ABSTRACT
Introduction: Childhood obesity has serious health risks including the development of metabolic syndrome, cardiovascular disease and mortality later in life. The critical growth period from 3 to 7 years provides a window of opportunity for interventions. The goal of this study is to evaluate a one year, multidisciplinary, low-intensity treatment program for young obese children, complemented with web-based modules, called “AanTafel!”, on body composition, cardiometabolic risk profile, quality of life (HRQoL), eating behavior and physical activity.
Methods: In the pre-post-test design all measures were taken at baseline, 4 months, at the end of treatment and 3 years after baseline.
Results: Thirteen boys and 27 girls with median BMI z-score of, respectively, 4.2 and 3.3 aged 3 to 8 started “AanTafel!”. Eighty percent (n = 32) completed treatment. BMI z-score decreased with 0.45 (end of treatment) and sustained after 3 years. At the start, 16.7% of the children had all four components of metabolic syndrome which decreased to 0%. HDL cholesterol significantly increased. Concentrations of the markers IL18, e-selectin, and sICAM significantly decreased indicating a reduction of inflammation.
Conclusion: “AanTafel!” is effective in improving health of obese young children. The reduction of overweight is clinically relevant and sustained after 3 years.
Introduction
Childhood obesity has serious health risks including development of metabolic syndrome and cardiovascular disease, lower health-related quality of life (HRQoL), and overall mortality later in life (Han et al. Citation2010). Research indicates the age interval from 3 to 7 years as a critical growth period with increased risk of persistent obesity (Williams and Goulding Citation2009). Among obese preschoolers, cardiometabolic risk factors are already common and HRQoL is impaired (van Grieken et al. Citation2013; Shashaj et al. Citation2014). With regards to reversing risks, treatment of young children has shown to be more effective than treatment of older children (Reinehr et al. Citation2010). Hence, young children represent a window of opportunity for interventions (van Hoek et al. Citation2014). Our systematic review with meta-analysis indicates that moderate and high intensity (>26 h) multicomponent treatment programs for overweight or obese young children result in clinically relevant effects (van Hoek et al. Citation2014). Low intensity (10–26 h) programs are potentially cost-effective; however, their effects are inconclusive compared to very low (<10 h) and moderate to high level of intensity programs (van Hoek et al. Citation2014).
In 2009 we developed the one-year, multidisciplinary, multicomponent treatment program “AanTafel!” for young 3–8-year-old obese children. “AanTafel!” is Dutch for going to the table, which is associated with positive domesticity. This treatment program is based on clinical guidelines, scientific literature, interviews with parents of young obese children and expert opinion (van Hoek et al. Citation2016). It has a low-intensity level and is complemented with a web-based learning module. A first pilot process evaluation showed that parents and therapists rated the program as acceptable. This study reports on the effectiveness of “AanTafel!” on overweight, cardiovascular risk profile, HRQoL, eating behavior and physical activity, using a pre-post-test design. We hypothesize that “AanTafel!” improves eating behavior and physical activity, reduce the degree of overweight based on the BMI z-score and improves cardio-metabolic risk profile and HRQoL.
Materials and methods
Screening
Participants were enrolled in a regional hospital (Gelderse Vallei Hospital, Ede, the Netherlands) between March 2010 and May 2013. Children, aged 3 to 8 years, referred by their general practitioner or youth health-care physician because of overweight/obesity according to the IOTF criteria (Cole et al. Citation2000), were invited to participate in “AanTafel!”. Children with endocrine, chromosomal or syndrome disorders were excluded from treatment.
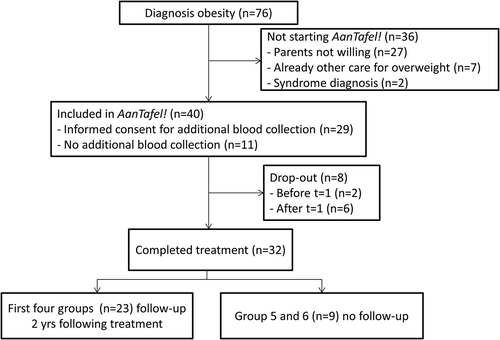
During the study period, 76 patients visited our hospital with the diagnosis of overweight. Forty patients started “AanTafel!” (). Reasons for not starting “AanTafel!” were parents not willing to start treatment (n = 27), already other care for overweight (n = 7), or syndrome diagnosis (n = 2). Written informed consent was obtained from each child’s parent or caregiver. The medical ethical committee of the Wageningen University approved the study protocol.
