hill carbonatite complex, Tanzania. J Afr Earth Sci. 5:589–598.
- Bedini E. 2009. Mapping lithology of the Sarfartoq carbonatite complex, southern West Greenland, using HyMap imaging spectrometer data. Remote Sens Environ. 113:1208–1219. doi: 10.1016/j.rse.2009.02.007
- Bell K, editor. 1989. Carbonatites: genesis and evolution. London (UK): Unwin Hyman.
- Bell K. 2001. Carbonatites: relationships to mantle-plume activity. In: Ernst RE, Buchan KL, editors. Mantle plumes: their identification through time. Geol Soc Am Spec Pap. 352:267–290.
- Bell K. 2005. Igneous rocks: carbonatites. In: Selley RC, Cocks LRM, Plimer IR, editors. Encyclopedia of geology. Vol. 3. Oxford (UK): Elsevier; p. 217–233.
- Bell K, Rukhlov AS. 2004. Carbonatites from the Kola Alkaline Province: origin, evolution and source characteristics. In: Wall F, Zaitsev AN, editors. Phoscorites and carbonatites from mantle to mine: the key example of the Kola Alkaline Province. Mineral Soc Ser. 10. Twickenham: Mineralogical Society of Great Britain and Ireland; p. 33–468.
- Bell K, Simonetti A. 2010. Source of parental melts to carbonatites-critical isotopic constraints. Mineral Petrol. 98:77–89. doi: 10.1007/s00710-009-0059-0
- Bell K, Tilton GR. 2001. Nd, Pb and Sr isotopic compositions of east African carbonatites: evidence for mantle mixing and plume inhomogeneity. J Petrol. 42:1927–1945. doi: 10.1093/petrology/42.10.1927
- Bell K, Tilton GR. 2002. Probing the mantle: the story from carbonatites. Eos Trans Am Geophys Union. 83(273):276–277.
- Berger VI, Singer DA, Orris GJ. 2009. Carbonatites of the world, explored deposits of Nb and REE – database and grade and tonnage models. US Geological Survey Open-File Report 2009–1139.
- Birkett TC, Simandl GJ. 1999. Carbonatite associated deposits: magmatic, replacement and residual. In: Simandl GJ, Hora ZD, Lefebure DV, editors. Selected British Columbia mineral deposit profiles. Vol. 3, Industrial minerals and gemstones. Victoria (BC): British Columbia Ministry of Energy, Mines and Petroleum Resources; British Columbia Geological Survey Open File 1999–10.
- Bistacchi A, Tibaldi A, Pasquarè FA, Rust D. 2012. The association of cone-sheets and radial dikes: data from the Isle of Skye (UK), numerical modelling, and implications for shallow magma chambers. Earth Planet Sci Lett. 339–340:46–56. doi: 10.1016/j.epsl.2012.05.020
- Boesche NK, Rogass C, Lubitz C, Brell M, Herrmann S, Mielke C, Tonn S, Appelt O, Altenberger U, Kaufmann H. 2015. Hyperspectral REE (rare earth element) mapping of outcrops – applications for neodymium detection. Remote Sens. 7:5160–5186. doi: 10.3390/rs70505160
- Bonadiman C, Beccaluva L, Coltorti C, Siena F. 2005. Kimberlite-like metasomatism and ‘garnet signature’ in spinel-peridotite xenoliths from Sal, Cape Verde archipelago: relics of a subcontinental mantle domain within the atlantic oceanic lithosphere? J Petrol. 46:2465–2493. doi: 10.1093/petrology/egi061
- Booysen R, Gloaguen R, Zimmermann R, Jakob S. 2017. Drone-borne hyperspectral remote sensing of REE deposits in Namibia [abstract]. 10th EARSeL SIG Imaging Spectroscopy Workshop; Apr 19–21; Zurich (CH).
- Born H. 1986. The Ipanema phosphate deposit, São Paulo, Brazil. In: Notholt AJG, Sheldon RP, Davidson DF, editors. Phosphate deposits of the world. Vol. 2, Phosphate Rock Resource. Cambridge (UK): Cambridge University Press; p. 116–119.
- Bose PN. 1884. Geolgoy of the Lower Narbada Valley between Nimáwar and Káwant. Geol Surv India Mem. 21:1–72.
- Bowden P. 1985. The geochemistry and mineralization of alkaline ring complexes in Africa (a review). J Afr Earth Sci. 3:17–39.
- Capponi L, Peroni RDL, Grasso CB, Fontes SB. 2009. Sampling protocol review of a phosphate ore mine to improve short term mine planning. In: The Southern African Institute of Mining and Metallurgy Paper 40. Fourth World Conference on Sampling and Blending; Oct 21–23; Cape Town, South Africa. Johannesburg (ZA): The Southern African Institute of Mining and Metallurgy. p. 65–72.
- Carvalho WT, Bressan SR. 1986. The phosphate deposit of Catalão I ultramafic alkaline complex, Goiás, Brazil. In: Notholt AJG, Sheldon RP, Davidson DF, editors. Phosphate deposits of the world. Vol. 2, Phosphate Rock Resource. Cambridge (UK): Cambridge University Press; p. 104–110.
- Castor SB. 2008. The Mountain Pass rare-earth carbonatite and associated ultrapotassic rocks, California. Can Miner. 46:779–806. doi: 10.3749/canmin.46.4.779
- Chakhmouradian AR, Mumin AH, Demény A, Elliott B. 2008. Postorogenic carbonatites at Eden Lake, Trans-Hudson Orogen (northern Manitoba, Canada): geological setting, mineralogy and geochemistry. Lithos. 103:503–526. doi: 10.1016/j.lithos.2007.11.004
- Chakhmouradian AR, Reguir EP, Kressall RD, Crozier J, Pisiak LK, Sidhu R, Yang P. 2015. Carbonatite-hosted niobium deposit at Aley, northern British Columbia (Canada): mineralogy, geochemistry and petrogenesis. Ore Geol Rev. 64:642–666. doi: 10.1016/j.oregeorev.2014.04.020
- Chakhmouradian AR, Reguir EP, Zaitsev AN, Couëslan C, Xu C, Jindřich Kynický J, Mumin AH, Yang P. 2017. Apatite in carbonatitic rocks: compositional variation, zoning, element partitioning and petrogenetic significance. Lithos. 274-275:188–213. doi: 10.1016/j.lithos.2016.12.037
- Chen W, Kamenetsky VS, Simonetti A. 2013. Evidence for the alkaline nature of parental carbonatite melts at Oka complex in Canada. Nat Commun. 4:2687. doi: 10.1038/ncomms3687
- Cheng Z, Zhang Z, Hou T, Santosh M, Chen L, Ke S, Xu L. 2017. Decoupling of Mg-C and Sr-Nd-O isotopes traces the role of recycled carbon in magnesiocarbonatites from the Tarim Large Igneous Province. Geochim Cosmochim Acta. 202:159–178. doi: 10.1016/j.gca.2016.12.036
- Clarke G. 1981. The Palabora complex – triumph over low grade ores. Ind Miner. 13:45–62.
- Clarke MGC, Roberts B. 1986. Carbonated melilitites and calcitized alkalicarbonatites from Homa Mountain, western Kenya: A reinterpretation. Geol Mag. 123:683–692. doi: 10.1017/S0016756800024195
- Cooper AF, Gittins J, Tuttle OF. 1975. The system Na2CO3-K2CO3-CaCO3 at 1 kilobar and its significance in carbonatite petrogenesis. Am J Sci. 275:534–560. doi: 10.2475/ajs.275.5.534
- Cordeiro PFO, Brod JA, Palmieri M, de Oliveira CG, Barbosa ESR, Santos RV, Gaspar JC, Assis LC. 2011. The Catalão I niobium deposit, central Brazil: resources, geology and pyrochlore chemistry. Ore Geol Rev. 41:112–121. doi: 10.1016/j.oregeorev.2011.06.013
- Corrigan D, Pehrsson S, Wodicka N, De Kemp E. 2009. The palaeoproterozoic trans-Hudson Orogen: a prototype of modern accretionary processes. In: Murphy JB, Keppie JD, Hynes AJ, editors. Ancient orogens and modern analogues. The Geological Society of London Special Publications 327. London (UK): The Geological Society; p. 457–479.