Intervention
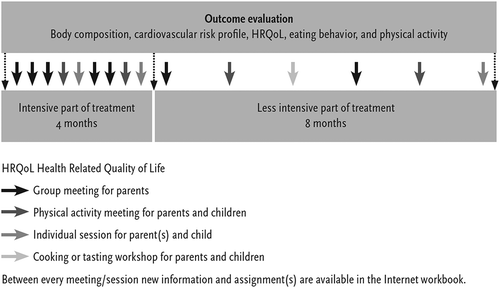
“AanTafel!” is developed by integrating evidence from science and practice in a five-step development process as reported earlier (van Hoek et al. Citation2016). Evidence from clinical guidelines (step I) was combined with a systematic review with meta-analysis (Step II) and an extended literature review (Step III). Furthermore, practice-based insights from parental interviews (Step IV) and involved therapists were added and subsequently the findings were integrated into the intervention “AanTafel!” (Step V). In sum, the resulting program “AanTafel!” provides 22.5 contact hours of physical exercise, nutrition education, and behavioral therapy (). The program focuses on parents, is tailored to age (3–8 years), and individual participants, includes individual- and group sessions and a web-based learning module. The first 4 months aim to increase awareness and to set goals for diet, eating behavior, and physical activity for the whole family; the second part aims to prevent relapses and to sustain the acquired behaviour in the family setting. The Web-based learning module provides information, short movie-clips and homework assignments, which participants have to complete before the next session. The team decided to include this module to reduce the number of high-cost individual sessions without reducing the amount of tailored feedback.
Evaluation scheme
The pre-post-test design involved measures of body composition, cardiometabolic risk profile, HRQoL, eating behavior and physical activity at baseline (t = 0), after the first 4 months of treatment (t = 4), and at the end of treatment (t = 12; ). BMI-z score was also measured at 3 years after baseline (t = 36) among the first 23 children who finished treatment.
To compare with natural course of BMI z-score in overweight or obese children, the Amsterdam Born Children and their Development prospective cohort study from fetal life onward served as reference population (van Eijsden et al. Citation2011). Weight and height were available from Youth Health Care (age 0 to 7). From the 8.266 pregnant women in this cohort study, 6575 consented to follow their child’s growth data. At the age of 5 years, 3321 children were measured (van Eijsden et al. Citation2011). In total 249 children were overweight (including 36 obese children) (Cole et al. Citation2000).
Outcome measures
Anthropometry
Trained staff measured children’s weight in underwear using an electronic calibrated scale (Seca 761), and height without shoes using a stadiometer (Holtain Ltd., Crymych, Cryfed, UK). Age and sex-specific BMI z-scores were calculated using Dutch growth curves of 2010 based on the LMS-methods (Talma et al. Citation2010). Biceps, triceps, subscapular, and suprailiacal skinfold thickness were measured with a skinfold caliper (Holtain Ltd., Crymych UK). Age and sex-specific z-scores for middle upper arm circumference (MUAC), waist circumference (WC), and the sum of the four skinfold thicknesses were calculated using the program Growth Analyser Research Calculation Tools (version 4.0, Growth Analyser B.V.) (Gerver and de Bruin Citation2001; Talma et al. Citation2010)
Cardiometabolic risk
Systolic and diastolic blood pressure were measured after 5-min rest in supine position with an automated blood pressure monitor (Welch Allyn VSM 300, Skaneateles Falls, NY, USA) at the left arm. The percentile scores of blood pressure were taking into account age, height, and sex (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescent Citation2004). Blood samples were taken after overnight fasting and analyzed for total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides (TG), glucose, glycated hemoglobin (HbA1c), and insulin. As a measure of insulin resistance, the homeostasis model assessment (HOMA IR) was used (glucose (mmol/L) * insulin (mU/L))/22.5 (Matthews et al. Citation1985). Individual components of the metabolic syndrome were recorded as defined by Ahrens et al. (Citation2014). The concentrations of the markers vascular endothelial growth factor (VEGF) and hsCRP were measured at the Wageningen University (V-PLEX Plus Human Biomarker 40-Plex Kit (MesoScale Diagnostics, Rockville, Maryland)). Interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-18 (IL-18), tumor necrosis factor (TNF), monocyte chemoattractant protein-1 (MCP-1), e-selectin, leptin, chemerin, plasminogen activator inhibitor-1 (PAI-1), retinol binding protein-4 (RBP-4), soluble intercellular adhesion molecule (sICAM), soluble vascular cell adhesion molecule (sVCAM), TIMP metallopeptidase inhibitor 1 (TIMP-1), cathepsin S, adiponectin were measured at the University Medical Center Utrecht using a previously developed and validated multiplex immunoassay (Schipper et al. Citation2010).
Health-related quality of life (HRQoL)
HRQoL in children of 5 years and older was measured by the generic parent-completed Child Health Questionnaire (CHQ-PF50, 14 domains) and in children below the age of 5 years by the Toddler version of the CHQ (Infant Toddler Quality of Life Questionnaire (ITQOL), 12 domains) (Raat et al. Citation2002, Citation2007). Items were summed and transformed on a 0–100 continuum that ranges from 0 (worst possible score) to 100 (best possible score) (Raat et al. Citation2002, Citation2007). The scores of different domains (CHQ-PF50) were combined to a physical and psychosocial summary score (Raat et al. Citation2002).
Eating behavior and physical activity
Eating behavior was measured by the parent-completed Children’s Eating Behaviour Questionnaire (CEBQ) (Sleddens et al. Citation2008). It covers the eight dimensions of eating style that are indicated to influence body weight. Dietary intake was assessed using a 3-day diary (including one weekend day). Daily energy and macronutrients intake were assessed using Evry Dietist software (Evry B.V. 2012) based on the Dutch food table (NEVO online 2011/3.0). Physical activity was assessed using the tri-axial accelerometers Actigraph (GT3x) on four consecutive days (including two weekend days). Actilife software (version 5, ActiGraph, Pensacola, Florida) was used to process the data. Median counts per minute and median time in moderate or vigorous physical activity (MVPA) per day were calculated according to age-specific validated cut-off points (Trost et al. Citation2011).
Statistical analysis
Analyses were performed by using IBM SPSS statistics for Windows version 22 (IBM Corp, Armork, NY). Normally distributed variables were reported as mean with standard deviation (SD), non-normal distributed variables were preferably reported as median with interquartile range (IQR), and independent t-test, respectively, Mann–Whitney U test were used to compare variables between groups. Paired t-test or the Wilcoxon signed-rank test in case of skewed variables were used to assess differences between time points. The last measure was used for missing data at t = 36 months (n = 6). A p-value less than 0.05 was considered statistically significant. To correct for disturbance in inflammatory markers in case of acute infection the results of cases with a CRP of >10 were not taken into account. If >25% of values of a variable were below the lower detection limit we excluded the variable from further analysis (chemerin, leptin and TIMP-1). If ≤25% of values were below the lower detection limit we used 50% of this limit as imputed value in subsequent analysis.
Results
Main outcome
BMI-z score
At baseline, 6 children were overweight and 34 children were obese (13, 7 and 14 children, respectively, had obesity grade 1, 2 or 3). The mean BMI z-score was 4.2 in boys and 3.3 in girls (). BMI z-score significantly decreased over time (). The mean reduction between t = 0 and t = 4 was 0.30 (0.30 SD), and 0.45 (SD 0.49) between t = 0 and t = 12. Boys and girls showed similar reductions in BMI z-score over time. Overall six out of the 40 children converted from obesity to overweight or from overweight to normal weight. Eight children (20%) dropped-out during treatment (). The children who completed treatment did not significantly differ from the drop-outs in age, BMI z-score or educational level. With last-observation carried forward the 12 month change in BMI z-score amounted to 0.35 (SD 0.49, p-value <0.001). Follow-up data of the first 23 children were available at 36 months. In this group, the reduction in BMI z-score sustained with 0.61 (SD 0.86) at t = 36.
Table 1. Baseline characteristics of children in “AanTafel!” evaluation study (n = 40).