- Currie K L, Ferguson J. 1971. A study of fenitisation around the alkaline carbonatite complex at Callander Bay, Ontario, Canada. Can J Earth Sci. 8:498–517. doi: 10.1139/e71-053
- Dalton JA, Presnall DC. 1998. Carbonatitic melts along the solidus of model lherzolite in the system CaO-MgO-Al2O3-SiO2-CO2 from 3 to 7 GPa. Contrib Mineral Petrol. 131:123–135. doi: 10.1007/s004100050383
- Deans T. 1966. Economic mineralogy of African carbonatites. In: Tuttle OF, Gittins J, editor. Carbonatites. New York (NY): Wiley; p. 358–413.
- Deans T, Roberts B. 1984. Carbonatite tuffs and lava clasts of the Tinderet foothills, western Kenya: a study of calcified natrocarbonatites. J Geol Soc Lond. 141:563–580. doi: 10.1144/gsjgs.141.3.0563
- Decree S, Boulvais P, Tack L, Andre L, Baele J-M. 2015. Fluorapatite in carbonatite related phosphate deposits: the case for the Matongo carbonatite (Burundi). Miner Depos. 50:1–14. doi: 10.1007/s00126-014-0576-6
- Demény A, Sitnikova MA, Karchevsky PI. 2004. Stable C and O isotope compositions of carbonatite complexes of the Kola Alkaline Province: phoscorite-carbonatite relationships and source compositions. In: Wall F, Zaitsev AN, editors. Phoscorites and carbonatites from mantle to mine: the key example of the Kola Alkaline Province. The Mineralogical Society of London Series 10. London (UK): The Mineralogical Society; p. 407–431.
- Denaeyer ME. 1966. Sur la présence d’une carbonatite ankéritique (rauhaugite) en bordure alcalin de Kirumba (Kivu) [On the occurrence of ankerite carbonatite (rauhaugite) on alkaline boarder of Kirumba (Kivu)]. C R Acad Sci. 263:9–12. French.
- Dickson JS. 2015. Eurochem reveals phosphate expansion plans at Kovdor and beyond. Ind Miner. 573:18.
- Drenth BJ. 2014. Geophysical expression of a buried niobium and rare earth element deposit: The Elk Creek carbonatite, Nebraska, USA. Interpretation. 2:SJ23–SJ33. doi: 10.1190/INT-2014-0002.1
- Drüppel K, Hoefs J, Okrusch M. 2005. Fenitizing processes induced by ferrocarbonatite magmatism at Swartbooisdrift, NW Namibia. J Petrol. 46:377–406. doi: 10.1093/petrology/egh081
- Duke GI. 2009. Black Hills-Alberta carbonatite-kimberlite linear trend: Slab edge at depth? Tectonophysics. 464:186–194. doi: 10.1016/j.tecto.2008.09.034
- Duke GI, Carlson RW, Frost CD, Hearn BC Jr, Eby GN. 2014. Continent-scale linearity of kimberlite-carbonatite magmatism, mid-continent North America. Earth Planet Sci Lett. 403:1–14. doi: 10.1016/j.epsl.2014.06.023
- von Eckermann H. 1942. Ett preliminärt meddelande om nya forskningsrön inom Alnö alkalina område [A preliminary notice of new research in Alno alkaline range]. Geologiska Föreningen i Stockholm Förhandlingar. 64:399–455. Swedish. doi: 10.1080/11035894209445103
- von Eckermann H. 1948. The alkaline district of Alnö Island. Sveriges Geologiska Undersèokning. Ser. Ca. 36:176p.
- von Eckermann H. 1966. Progress of research on the Alnö carbonatite. In: Tuttle OF, Gittins J, editor. Carbonatites. New York (NY): J Wiley and Sons, Interscience publications; p. 3–31.
- Eggler DH. 1978. The effect of CO2 upon partial melting of peridotite in the system Na2O-CaO-Al2O3-MgO-SiO2-CO2 to 35 kb, with an analysis of melting in a peridotite-H2O-CO2 system. Am J Sci. 278:305–343. doi: 10.2475/ajs.278.3.305
- Elliott HAL, Wall F, Chakhmouradian AR, Siegfried PR, Dahlgren S, Weatherley S, Finch AA, Marks MAW, Dowman E, Deady E. 2018. Fenites associated with carbonatite complexes: a review. Ore Geol Rev. 93:38–59. doi: 10.1016/j.oregeorev.2017.12.003
- Epshteyn YM, Kaban’kov VY. 1984. The depth of emplacement and mineral potential of ultramafic, ijolite, and carbonatite plutons. Int Geol Rev. 26:1402–1415. doi: 10.1080/00206818409466660
- Ernst RE, Bell K. 2010. Large igneous provinces (LIPS) and carbonatites. Mineral Petrol. 98:55–76. doi: 10.1007/s00710-009-0074-1
- European Commission. 2017. Report on critical raw materials for the EU. [accessed 2018 June 10]. https://publications.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1/language-en.
- Fajber R, Simandl GJ, Luck P. 2015. Exploration for carbonatite-hosted niobium-tantalum deposits using biogeochemical methods (orientation survey), Blue River Area, British Columbia, Canada. In: Lasemi Z, editor. Proceedings of the 47th Forum on the Geology of Industrial Minerals; May 15–17; Champaign (IL): Illinois State Geological Survey, Circular 587.
- Ferrero S, Wunder B, Ziemann MA, Wälle M, O’Brien PJ. 2016. Carbonatitic and granitic melts produced under conditions of primary immiscibility during anataxis in the lower crust. Earth Planet Sci Lett. 454:121–131. doi: 10.1016/j.epsl.2016.08.043
- Ford KL, Dilabio RNW, Rencz AN. 1988. Geological, geophysical and geochemical studies around the Allan Lake carbonatite, Algonquin Park, Ontario. J Geochem Explor. 30:99–121. doi: 10.1016/0375-6742(88)90054-4
- Fourie PJ, De Jager DH. 1986. Phosphate in the Phalaborwa complex. In: Anhaeusser CR, Maske S, editors. Mineral deposits of Southern Africa. Vol. II. Johannesburg (ZA): The Geological Society of South Africa; p. 2239–2253.
- Frolov AA. 1971. Vertical zonation in deposition of ore, as in ultrabasic-alkaline rocks and carbonatites. Int Geol Rev. 13:685–695. doi: 10.1080/00206817109475486
- Garson MS. 1962. The Tundulu carbonatite ring-complex in southern Nyasaland. Nyasaland [Malawi]: Nyasaland Geological Survey Department, memoir 2.
- Garson MS, Smith WC. 1958. Chilwa Island. Nyasaland [Malawi]: Nyasaland Geological Survey Department, memoir 1.
- Gittins J, Harmer RE. 1997. What is ferrocarbonatite? A revised classification. J Afr Earth Sci. 25:159–168. doi: 10.1016/S0899-5362(97)00068-7
- Gold DP, Vallée M, Charette JP. 1967. Economic geology and geophysics of the Oka Alkaline complex, Quebec. Can Inst Min Metall Bull. 60:1131–1144.
- Guarino V, Azzone RG, Brotzu P, Gomes CB, Melluso L, Morbidelli L, Ruberti E, Tassinari CCG, Brilli M. 2012. Magmatism and fenitization in the Cretaceous potassium-alkaline-carbonatitic complex of Ipanema São Paulo State, Brazil. Mineral Petrol. 104:43–61. doi: 10.1007/s00710-011-0168-4
- Gunthorpe RD, Bueger AD. 1986. Geology and economic evaluation of the Otjisazu alkaline igneous complex, central South West Africa/Namibia. In: Anhaeusser CR, Maske S, editor. Mineral deposits of Southern Africa. Johannesburg (ZA): Geological Society of South Africa; p. 2255–2260.