Table 2. Biomedical parameters during “AanTafel!” evaluation study.
Reference population
Two-hundred forty-nine children of the ABCD study had overweight or obesity at age 3, 4 or 5 years (). At the age of 3 or 4 years, the change in BMI z-score during the next 12 months amounted to −0.13 (SD 0.65). At the age of 5 years, the BMI z-score reduced less. Among the few children with obesity, the reductions were slightly different (−0.17 for age 3 year, −0.05 for age 4 and 5 years, ). Overall, the reduction in BMI z-score was significantly larger in our children aged 3-5 yr (-0.42, SD 45, n = 21) compared to observation in the obese children of reference group (p = 0.01).
Table 3. Characteristics (mean and SD) at baseline and after 1 year of follow-up in overweight (including obese) children and obese children from the ABCD study (van Eijsden et al. Citation2011).
Secondary outcomes
Anthropometry
The z-scores of the waist circumference and sum of four skinfold thickness significantly decreased in the intensive phase of treatment and remained stable in the less intensive phase of treatment ().
Cardiometabolic risk profile
Girls had higher systolic blood pressure levels and lower HDL-cholesterol compared to boys. The drop-outs had higher systolic blood pressure percentile (97.5 vs 75.0, p = 0.01) and HOMA IR (1.65 vs 0.47, p = 0.033) than the completers. HDL-cholesterol significantly increased during treatment (). In addition, a small increase in TC was found. No changes were found in HbA1c, HOMA-IR or blood pressure. At baseline, 16.7% of the children had all four components of metabolic syndrome decreasing to 0% at the end of treatment (). The percentage of children with no or one component of metabolic syndrome increased from 30.5% to 70.8% at the end of treatment. Concentrations of IL18, e-selectin, and sICAM significantly decreased from baseline to the end of treatment (). TNF and PAI-1 showed significant reductions in the less-intensive phase. A significant increase was seen in IL-8 in the intensive phase of treatment and sVCAM in the less-intensive phase.
Figure 3. Prevalence of metabolic syndrome components during the “AanTafel!” evaluation study at baseline (t = 0), 4 months (t = 4), and 12 months (t = 12).
t = 0 to t = 4, p-Value = 0.021; t = 4 to t = 12, p-Value = 0.95; t = 0 to t = 12, p-Value = 0.05
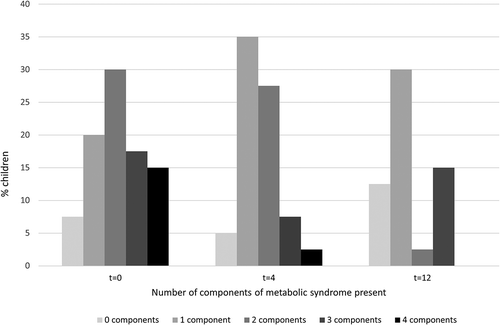
Table 4. Cardiometabolic markers during the “AanTafel!” evaluation study.
HRQoL
For all domains, except parental impact of time, the scores showed a non-significant improvement during treatment. The psychosocial summary score increased from 48.9 at baseline to 50.1 at the end of treatment with significant improvement after the intensive phase (Supplemental Table 1). The physical summary score increased from 49.7 to 53.2. This improvement is clinically relevant (Norman et al. Citation2003).
Tertiary outcomes
Eating behavior
The dimensions desire to drink and food fussiness of the CEBQ showed a significant decrease during overall treatment (Supplemental Table 2). No significant changes were found in the other dimensions.
Dietary intake
At baseline, a higher energy intake was reported than recommended by the Dutch Health Council (Supplemental Table 3) (Health Council of the Netherlands Citation2006). Median energy intake significantly decreased (approximately 300 kcal). Median fat intake reduced during the intensive phase. During treatment, the intake of sugar-containing sweet drinks decreased from 2.5 drinks per day to 1.2 drinks per day. Furthermore, the use of breakfast increased from 82% to 100% of the children.
Physical activity level
No difference was seen in the median counts per minute during the treatment (241 counts/min at baseline to 258 counts/min (p = 0.387)) and median time in MVPA per day (43 min at baseline to 38 min at 12 months (p = 0.178, data not shown)).