- Guzmics T, Mitchell RH, Szabo C, Berkesi M, Milke R, Abart R. 2011. Carbonatite melt inclusions in coexisting magnetite, apatite and monticellite in Kerimasi calciocarbonatite, Tanzania: melt evolution and petrogenesis. Contrib Mineral Petrol. 161:177–196. doi: 10.1007/s00410-010-0525-z
- Hagni RD. 1999. Mineralogy of beneficiation problems involving fluorspar concentrates from carbonatite-related fluorspar deposits. Mineral Petrol. 67:33–44. doi: 10.1007/BF01165114
- Hannington MD. 2014. Volcanogenic massive sulphide deposits. In: Holland H, Turekian K, editor. Treatise on geochemistry. Amsterdam (Netherlands): Elsevier; p. 463–487.
- Harmer RE, Gittins J. 1998. The case for primary, mantle-derived carbonatite magma. J Petrol. 39:1895–1903. doi: 10.1093/petroj/39.11-12.1895
- Hay RL. 1983. Natrocarbonatite tephra of Kerimasi volcano, Tanzania. Geology. 11:599–602. doi: 10.1130/0091-7613(1983)11<599:NTOKVT>2.0.CO;2
- Hedenquist JW, Taran YA. 2013. Modeling the formation of a dvanced argillic lithocaps: volcanic vapor condensation above porphyry intrusions. Econ Geol. 108:1523–1540. doi: 10.2113/econgeo.108.7.1523
- Heinrich EWM. 1958. Mineralogy and geology of radioactive raw materials. New York (NY): McGraw-Hill.
- Heinrich EWM. 1966. The geology of carbonatites. New York (NY): Huntington, Rand McNally & Company.
- Heinrich EWM. 1970. The Palabora carbonatitic complex – a unique copper deposit. Can Mineral. 10:585–598.
- Heinrich EWM. 1980. The geology of carbonatites. Huntington (NY): Robert E. Kreiger Publishing Company.
- Heinrich EWM. 1985. Infinite variations on a fenite theme. Indian Mineral. Sukheswala Volume:151–162.
- Hornig-Kjarsgaard I. 1998. Rare earth elements in Sövitic carbonatites and their mineral phases. J Petrol. 39:2105–2121. doi: 10.1093/petrology/39.11.2105
- Horstmann UE, Verwoerd WJ. 1997. Carbon and oxygen isotope variations in southern African carbonatites. J Afr Earth Sci. 25:115–136. doi: 10.1016/S0899-5362(97)00065-1
- Hou Z, Tian S, Yuan Z, Xie Y, Yin S, Yi L, Fei H, Yang Z. 2006. The Himalayan collision zone carbonatites in western Sichuan, SW China: petrogenesis, mantle source and tectonic implication. Earth Planet Sci Lett. 244:234–250. doi: 10.1016/j.epsl.2006.01.052
- Hou Z, Yan Liu Y, Tian S, Yang Z, Xie Y. 2015. Formation of carbonatite-related giant rare-earth-element deposits by the recycling of marine sediments. Nature-Scientific Reports. 5:10231. doi: 10.1038/srep10231
- Hulett SRW, Simonetti A, Rasbury ET, Hemming NG. 2016. Recycling of subducted crustal components into carbonatite melts revealed by boron isotopes. Nat Geosci. 9:904–908. doi: 10.1038/ngeo2831
- Issa Filho A, Lima PRA, Souza OM. 1984. Aspects of geology of the Barreiro Carbonatitic Complex, Araxá, MG, Brazil. In: Carbonatitic complexes of Brazil: geology. Sao Paulo: Companhia Brasileira de Metalurgia e Mineração; p. 21–44.
- Issa Filho A, Riffel BF, de Faria Sousa CA. 2015. Some aspects of the mineralogy of CBMM niobium deposit and mining and pyrochlore ore processing – Araxá, MG – Brazil. In: Proceedings of the International Symposium Niobium, Niobium Science & Technology. Sao Paulo: Companhia Brasileira de Metalurgia e Mineração. p. 53–65.
- Ivanyuk GY, Kalashnikov AO, Pakhomovsky YA, Mikhailova JA, Yakovenchuk VN, Konopleva NG, Sokharev VA, Bazai AV, Goryainov PM. 2016. Economic minerals of the Kovdor baddeleyite-apatite-magnetite deposit, Russia: mineralogy, spatial distribution and ore processing optimization. Ore Geol Rev. 77:279–311. doi: 10.1016/j.oregeorev.2016.02.008
- Kalashnikov AO, Yakovenchuk VN, Pakhomovsky Y, Bazai AV AV, Sokharev VA, Konopleva NG, Mikhailova JA, Goryainov PM, Ivanyuk GY. 2016. Scandium of the Kovdor baddeleyite–apatite–magnetite deposit (Murmansk Region, Russia): mineralogy, spatial distribution, and potential resource. Ore Geol Rev. 72:532–537. doi: 10.1016/j.oregeorev.2015.08.017
- Kamenetsky VS, Mitchell RH, Maas R, Giuliani A, Gaboury D, Zhitova L. 2015. Chlorine in mantle-derived carbonatite melts revealed by halite in the St.-Honoré intrusion (Québec, Canada). Geology. 43:687–690. doi: 10.1130/G36843.1
- Keller J, Hoefs J. 1995. Stable isotope characteristics of recent natrocarbonatite from Oldoinyo Lengai. In: Bell K, Keller J, editor. IAVCEI, proceedings in volcanology vol. 4, carbonatite volcanism: Oldoinyo Lengai and the petrogenesis of natrocarbonatites; Mainz, Germany. Berlin: Springer-Verlag; p. 113–123.
- Keller J, Krafft M. 1990. Effusive natrocarbonatite activity of Oldoinyo Lengai, June 1988. Bull Volcanol. 52:629–645. doi: 10.1007/BF00301213
- Keller J, Zaitsev AN. 2012. Geochemistry and petrogenetic significance of natrocarbonatites at Oldoinyo Lengai, Tanzania: composition of lavas from 1988 to 2007. Lithos. 148:45–53. doi: 10.1016/j.lithos.2012.05.022
- Kjarsgaard BA, Hamilton DL. 1989. The genesis of carbonatites by immiscibility. In: Bell K, editor. Carbonatites: genesis and evolution. London (UK): Unwin Hyman; p. 388–404.
- Klausen MB. 2004. Geometry and mode of emplacement of the Thverartindur cone sheet swarm, SE Iceland. J Volcanol Geotherm Res. 138:185–204. doi: 10.1016/j.jvolgeores.2004.05.022
- Knauth LP, Kennedy MJ. 2009. The late Precambrian greening of Earth. Nature. 460:728–732.
- Kogarko LN, Plant DA, Henderson CMB, Kjarsgaard BA. 1991. Na-rich carbonate inclusions in perovskite and calzirtite from the Guli intrusive Ca-carbonatite, polar Siberia. Contrib Mineral Petrol. 109:124–129. doi: 10.1007/BF00687205
- Koide H, Bhattacharji S. 1975. Formation of fractures around magmatic intrusions and their role in ore localization. Econ Geol. 70:781–799. doi: 10.2113/gsecongeo.70.4.781
- Korzhinskii DS. 1970. Theory of metasomatic zoning. Oxford: Clarendon Press. 162 p.
- Krasnova NI, Petrov TG, Balaganskaya EG, Garcia D, Moutte J, Zaitsev AN, Wall F. 2004. Introduction to phoscorites: occurrence, composition, nomenclature and petrogenesis. In: Wall F, Zaitsev AN, editor. Phoscorites and carbonatites from mantle to mine: the Key example of the Kola Alkaline Province. London (UK): The Mineralogical Society of London; p. 1–36.
- Kresten P. 1980. The Alnö complex: Tectonics of dyke emplacement. Lithos. 13:153–158. doi: 10.1016/0024-4937(80)90016-X
- Kresten P. 1988. The chemistry of fenitization: examples from Fen, Norway. Chem Geol. 68:329–349. doi: 10.1016/0009-2541(88)90030-7
- Kresten P, Morogan V. 1986. Fenitization at the Fen complex, southern Norway. Lithos. 19:27–42. doi: 10.1016/0024-4937(86)90013-7
- Kulla G, Hardy J. 2015. NI 43-101 Blue River Tantalum-Niobium Project, British Columbia, Canada. Report prepared for Commerce Resources Corp.
- Kunzendorf H, Secher K. 1987. Dispersion of niobium and phosphorus in soil overlying the Qaqarssuk Carbonatite Complex, Southern West Greenland. J Geochem Explor. 28:285–296. doi: 10.1016/0375-6742(87)90053-7
- Lafleur PJ, Ayad AB. 2012. NI 43-101 technical report to present the mineral resources of the rare earth elements zone Niobec Mine. Prepared for IAMGOLD Corporation.
- Lai X, Yang X, Liu Y, Yan Z. 2016. Genesis of the Bayan Obo Fe–REE–Nb deposit: evidences from Pb–Pb age and microanalysis of the H8 formation in Inner Mongolia, North China Craton. J Asian Earth Sci. 120:87–99. doi: 10.1016/j.jseaes.2016.01.024
- Laznicka P. 2006. Giant metallic deposits: future sources of industrial metals. Berlin: Springer.
- Le Bas MJ. 1977. Carbonatite-Nephelinite volcanism. New York (NY): Wiley.
- Le Bas MJ. 1981. Carbonatite magmas. Miner Mag. 44:133–140. doi: 10.1180/minmag.1981.044.334.02
- Le Bas MJ. 1987. Nephelinites and carbonatites. In: Fitton JG, Upton BGJ, editors. Alkaline igneous rocks. London (UK): The Geological Society of London, Special Publication 30; p. 53–83.
- Le Bas MJ. 2008. Fenites associated with carbonatites. Can Mineral. 46:915–932. doi: 10.3749/canmin.46.4.915
- Le Bas MJ, Ba-bttat MAO, Taylor RN, Milton JA, Windley BF, Evins PM. 2004. The carbonatite-marble dykes of Abyan Province, Yemen Republic: the mixing of mantle and crustal carbonate materials revealed by isotope and trace element analysis. Mineral Petrol. 82:105–135. doi: 10.1007/s00710-004-0056-2
- Le Maître RW. 2002. Igneous rocks: a classification and glossary of terms. Cambridge (UK): Cambridge University Press.
- Lee W-J, Wyllie PJ. 1998. Processes of crustal carbonatite formation by liquid immiscibility and differentiation, elucidated by model systems. J Petrol. 39:2005–2013. doi: 10.1093/petroj/39.11-12.2005
- Leelanandam C, Burke K, Ashwal LD, Webb SJ. 2006. Proterozoic mountain building in peninsular India:an analysis based primarily on alkaline rock distribution. Geolog Mag. 143:195–212. doi: 10.1017/S0016756805001664
- Lentz DR. 1999. Carbonatite genesis:a re-examination of the role of intrusion-related pneumatolytic skarn processes in limestone melting. Geology. 27:335–338. doi: 10.1130/0091-7613(1999)027<0335:CGAROT>2.3.CO;2
- Lentz D, Eby N, Lavoie S, Park A. 2006. Diatremes, dykes, and diapirs: revisiting the ultra-alkaline to carbonatitic magmatism of the Monteregian Hills. GAC/MAC Joint Annual Meeting, Montréal, Québec, Post-conference Field Trip B4.
- Lie A, Østergaard C. 2011. Fen carbonatite complex:results of a mobile metal ion survey, Ulefoss, South Norway. Compiled and prepared by 21st NORTH, Svendborg, 2011 Sep 20 in commission for REE Minerals, Norway.
- Liu S, Fan H-R, Yang K-F, Hu F-F, Rusk B, Liu X, Li X-C, Yang Z-F, Wang Q-W, Wang K-Y. 2018. Fenitization in the giant bayan Obo REE-Nb-Fe deposit: implications for REE mineralization. Ore Geol Rev. 94:290–309. doi: 10.1016/j.oregeorev.2018.02.006
- Lottermoser BG. 1990. Rare-earth element mineralisation within the Mt. Weld carbonatite laterite, western Australia. Lithos. 24:151–167. doi: 10.1016/0024-4937(90)90022-S
- Mackay DAR, Simandl GJ. 2014a. Geology, market and supply chain of niobium and tantalum – a review. Miner Depos. 49:1025–1047. doi: 10.1007/s00126-014-0551-2
- Mackay DAR, Simandl GJ. 2014b. Portable X-ray fluorescence to optimize stream sediment chemistry and indicator mineral surveys, case 1: carbonatite-hosted Nb deposits, Aley carbonatite, British Columbia, Canada. In: Geological Fieldwork 2013. British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2014–1; p. 183–194.
- Mackay DAR, Simandl GJ. 2015. Pyrochlore and columbite-tantalite as indicator minerals for specialty metal deposits. Geochem:Explor Environ Anal. 15:167–178.
- Mackay DAR, Simandl GJ, Ma W, Gravel J, Redfearn M. 2015. Indicator minerals in exploration for specialty metal deposits:A QEMSCAN® approach. In: Simandl GJ, Neetz M, editors. Symposium on strategic and critical materials proceedings; Nov 13–14; Victoria, British Columbia. British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015–3. p. 211–217.
- Mackay DAR, Simandl GJ, Ma W, Redfearn M, Gravel J. 2016. Indicator mineral-based exploration for carbonatites and related specialty metal deposits - a QEMSCAN orientation survey, British Columbia, Canada. J Geochem Explor. 165:159–173. doi: 10.1016/j.gexplo.2016.03.005
- Mann AW. 2007. Ligand Based Soil Extraction Geochemistry. In: Milkereit B, editor. Proceedings of Exploration 07, Fifth Decennial International Conference on Mineral Exploration, Exploration in the new millenium; Sep 9–12; Toronto, Canada. Toronto (ON): Decennial Mineral Exploration Conferences. p. 281–289.
- Mantovani MSM, Louro VHA, Ribeiro VB, Requejo HS, dos Santos RPZ. 2016. Geophysical analysis of Catalão I alkaline-carbonatite complex in Goiás, Brazil. Geophys Prospect. 64:216–227. doi: 10.1111/1365-2478.12283
- Mao M, Rukhlov AS, Rowins SM, Spence J, Coogan LA. 2016. Apatite trace-element compositions: A robust new tool for mineral exploration. Econ Geol. 111:1187–1222. doi: 10.2113/econgeo.111.5.1187
- Maravic H, Morteani G. 1980. Petrology and geochemistry of the carbonatite and syenite complex of Lueshe (N.E. Zaire). Lithos. 13:159–170. doi: 10.1016/0024-4937(80)90017-1
- Mariano AN. 1989a. Economic geology of rare earth minerals. In: Lipman BR, McKay GA, editors. Geochemistry and mineralogy of rare earth elements. Chantilly (VA): Mineralogical Society of America, Reviews in mineralogy, v. 21; p. 303–337.
- Mariano AN. 1989b. Nature of economic mineralization in carbonatites and related rocks. In: Bell K, editor. Carbonatites: genesis and evolution. London (UK): Unwin Hyman; p. 149–176.
- McDonough WF, Sun S-s. 1995. The composition of the Earth. Chem Geol. 120:223–253. doi: 10.1016/0009-2541(94)00140-4
- Mars JC, Rowan LC. 2011. ASTER spectral analysis and lithologic mapping of the Khanneshin carbonatite volcano, Afghanistan. Geosphere. 7:276–289. doi: 10.1130/GES00630.1
- Marshall L. 2012. Hitting paydirt: Mines, Colorado School of Mines Magazine. [accessed 2017 Apr 20]. http://minesmagazine.com/6211/.