Discussion
This study showed that the multicomponent, multidisciplinary treatment program “AanTafel!” with low-intensity face-to-face contact and a complementary web-based learning module reduces weight gain in overweight and obese young children at short and long term. Furthermore, improvement of the cardiometabolic risk profile was found. Eating behavior improved, HRQoL showed a non-significant improvement and physical activity measured by accelerometry did not appear to change much.
The strength of this study is that it evaluates a treatment program based on multiple indicators, providing insight in the physical and psychosocial health and well-being of the children in short and long term. This is recommended in the literature, but not common practice (van Hoek et al. Citation2014). Another strength is that the evaluation is carried out in regular clinical practice rather than a research setting, and provides insight in the effect of treatment in a “real-life” setting (Singal et al. Citation2014). The study population is representative for the obese help-seeking population in practice. Therefore, the clinical relevant decrease of BMI z-score in short and long term is promising.
A limitation of our study on the other hand is that the conditions are less controlled comparing to a randomized controlled trial (RCT). Since patients were not randomized the more motivated families are probably in the AanTafel! group. This could have introduced bias. We used the reference population to compare the change in our patient group with the natural course of BMI- z score in overweight and obese children in the general population. In an RCT the likelihood of observing an intervention effect is maximized. However, this effect is often not representative to the effect of clinical intervention due to low external validity (Singal et al. Citation2014). The drop-out rate of 20% in our study is comparable to what is found in the literature (Kleber et al. Citation2009; Stark et al. Citation2011), and seemed to be similar as RCT’s on treating childhood obesity (Bocca et al. Citation2012). However, our sample is small and therefore results should be taken with caution.
A mean reduction in BMI z-score of 0.45 was found comparable with that of multicomponent moderate or high-intensity programs treating overweight (including obese) young children (Kleber et al. Citation2009; Stark et al. Citation2011; Bocca et al. Citation2012; van Hoek et al. Citation2014). A reduction of 0.5 in BMI z-score is clinically relevant given that it is associated with a reduction in cardiovascular risk factors and insulin resistance in children 4–15 years of age (Reinehr and Andler Citation2004; Reinehr et al. Citation2004).
Our intervention was of low intensity and hence, can be regarded as more cost-effective. The web-based learning module seemed to play a role in maintaining results with a treatment program of lower intensity as the short-term results are persisting on the long term.
At baseline, more than 30% of our study population had three of the four components defining metabolic syndrome compared to 1.5% in a population-based survey with European children 2.0–10.9 years (Ahrens et al. Citation2014). During intervention, this decreased to less than 15%. In addition, 16.7% of our study population had all four components of the metabolic syndrome decreasing to 0% at the end of treatment. Kleber at al. evaluated a multicomponent moderate to high-intensity treatment program (Obeldicks Mini) for young obese children with comparable result in BMI z-score (−0.46). An increase in HDL and triglycerides was observed as well (Kleber et al. Citation2009). Kleber at al. observed a significant decrease of HOMA IR of approximately 15%. In our study, no change in HOMA IR was observed. However, the mean HOMA IR at baseline in the Obeldicks Mini was 2.49, compared to mean HOMA IR of 1.01 in our study.
Importantly, the reduction in BMI z-score remains at long term. This results in lower chronic inflammation and better cardiometabolic risk profile reducing the risk of cardiometabolic diseases in the future. Evidence on long-term effects of overweight treatment programs for children is limited (Whitlock et al. Citation2010). To examine whether our result is not due to secular changes, we described the natural course of overweight children using a reference cohort. A small decrease in BMI z-score in 3 and 4 years old overweight or obese children and a stable BMI z-score in the 5-year-old children was found. Other studies showed that without treatment the BMI z-score of overweight and obese children does not change (van Hoek et al. Citation2014). The observed change in BMI z-score in our intervention group can therefore be seen as treatment effect.