- Martin RF. 2006. A-type granites of crustal origin ultimately result from open-system fenitization-type reactions in an extensional environment. Lithos. 91:125–136. doi: 10.1016/j.lithos.2006.03.012
- McLeish DF, Kressall R, Chakhmouradian A, Crozier J, Johnston ST, Mortensen JK. 2010. The Aley Carbonatite Complex – Part I Structural Evolution of a Cordilleran Niobium Deposit. In: Simandl GJ, Lefebure DV, editors. International workshop on the geology of rare metals, extended abstracts volume; Nov 9–10; Victoria, Canada. Victoria (BC): British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Open File 2010-10. p. 21–24.
- Menge GFW. 1986. Sodalite carbonatite deposits of Swartbooisdrif, South West Africa/Namibia. In: Anhaeusser CR, Maske S, editor. Mineral deposits of Southern Africa, vol. II. Johannesburg (ZA): The Geological Society of South Africa; p. 2261–2268.
- Mian I, Le Bas MJ. 1986. Sodic amphiboles in fenites from the Loe Shilman carbonatite complex, NW Pakistan. Mineral Mag. 50(356):187–197. doi: 10.1180/minmag.1986.050.356.01
- Migdisov A, Williams-Jones AE, Brugger J, Caporuscio FA. 2016. Hydrothermal transport, deposition, and fractionation of the REE: experimental data and thermodynamic calculations. Chem Geol. 439:13–42. doi: 10.1016/j.chemgeo.2016.06.005
- Milani L, Bolhar R, Frei D, Harlov DE, Samuel VO. 2017. Light rare earth element systematics as a tool for investigating the petrogenesis of phoscorite-carbonatite associations, as exemplified by the Phalaborwa Complex, South Africa. Miner Deposita. 52:1105–1125. doi: 10.1007/s00126-016-0708-2
- Millonig LJ, Gerdes A, Groat LA. 2012. U-Th-Pb geochronology of meta-carbonatites and meta-alkaline rocks in the southern Canadian Cordiller; a geodynamic perspective. Lithos. 152:202–217. doi: 10.1016/j.lithos.2012.06.016
- Mitchell RH. 2005. Carbonatites and carbonatites and carbonatites. Can Mineral. 43:2049–2068. doi: 10.2113/gscanmin.43.6.2049
- Mitchell RH, Belton F. 2004. Niocalite–cuspidine solid solution and manganoan monticellite from natrocarbonatite, Oldoinyo Lengai, Tanzania. Mineral Mag. 68:787–799. doi: 10.1180/0026461046850219
- Morogan V. 1989. Mass transfer and REE mobility during fenitization at Anö, Sweden. Contrib Mineral Petrol. 103:29–34. doi: 10.1007/BF00371362
- Morogan V. 1994. Ijolite versus carbonatite as sources of fenitization. Terra Nova. 6:166–176. doi: 10.1111/j.1365-3121.1994.tb00650.x
- Morogan V, Lindblom S. 1995. Volatiles associated with the alkaline – carbonatite magmatism at Alnö, Sweden: a study of fluid and solid inclusions in minerals from the Langarsholmen ring complex. Contrib Mineral Petrol. 122:262–274. doi: 10.1007/s004100050126
- Morrison GW. 1980. Characteristics and tectonic setting of the shoshonite rock association. Lithos. 13:97–108. doi: 10.1016/0024-4937(80)90067-5
- Moser DE, Bowman JR, Wooden J, Valley JW, Mazdab F, Kita N. 2008. Creation of a continent recorded in zircon zoning. Geology. 36:239–242. doi: 10.1130/G24416A.1
- Neave DA, Black M, Riley TR, Gibson SA, Ferrier G, Wall F, Broom-Fendley S. 2016. On the feasibility of imaging carbonatite-hosted rare earth element deposits using remote sensing. Econ Geol. 111:641–665. doi: 10.2113/econgeo.111.3.641
- Nelson DR, Chivas AR, Chappell BW, MuCulloch MT. 1988. Geochemical and isotopic systematics in carbonatites and implications for the evolution of ocean-island sources. Geochim Cosmochim Acta. 52:1–17. doi: 10.1016/0016-7037(88)90051-8
- Neumann R, Medeiros EB. 2015. Comprehensive mineralogical and technological characterisation of the Araxá (SE Brazil) complex REE (Nb-P). Int J Miner Process. 144:1–10. doi: 10.1016/j.minpro.2015.08.009
- Ngwenya BT, Bailey DK. 1990. Kaluwe carbonatite, Zambia: an alternative to natrocarbonatite. J Geol Soc Lond. 147:213–216. doi: 10.1144/gsjgs.147.2.0213
- Nielsen TFD. 1980. The petrology of a melitolite, melteigite, carbonatite and syenite ring dike system, in the Gardiner complex, East Greenland. Lithos. 13:181–197. doi: 10.1016/0024-4937(80)90019-5
- O’Brien H, Heilimo E, Heino P. 2015. The Archean Siilinjärvi carbonatite complex. In: Maier DW, Lahtinen R, O’Brien H, editor. Mineral deposits of Finland. Amsterdam (NL): Elsevier; p. 327–343.
- Ontario Mining Association. 2015. Mining 101. [cited 2017 Apr 20]. http://www.oma.on.ca/en/ontariomining/Mining101.asp?hdnContent.
- Pecora WT. 1956. Carbonatites, a review. Geological Society of America Bulletin. 67:1537–1556. doi: 10.1130/0016-7606(1956)67[1537:CAR]2.0.CO;2
- Pell J. 1994. Carbonatites, nepheline syenites, kimberlites and related rocks in British Columbia. Victoria (BC): British Columbia Ministry of Energy, Mines and Petroleum Resources, Bulletin 88.
- Pell J. 1996. Mineral deposits associated with carbonatites and related alkaline igneous rocks. In: Mitchell RH, editor. Unsaturated alkaline rocks: mineralogy, petrogenesis, and economic potential. Quebec (CA): Mineralogical Association of Canada, Short Course Series v. 24; p. 271–310.
- Perttunen M, Vartiainen H. 1992. Glaciofluvial transport of clasts and heavy minerals from the Sokli carbonatite complex, Finnish Lapland. Geological Survey of Finland Bulletin 366.
- Pirajno F. 1994. Mineral resources of anorogenic alkaline complexes in Namibia: a review. Aust J Earth Sci. 41:157–168. doi: 10.1080/08120099408728123
- Pirajno F. 2000. Ore deposits and mantle plumes. Dordrecht (NL): Kluwer Academic Publishers.
- Pirajno F. 2015. Intracontinental anorogenic alkaline magmatism and carbonatites, associated mineral systems and the mantle plume connection. Gondwana Res. 27:1181–1216. doi: 10.1016/j.gr.2014.09.008
- Pirajno F, González-Álvarez I, Chen W, Kyser KT, Simonetti A, Leduc E, leGras M. 2014. The Gifford creek ferrocarbonatite complex, Gascoyne Province, Western Australia: associated fenitic alteration and a putative link with the ∼1075 Ma Warakurna LIP. Lithos. 202–203:100–119. doi: 10.1016/j.lithos.2014.05.012
- Preston G. 2015. Roundup takeaway 1: Outsmart tough odds, Mining.com. [accessed 2017 Apr 20]. http://www.mining.com/web/roundup-takeaway-1-outsmart-tough-odds/.
- Prokopyev IR, Doroshkevich AG, Ponomarchuk AV, Sergeev SA. 2017. Mineralogy, age and genesis of apatite-dolomite ores at the Seligdar apatite deposit (Central Aldan, Russia). Ore Geol Rev. 81:296–308. doi: 10.1016/j.oregeorev.2016.10.012
- Puustinen K. 1970. The carbonatite of Siilinjärvi in the Precambrian of Eastern Finland: a preliminary report. Lithos. 3:89–92. doi: 10.1016/0024-4937(70)90090-3
- Ray JS, Ramesh R. 2006. Stable carbon and oxygen isotopic compositions of Indian carbonatites. Int Geol Rev. 48(1):17–45. doi: 10.2747/0020-6814.48.1.17
- Richardson DG, Birkett TC. 1996a. Carbonatite associated deposits. In: Eckstrand OR, Sinclair WD, Thorpe RI, editor. Geology of Canada 8: geology of Canadian deposit types. Ottawa (CA): Geological Survey of Canada; p. 541–558.
- Richardson DG, Birkett TC. 1996b. Residual carbonatite-associated deposits. In: Eckstrand OR, Sinclair WD, Thorpe RI, editor. Geology of Canada 8: geology of Canadian mineral deposit types. Ottawa (CA): Geological Survey of Canada; p. 108–119.
- Reid DL, Cooper AF. 1992. Oxygen and carbon isotope patterns in the Dicker Willem carbonatite complex, southern Namibia. Chem Geol. 94:293–305. doi: 10.1016/S0009-2541(10)80031-2
- Robins B, Tysseland M. 1983. The geology, geochemistry and origin of ultrabasic fenites associated with the pollen carbonatite (Finnmark, Norway). Chem Geol. 40:65–95. doi: 10.1016/0009-2541(83)90092-X
- Rowan LC, Kingston MJ, Crowley JK. 1986. Spectral reflectance of carbonatite and related alkalic igneous rocks from four North American localities. Econ Geol. 81:857–871. doi: 10.2113/gsecongeo.81.4.857
- Rowan LC, Mars JC. 2003. Lithologic mapping in the Mountain Pass, California area using Advanced Spaceborne Thermal Emission and Reflection Radiometer (ASTER) data. Remote Sens Environ. 84:350–366. doi: 10.1016/S0034-4257(02)00127-X
- Rugless CS, Pirajno F. 1996. Geology and geochemistry of the copperhead albitite ‘carbonatite’ complex, east Kimberley, Western Australia. Aust J Earth Sci. 43:311–322. doi: 10.1080/08120099608728258
- Rudashevsky NS, Kretser YL, Rudashevsky VN, Sukharzhevskaya ES. 2004. A review and comparison of PGE, noble-metal and sulphide mineralization in phoscorites and carbonatites from Kovdor and Phalaborwa. In: A Zaitsev, F Wall, editor. Phoscorites and carbonatites from mantle to mine: the Key example of the Kola Alkaline Province. Mineralogical Society Series, 10. London: Mineralogical Society; p. 363–393. ISBN 0 903056 22 4.
- Rukhlov AS, Bell K. 2010. Geochronology of carbonatites from the Canadian and Baltic Shields, and the Canadian Cordillera: clues to mantle evolution. Mineral Petrol. 98:11–54. doi: 10.1007/s00710-009-0054-5
- Rukhlov AS, Bell K, Amelin Y. 2015. Carbonatites, isotopes and evolution of the subcontinental mantle: an overview. In: Simandl GJ, Neetz M, editors. Symposium on strategic and critical materials proceedings; Nov 13–14; Victoria, British Columbia. Victoria (BC): British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015–3; p. 39–64.
- Sabins FF. 1997. Remote sensing: principles and interpretation. New York (NY): WH Freeman and Company.
- Sage RP, Watkinson DH. 1991. Alkalic rock-carbonatite complexes of the Superior Structural Province, Northern Ontario, Canada. Chron Rech Min. 504:5–19.
- Samoilov VS. 1991. The main geochemical features of carbonatites. J Geochem Explor. 40:251–262. doi: 10.1016/0375-6742(91)90041-R
- dos Santos EJ, Neto JAS, Carmona LCM, Armstrong R, Santos LCML, Mendes LUDS. 2013. The metacarbonate rocks of Itatuba (Paraíba): a record of sedimentary recycling in a Paleoproterozoic collision zone of the Borborema province, NE Brazil. Precambrian Res. 224:454–471. doi: 10.1016/j.precamres.2012.09.021
- Santos RV, dos Santos EJ, Neto JAS, Carmona LCM, Sial AN, Mancini LH, Santos LCML, Nascimento GH, Mendes LUDS, Anastácio EMF. 2013. Isotope geochemistry of Paleoproterozoic metacarbonates from Itatuba, Borborema Province, Northeastern Brazil: evidence of marble melting within a collisional suture. Gondwana Res. 23:380–389. doi: 10.1016/j.gr.2012.04.010
- Sarapää O, Ani TA, Lahti SI, Lauri LS, Sarala P, Torppa A, Kontinen A. 2013. Rare earth exploration potential in Finland. J Geochem Explor. 133:25–41. doi: 10.1016/j.gexplo.2013.05.003
- Satterly J. 1970. Aeromagnetic maps of carbonatite-alkalic complexes in Ontario. Ontario Department of Mines and Northern Affairs, Preliminary Map no. P452 (revised). [accessed 2017 Apr 20]. http://www.geologyontario.mndmf.gov.on.ca/mndmfiles/pub/data/imaging/P0452/P0452.pdf.
- Schleicher H, Todt W, Viladkar GS, Schmidt F. 1997. Pb/Pb age determinations on the Newania and Sevattur carbonatites of India: evidence for multi-stage histories. Chem Geol. 140:261–273. doi: 10.1016/S0009-2541(97)00022-3
- Schultz F, Lehmann B, Tawackoli S, Rössling R, Belyatsky B, Dulski P. 2004. Carbonatite diversity in the Central Andes: the Ayopaya alkaline province, Bolivia. Contrib Mineral Petrol. 148:391–408. doi: 10.1007/s00410-004-0612-0
- Secher K, Larsen LM. 1980. Geology and mineralogy of the Sarfartôq carbonatite complex, southern West Greenland. Lithos. 13:199–212. doi: 10.1016/0024-4937(80)90020-1
- Seo J, Choi S-G, Park J-W, Whattam S, Kim DW, Ryu IC, Oh CW. 2016. Geochemical and mineralogical characteristics of the Yonghwa phoscorite–carbonatite complex, South Korea, and genetic implications. Lithos. 262:606–619. doi: 10.1016/j.lithos.2016.08.006
- Sharygin VV, Zhitova LM, Nigmatulina EN. 2011. Fairchildite K2Ca(CO3)2 in phoscorites from Phalaborwa, South Africa:the first occurrence in alkaline carbonatite complexes. Russ Geol Geophys. 52:208–219. doi: 10.1016/j.rgg.2010.12.015
- Shives RBK. 2015. Using gamma ray spectrometry to find rare metals. In: Simandl GJ, Neetz M, editors. Symposium on strategic and critical materials proceedings; Nov 13–14; Victoria, British Columbia. Victoria (BC): British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015–3. p. 199–209.
- Sillitoe RH. 2010. Porphyry copper systems. Econ Geol. 105:3–41. doi: 10.2113/gsecongeo.105.1.3
- Simandl GJ. 2014. Geology and market-dependent significance of rare earth element resources. Mineral Depos. 49(8):889–904. doi: 10.1007/s00126-014-0546-z
- Simandl GJ, Burt RO, Trueman DL, Paradis S. 2018. Economic geology models 2. Tantalum and niobium: deposits, resources, exploration methods and market – a primer for geoscientists. Geosci Can. 45:85–96. doi: 10.12789/geocanj.2018.45.135
- Simandl GJ, Fajber R, Paradis S. 2014a. Portable X-ray fluorescence in the assessment of rare earth element-enriched sedimentary phosphate deposits. Geochem Explor Environ Anal. 14:161–169. doi: 10.1144/geochem2012-180
- Simandl GJ, Mackay DAR, Ma X, Luck P, Gravel J, Akam C. 2017. The direct indicator mineral concept and QEMSCAN® applied to exploration for carbonatite and carbonatite-related ore deposits. In: Ferbey T, Plouffe A, Hickin AS, editors. Indicator minerals in till and stream sediments of the Canadian cordillera. Geological Association of Canada Special Paper Volume 50, and Mineralogical Association of Canada Topics in Mineral Sciences Volume 47. p. 175–190.