Little is known about the effects of an overweight treatment program on markers of cardiovascular risk that mediate low-grade inflammation, endothelial dysfunction, or coagulation in young children. In 3 to 5-year-old children, a decrease in serum TNF concentration was found in a multidisciplinary intervention group (n = 33), but no changes were observed in levels of adiponectin, leptin, hsCRP and IL-6 (Bocca et al. Citation2012). In prepubertal children aged 6–9 years (n = 24) with a decrease in BMI z-score of ≥0.5, a decrease in CRP and borderline significant decrease in IL-6 was observed (Martos et al. Citation2009). No changes in sICAM were observed (Martos et al. Citation2009). We observed significant decreases in IL-18, e-selectin, and sICAM during treatment. This indicates that successful treatment of obese prepubertal children improves inflammation status. However, additional studies are needed to elucidate which markers are most sensitive for changes of cardiometabolic risk in these children.
The children in our study had lower HRQoL than the general Dutch population, especially on the domains emotional parental impact, physical functioning, and general health (Norman et al. Citation2003; Spuijbroek et al. Citation2011). The physical summary score of our population was also clinically relevant lower compared to the general- and to a severe obese non-treatment-seeking population (van Grieken et al. Citation2013). Young obese children have a generally low HRQoL and give an addition call for effective treatment. During our treatment program, HRQoL increased over time in all domains, except from the domain parental impact time, which may be due to time investment in the treatment of their obese child.
Children in our study were less active than recommended, less than 60 min/day MVPA. We found a non-significant decrease in time spent in MVPA and a non-significant increase in mean counts per minute. These conflicting results may be caused by the choices of higher cutoff levels in older children. Another reason could be that children have increased their low-intensity physical activity (walking or cycling) instead of MVPA, as was intended in this treatment program. Many parents stimulated their child to participate in organized sports like swimming and judo. However, during both sports the accelerometer cannot be used and therefore no data was available. These missing data may have resulted in the absence of significant changes in the level of physical activity.
Conclusions
We observed that a treatment program based on literature review, parental interviews and therapist practice-based insights from parental interviews and involved therapists with low intensity and a complementary web-based learning module is an effective strategy to improve the health and well-being of obese children aged 3 to 8 years on short and long term. The BMI z-score showed a clinically relevant decrease, not only at short term but also at 2 year follow-up. Approximately, one sixth of our treatment group had all four components of metabolic syndrome at baseline decreasing to 0% at the end of treatment, illustrating its potential for cardiometabolic risk management in obese children.
Author’s Contributions
Dr. van Hoek was the principle investigator of the evaluation of the treatment program, performed the data collection, analysis and drafted the initial manuscript, and approved the final manuscript as submitted.
Prof. dr. ir. Feskens, dr. ir. Bouwman and dr. Janse contributed to the study design, analytical framework, analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Ms Verburgt contributed to the collection of data on eating behavior, revised the manuscript, and approved the final manuscript as submitted.
Dr. Schipper and dr. de Jager carried out the initial laboratory measures, reviewed and revised the manuscript and approved the final manuscript as submitted.
Dr. T.G.M. Vrijkotte contributed data from the ABCD-study, reviewed and revised the manuscript and approved the final manuscript as submitted.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Health and Safety
All mandatory laboratory health and safety procedures have been complied with in the course of conducting any experimental work reported in this paper. This paper contains all appropriate warnings on any hazards that may be involved in carrying out the experiments or procedures we described.
Supplemental Material
Download MS Word (58.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, AJ, upon reasonable request [email protected].
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ahrens W, Moreno LA, Marild S, Molnár D, Siani A, De Henauw S, Böhmann J, Günther K, Hadjigeorgiou C, Iacoviello L, et al. 2014. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes (Lond). 38(Suppl 2):S4–14.
- Bocca G, Corpeleijn E, Stolk RP, Sauer PJJ. 2012. Results of a multidisciplinary treatment program in 3-year-old to 5-year-old overweight or obese children: a randomized controlled clinical trial. Arch Pediatr Adolesc Med. 166:1109–1115.
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. 2000. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 320:1240–1243.
- Gerver WJM, de Bruin R. 2001. Paediatric morfometrics; a reference manual. Maastricht: Universitaire Pers Maastricht.
- Han JC, Lawlor DA, Kimm SY. 2010. Childhood obesity. Lancet. 375:1737–1748.
- Health Council of the Netherlands. 2006. Guidelines for a healthy diet. The Hague: Health Council of the Netherlands.
- Kleber M, Schaefer A, Winkel K, Hoffmann D, Wunsch R, Kersting M, Reinehr T. 2009. Lifestyle intervention “Obeldicks Mini” for obese children aged 4 to 7 years. Klin Padiatr. 221:290–294.
- Martos R, Valle M, Morales RM, Cañete R, Gascón F, Urbano MM. 2009. Changes in body mass index are associated with changes in inflammatory and endothelial dysfunction biomarkers in obese prepubertal children after 9 months of body mass index SD score loss. Metabolism. 58:1153–1160.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28:412–419.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescent. 2004. The fourth report on the diagnosis, evaluation, and treatment of hogh blood pressure in children and adolecents. Pediatrics. 114:555–576.
- Norman GR, Sloan JA, Wyrwich KW. 2003. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 41:582–592.
- Raat H, Landgraf JM, Bonsel GJ, Gemke RJBJ, Essink‐Bot ML. 2002. Reliability and validity of the child health questionnaire-child form (CHQ-CF87) in a Dutch adolescent population. Qual Life Res. 11:575–581.
- Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot M-L. 2007. Reliability and validity of the infant and toddler quality of life questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 16:445–460.
- Reinehr T, Andler W. 2004. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 89:419–422.
- Reinehr T, Kiess W, Kapellen T, Andler W. 2004. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 114:1569–1573.
- Reinehr T, Kleber M, Lass N, Toschke AM. 2010. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 91:1165–1171.
- Schipper HS, de Jager W, van Dijk ME, Meerding J, Zelissen PMJ, Adan RA, Prakken BJ, Kalkhoven E. 2010. A multiplex immunoassay for human adipokine profiling. Clin Chem. 56:1320–1328.
- Shashaj B, Bedogni G, Graziani MP, Tozzi AE, DiCorpo ML, Morano D, Tacconi L, Veronelli P, Contoli B, Manco M. 2014. Origin of cardiovascular risk in overweight preschool children: a cohort study of cardiometabolic risk factors at the onset of obesity. JAMA Pediatr. 168:917–924.
- Singal AG, Higgins PD, Waljee AK. 2014. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 5:e45.
- Sleddens EF, Kremers SP, Thijs C. 2008. The children’s eating behaviour questionnaire: factorial validity and association with body mass index in Dutch children aged 6–7. Int J Behav Nutr Phys Ac. 5:49.
- Spuijbroek AT, Oostenbrink R, Landgraf JM, Rietveld E, de Goede-Bolder A, van Beeck EF, van Baar M, Raat H, Moll HA. 2011. Health-related quality of life in preschool children in five health conditions. Qual Life Res. 20:779–786.
- Stark LJ, Spear S, Boles R, Kuhl E, Ratcliff M, Scharf C, Bolling C, Rausch J. 2011. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity. 19:134–141.
- Talma HSY, Bakker B, Hirasing RA, Schonbeck, Y, Buuren, SV. 2010. Nederlandse Groeidiagrammen 2010: handleiding bij het meten en wegen van kinderen en het invullen van groeidiagrammen [Growth diagrams 2010: manual for measuring and weighing of children and the use of growth diagrams]. Leiden: TNO kwaliteit van Leven
- Trost SG, Loprinzi PD, Moore R, Pfeiffer KA. 2011. Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc. 43:1360–1368.
- van Eijsden M, Vrijkotte TG, Gemke RJ, van der Wal MF. 2011. Cohort profile: the Amsterdam born children and their development (ABCD) study. Int J Epidemiol. 40:1176–1186.
- van Grieken A, Veldhuis L, Renders CM, Landgraf JM, Hirasing RA, Raat H. 2013. Impaired parent-reported health-related quality of life of underweight and obese children at elementary school entry. Qual Life Res. 22:917–928.
- van Hoek E, Bouwman LI, Koelen MA, Lutt MAJ, Feskens EJM, Janse AJ. 2016. Development of a Dutch intervention for obese young children. Health Promot Int. dav115.
- van Hoek E, Feskens EJ, Bouwman LI, Janse AJ. 2014. Effective interventions in overweight or obese young children: systematic review and meta-analysis. Child Obes. 10:448–460.
- Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. 2010. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 125:e396–418.
- Williams SM, Goulding A. 2009. Patterns of growth associated with the timing of adiposity rebound. Obesity. 17:335–341.