- Simandl GJ, Paradis S, Simandl L, Dahrouge J. 2010. Vermiculite in the Blue River Area, East-Central British Columbia, Canada. In: Geological Fieldwork 2009. Victoria (BC):British Columbia Ministry of Energy, Mines and Natural Gas, British Columbia Geological Survey Paper 2010–1; p. 83–91.
- Simandl GJ, Paradis S, Stone RS, Fajber R, Kressall R, Grattan K, Crozier J, Simandl LJ. 2014b. Applicability of handheld X-ray fluorescence spectrometry in the exploration and development of carbonatite-related niobium deposits: a case study of the Aley carbonatite, British Columbia, Canada. Geochem Explor Environ Anal. 14:211–221. doi: 10.1144/geochem2012-177
- Simandl GJ, Stone RS, Paradis S, Fajber R, Reid HM, Grattan K. 2014c. An assessment of a handheld X-ray fluorescence instrument for use in exploration and development with an emphasis on REEs and related specialty metals. Mineral Depos. 49:999–1012. doi: 10.1007/s00126-013-0493-0
- Smith MP. 2007. Metasomatic silicate chemistry at the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China: contrasting chemistry and evolution of fenitizing and mineralizing fluids. Lithos. 93:126–148. doi: 10.1016/j.lithos.2006.06.013
- Smith MP, Campbell LS, Kynicky J. 2015. A review of the genesis of the world class Bayan Obo Fe-REE-Nb deposits, Inner Mongolia, China: multistage processes and outstanding questions. Ore Geol Rev. 64:459–476. doi: 10.1016/j.oregeorev.2014.03.007
- Solgadi F, Groulier P-A, Moukhsil A, Ohnenstetter D, André-Mayer A-S, Zeh A. 2015. Nb-Ta-REE mineralization associated with the Crevier alkaline intrusion. In: Simandl GJ, Neetz M, editors. Symposium on strategic and critical materials proceedings; Nov 13–14; Victoria, British Columbia. Victoria (BC): British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015–3. p. 69–74.
- Song W, Xu C, Chakhmouradian AR, Kynicky J, Huang K, Zhang Z. 2017. Carbonatites of Tarim (NW China): first evidence of crustal contribution in carbonatites from a large igneous province. Lithos. 282:1–9. doi: 10.1016/j.lithos.2017.02.018
- Song W, Xu C, Veksler IV, Kynicky J. 2016. Experimental study of REE, Ba, Sr, Mo and W partitioning between carbonatitic melt and aqueous fluid with implications for rare metal mineralization. Contrib Mineral Petrol. 171(1):91. doi: 10.1007/s00410-015-1217-5
- van Straaten P. 2002. Rocks for crops: agrominerals of sub-Saharan Africa. Nairobi: International Centre for Research in Agroforestry (ICRAF).
- Swanepoel E. 2014. Iluka in Brazil titanium deal with Vale: Creamer Media’s Mining Weekly. [accessed 2017 Apr 20]. http://www.miningweekly.com/print-version/iluka-in-brazil-titanium-deal-with-vale-2014-06-04.
- Taylor HP, Frechen J, Degens ET. 1967. Oxygen and carbon isotope studies of carbonatites from the Laacher See district, West Germany and the Alnö district, Sweden. Geochim Cosmochim Acta. 31:407–430. doi: 10.1016/0016-7037(67)90051-8
- Thomas MD, Ford KL, Keating P. 2011. Exploration geophysics for intrusion-hosted rare earth metals [Poster]. Geological Survey of Canada Open File 6828. [accessed 2017 Apr 20]. http://ftp.maps.canada.ca/pub/nrcan_rncan/publications/ess_sst/288/288092/gscof_6828_e_2011_pr01.pdf.
- Thomas MD, Ford KL, Keating P. 2016. Review paper: exploration for intrusion-hosted rare metals. Geophys Prospect. 64:1275–1304. doi: 10.1111/1365-2478.12352
- Thompson RN, Smith PM, Gibson SA, Mattey DP, Dickin AP. 2002. Ankerite carbonatite from Swartbooidrif, Namibia: the first evidence of magmatic ferrocarbonatite. Contrib Mineral Petrol. 143:377–396. doi: 10.1007/s00410-002-0350-0
- Tilton GR, Bryce JG, Mateen A. 1998. Pb-Sr-Nd isotope data from 30 and 300 Ma collision zone carbonatites in northwest Pakistan. J Petrol. 39:1865–1874. doi: 10.1093/petroj/39.11-12.1865
- Timofeev A, Migdisov AA, Williams-Jones AE. 2015. An experimental study of the solubility and speciation of niobium in fluoride-bearing aqueous solutions at elevated temperature. Geochim et Cosmochim Acta. 158:103–111. doi: 10.1016/j.gca.2015.02.015
- Timofeev A, Migdisov AA, Williams-Jones AE. 2017. An experimental study of the solubility and speciation of tantalum in fluoride-bearing aqueous solutions at elevated temperature. Geochim Cosmochim Acta. 197:294–304. doi: 10.1016/j.gca.2016.10.027
- Tohver E, Teixeira W, Van Der Pluijm B, Geraldes MC, Bettencourt JS, Rizzotto G. 2006. Restored transect across the exhumed Grenville orogen of Laurentia and Amazonia, with implications for crustal architecture. Geology. 34:669–672. doi: 10.1130/G22534.1
- Torró L, Villanova C, Castillo M, Campeny M, Gonçalves AO, Melgarejo JC. 2012. Niobium and rare earth minerals from the Virulundo carbonatite, Namibe, Angola. Miner Mag. 76:393–409. doi: 10.1180/minmag.2012.076.2.08
- Traversa G, Gomes CB, Brotzu P, Buraglini N, Morbidelli L, Principato MS, Ronca S, Ruberti E. 2001. Petrography and mineral chemistry of carbonatites and mica-rich rocks from the Araxá complex (Alto Paranaíba Province, Brazil). An Acad Bras Ciênc. 73:71–98. doi: 10.1590/S0001-37652001000100008
- Tremblay J, Bédard LP, Matton G. 2015. A petrographic study of Nb-bearing minerals at the Saint-Honoré niobium deposit. In: Simandl GJ, Neetz M, editors. Symposium on strategic and critical materials proceedings; Nov 13–14; Victoria, British Columbia. Victoria (BC): British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015–3. p. 75–81.
- Trofanenko J, Williams-Jones AE, Simandl GJ, Migdisov AA. 2016. The nature and origin of the REE mineralization in the Wicheeda Carbonatite, British Columbia, Canada. Econ Geol. 111:199–223. doi: 10.2113/econgeo.111.1.199
- Turner D, Rivard B, Groat L. 2015. Visible to shortwave infrared reflectance spectroscopy of rare earth element minerals. In: Simandl GJ, Neetz M, editors. Symposium on strategic and critical materials proceedings; Nov 13–14; Victoria, British Columbia. Victoria (BC): British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015–3. p. 219–229.
- Turner N, Mills D, Fedikow M, Prince P. 2007. The evaluation of geological exploration samples using multi-element mobile metal ion (MMI-M) selective weak extraction and inductively coupled plasma mass spectrometry (ICP-MS). In: Milkereit B, editor. Proceedings of exploration 07, Fifth Decennial International Conference on Mineral Exploration, Exploration in the new millenium; Sep 9–12; Toronto, Canada. Toronto (ON):Decennial Mineral Exploration Conferences. p. 973–977.
- Twyman J, Gittins J. 1987. Alkalic carbonatite magmas: parental or derivative? In: Fitton JG, Upton BG, editors. Alkaline Igneous Rocks. Geol Soc Lond Spec Pub. 30:85–94.
- Van Gosen B, Lowers H. 2007. The Iron Hill (Powderhorn) carbonatite complex, Gunnison County, CO – a potential source of several uncommon mineral resources. Society for Mining, Metallurgy, and Exploration Annual Meeting; Denver, Colorado. p. 298–305.
- Van Gosen BS. 2009. The Iron Hill (Powderhorn) Carbonatite Complex, Gunnison County, Colorado – a potential source of several uncommon mineral resources, US Department of the Interior, US Geological Survey, Open-File Report 2009–1005.
- Veizer JEA. 1992. Temporal distribution of carbonatites. Geology. 20:1147–1149. doi: 10.1130/0091-7613(1992)020<1147:TDOC>2.3.CO;2
- Veksler IV, Lentz D. 2006. Parental magmas of plutonic carbonatites, carbonate-silicate immiscibility and decarbonation reactions: evidence from melt and fluid inclusions. In: Webster JD, editor. Melt inclusions in Plutonic Rocks. Montreal (QC): Mineralogical Association of Canada, Short Course 36; p. 123–149.
- Verbaan N, Bradley K, Brown J, Mackie S. 2015. A review of hydrometallurgical flowsheets considered incurrent REE projects. In: Simandl, G.J. and Neetz, M., editors, Symposium on Strategic and Critical Materials Proceedings, Victoria, British Columbia. British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015-3, p. 147–162.
- Verplank PL, Mariano AN, Mariano AN Jr. 2016. Rare earth element ore geology of carbonatites. Rev Econ Geol Soc Econ Geol. 18:5–32.
- Verwoerd WJ. 1986. Mineral deposits associated with carbonatites and alkaline rocks. In: Anhaeusser CR, Maske S, editors. Mineral deposits of Southern Africa, vol. II. Johannesburg (ZA): The Geological Society of South Africa; p. 2173–2191.
- Verwoerd WJ. 1990. The Salpeterkop ring structure, Cape Province, South Africa. Tectonophysics. 171:275–285. doi: 10.1016/0040-1951(90)90105-H
- Vuotovesi T, Peuraniemi V, Nuutilainen J. 1980. On prospecting for pyrochlore mineralization by humus analyses at Sokli. In: Tanskanen H, editor. Methods of geochemical sampling and boulder prospecting in the eastern part of Baltic shield. Proceedings of a Finnish-Soviet seminar; 1979 Sept 3–5; Leningrad, Russia. Committee for Scientific and Technical Co-operation between Finland and Soviet Union. p. 235–245.
- Walter TR, Troll VR. 2001. Formation of caldera periphery faults: an experimental study. Bull Volcanol. 63:191–203. doi: 10.1007/s004450100135
- Weidendorfer D, Schmidt MW, Mattson HB. 2017. A common origin of carbonatite magmas. Geology. 45:507–510. doi: 10.1130/G38801.1
- Williams-Jones AE, Migdisov AA, Sampson IM. 2012. Hydrothermal mobilization of the rare earth elements – a tale of ‘ceria’ and ‘yttria’. Elements. 8:355–360. doi: 10.2113/gselements.8.5.355
- Williams-Jones AE, Palmer DAS. 2002. The evolution of aqueous–carbonic fluids in the Amba Dongar carbonatite, India: implications for fenitisation. Chem Geol. 185:283–301. doi: 10.1016/S0009-2541(01)00409-0
- Winterburn P. 2015. Exploration geochemistry: principles and practices for strategic commodities Nb, Ta, Zr, and rare earth elements. In: Simandl GJ, Neetz M, editors. Symposium on strategic and critical materials proceedings; Nov 13–14; Victoria, British Columbia. Victoria (BC): British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015–3. p. 193–197.
- Woodard J, Hetherington CJ. 2014. Carbonatite in a post-collisional tectonic setting: geochronology and emplacement conditions at Naantali, SW Finland. Precambrian Res. 240:94–107. doi: 10.1016/j.precamres.2013.10.017
- Woodard J, Hölttä P. 2005. The Naantali alvikite vein-dykes:a new carbonatite in southwestern Finland. Geological Survey of Finland Special Paper 38. p. 5–10.
- Woolley AR. 1969. Some aspects of fenitization with particular reference to Chilwa Island and Kangankunde, Malawi. Bull Br Museum (Nat History) Miner. 2(4). p. 191–219.
- Woolley AR. 1982. A discussion of carbonatite evolution and nomenclature, and the generation of sodic and potassic fenites. Mineral Mag. 46:13–17. doi: 10.1180/minmag.1982.046.338.03
- Woolley AR, Bailey DK. 2012. The crucial role of lithospheric structure in the generation and release of carbonatites: geological evidence. In:Downes H, Wall F, Demény A, Szabo C, editors. Continuing the carbonatite controversy. Mineral Mag. 76:259–270.
- Woolley AR, Kempe DRC. 1989. Carbonatites: nomenclature, average chemical compositions and element distribution. In: Bell K, editor. Carbonatites: genesis and evolution. London (UK): Unwin Hyman; p. 1–14.
- Woolley AR, Kjarsgaard A. 2008a. Carbonatite occurrences of the world: map and database. Geological Survey of Canada Open File 5796.
- Woolley AR, Kjarsgaard A. 2008b. Paragenetic types of carbonatite as indicated by the diversity and relative abundances of associated silicate rocks: evidence from a global database. Can Mineral. 46:741–752. doi: 10.3749/canmin.46.4.741
- Wu F-Y, Arzamastsev AA, Mitchell RH, Li Q-L, Sun J, Yang Y-H, Wang R-C. 2013. Emplacement age and Sr–Nd isotopic compositions of the Afrikanda alkaline ultramafic complex, Kola Peninsula, Russia. Chem Geol. 353:210–229. doi: 10.1016/j.chemgeo.2012.09.027
- Wyllie PJ, Huang WL. 1976a. Carbonation and melting reactions in the system CaO–MgO–SiO2–CO2 at mantle pressures with geophysical and petrological applications. Contrib Mineral Petrol. 54:79–107. doi: 10.1007/BF00372117
- Wyllie PJ, Huang WL. 1976b. High CO2 solubilities in mantle magmas. Geology. 4:21–24. doi: 10.1130/0091-7613(1976)4<21:HCSIMM>2.0.CO;2
- Wyllie PJ, Lee W-J. 1998. Model system controls on conditions from the mantle. J Petrol. 39:1885–1893. doi: 10.1093/petroj/39.11-12.1885
- Yegorov LS. 1993. Phoscorites of the Maymecha-Kotuy ijolite-carbonatite association. Int Geol Rev. 35:346–358. doi: 10.1080/00206819309465533
- Zaitsev AN. 2010. Nyerereite from calcite carbonatite at the Kerimasi volcano, northern Tanzania. Geol Ore Depos. 52:630–640. doi: 10.1134/S1075701510070159
- Zaitsev NA, Keller J. 2006. Mineralogical and chemical transformation of Oldoinyo Lengai natrocarbonatites, Tanzania. Lithos. 91(1-4):191–207. doi: 10.1016/j.lithos.2006.03.018
- Zaitsev NA, Keller J, Spratt J, Perova EN, Kearsley A. 2008. Nyerereite – pirssonite – calcite – shortite relationships in altered natrocarbonatites, Oldoinyo Lengai, Tanzania. Can Mineral. 46:843–860. doi: 10.3749/canmin.46.4.843
- Zaitsev AN, Wenzel T, Vennemann T, Markl G. 2013. Tinderet Volcano, Kenya: an altered natrocarbonatite locality? Mineral Mag. 77:213–226. doi: 10.1180/minmag.2013.077.3.01
- Zharikov VA, Pertsev NA, Rusinov VL, Callegari E, Fettes DJ. 2007. Metasomatism and metasomatic rocks - Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks: Web version 01.02.07. [accessed 2018 Apr 20]. https://www.bgs.ac.uk/scmr/products.html.
- Zimmermann R, Brandmeier M, Andreani L, Mhopjeni K, Gloaguen R. 2016. Remote sensing exploration of Nb-Ta-LREE-enriched carbonatite (Epembe/Namibia). Remote Sens. 8:620. doi: 10.3390/rs8080620