ABSTRACT
Objective: Epicardial adipose tissue thickness (EATT) is suggested to play a role in the development of cardiovascular disease. In adolescents it is correlated with BMI z-score, cardiovascular risk factors, and pro- and anti-inflammatory markers. EATT of overweight/obese children was compared with EATT of normal weight peers (cross-sectional design). We investigated the association between EATT, cardiovascular risk factors and pro- and anti-inflammatory markers and the effect of a one year, multidisciplinary, treatment program on EATT in overweight/obese children (longitudinal design).
Methods: EATT was measured by echocardiography (25 obese, 8 overweight and 15 normal weight children; median age 5.1 years). In the overweight/obese children blood pressure, lipid profile, glucose, insulin, high sensitive CRP, and adiponectin concentrations were measured. In overweight/obese children participating in a multidisciplinary treatment program, measurements were repeated after 4 and 12 months.
Results: EATT was significantly higher in the overweight (median 1.38mm) and obese (median 1.57mm) children compared to normal weight children (median 0.87mm). Among obese children EATT was significantly inversely associated with adiponectin (r = −0.485).
Conclusions: EATT is increased in overweight/obese children and is inversely associated with adiponectin. Echocardiographic measurement of EATT is easy and might serve as a simple tool for cardio-metabolic risk stratification.
Introduction
Obesity among children and adolescents is widely considered as an important public health problem (Haslam and James Citation2005). Obese children are at increased risk of becoming obese adults (Dietz Citation1994; Singh et al. Citation2008) and is associated with increased cardiovascular mortality and morbidity (Mossberg Citation1989). Adipose tissue, especially visceral fat, has distinct metabolic characteristics by producing and secreting inflammatory metabolites resulting in low-grade systemic inflammation contributing to the development of metabolic- and cardiovascular disease (Han et al. Citation2010; Cheng et al. Citation2010; Weiss Citation2011; Choi et al. Citation2013). Even in childhood obesity is associated with inflammation, oxidative stress and endothelial dysfunction (Montero et al. Citation2012).
Since several years epicardial adipose tissue thickness (EATT) is subject of increasing research. Epicardial adipose tissue (EAT) is located between the visceral layer of the pericardium and the outer wall of the myocardium. No structure besides a fascia separates the EAT from the myocardium and coronary vessels (Sacks and Fain Citation2011; Echavarria-Pinto et al. Citation2013). It shares the same microcirculation and innervation as the myocardium and can therefore interact with the myocardium through vasocrine and paracrine pathways (Echavarria-Pinto et al. Citation2013; Cabrera-Rego et al. Citation2014). Under normal physiological conditions EAT has a role in producing energy, providing direct heating to the myocardium, and protecting the coronary arteries (Iacobellis and Bianco Citation2011; Iacobellis Citation2015). An imbalance between cardioprotective and harmful adipokines secreted by epicardial fat is strongly linked to the development of coronary arteriosclerosis (Shimabukuro et al. Citation2013). Increased EATT is correlated to visceral adipose tissue, body mass index (BMI), and coronary artery disease (CAD) in adults (Sacks and Fain Citation2011; Iacobellis and Bianco Citation2011; Echavarria-Pinto et al. Citation2013; Rabkin Citation2014).
Several studies in children and adolescents showed that EATT measured by echocardiography is correlated with BMI z-score and other cardiovascular risk factors (Akyol et al. Citation2013; Schusterova et al. Citation2014). In adolescents EATT was associated with pro- and anti-inflammatory markers like leptin, adiponectin, and high sensitive C reactive protein (hsCRP) (Ito et al. Citation2013; Ahrens et al. Citation2014). Others showed that EATT was associated with carotid intima-media thickness, a surrogate marker of early atherosclerosis, in children from the age of nine years (Akyol et al. Citation2013; Cabrera-Rego et al. Citation2014). It is unknown whether EATT is already increased at younger age.
The first aim of our study was therefore to analyze whether EATT is associated with obesity in young children, using a cross-sectional study of normal weight and obese children. The second aim was to examine the cross-sectional association between EATT, cardiovascular risk factors and pro- and anti-inflammatory markers in obese children, and the third aim was to study if a one year, multidisciplinary, low intensity treatment program modifies EATT in obese children. For this we measured EATT in a longitudinal study of obese children participating in a treatment program.
Subjects and methods
Participants were enrolled in a regional hospital (Gelderse Vallei Hospital, Ede, the Netherlands) between March 2010 and January 2015. Children, aged 3 to 8 years, referred by their general practitioner or youth health care physician because of overweight/obesity according to the World Obesity Federation (WOF), were invited to participate in the one year outpatient treatment program “AanTafel!”(Cole et al. Citation2000). Children with endocrine, chromosomal or syndrome disorders were excluded from treatment. “AanTafel” is developed by integrating evidence from science and practice in a five step development process as reported earlier (van Hoek et al. Citation2016). In sum, “AanTafel!” provides 22.5 contact hours of physical exercise, nutrition education, and behavioural therapy. The program focuses on parents and children, is tailored to age (3–8 years), includes individual- and group sessions and a web-based learning module. The first 4 months aim to increase awareness and to set goals for diet, eating behaviour, and physical activity; the second part (8 months) aims to prevent relapses and to sustain the acquired behaviour in the family setting. The Web-based learning module provides information, short movie-clips and homework assignments, which participants have to complete before the next session. The treatment team decided to include this module to reduce the number of high-cost individual sessions without reducing the amount of tailored feedback.
Healthy normal weight children were recruited at the outdoor clinic of our hospital. Children diagnosed with an innocent heartmurmer (confirmed by echographic investigation accomplished by a paediatric cardiologist), having normal weight and no morbidity were eligible to participate in the study.
Echocardiographic investigations were performed using GE Healthcare Vivid S6 (General Electric Company, United Kingdom) by three experienced and trained echo cardiographers. Epicardial fat was identified as the echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium. EATT was measured in left decubitus position in the parasternal long axis perpendicular to the aortic annulus, which was used as the anatomical landmark, according to the method proposed by Iacobellis et al (Citation2003). All measurements were taken end-systolic when the EATT is highest. The average value of three cardiac cycles was calculated. The coefficient of interobserver variation was 14.4% (EvH) and the coefficient of intraobserver variation was 19.5% (blinded measurements by LPK and EvH). Measurements were performed in anonymized images by one observer (EvH).
Height and weight was measured at baseline (t = 0), after the intensive phase (t = 1) and at the end of treatment (t = 2). Trained staff measured children’s weight without shoes, using an electronic, calibrated scale (Seca 761) and height without shoes using a stadiometer (Holtain limited, Crymych, Cryfed, UK). Age and sex-specific BMI z-scores were calculated (Talma et al. Citation2010). In overweight and obese children blood pressure was measured after 5 minutes rest in supine position with an automated blood pressure monitor (Welch Allyn VSM 300, Skaneateles Falls, NY, USA) at the left arm. Repeated measurements were performed until the value was stable. Blood samples were taken after overnight fasting. Blood samples were analyzed for total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, glucose, and insulin. All analyses were done using Dimension Vista 1500 automated analyzer (Siemens, Erlangen, Germany) in plasma with enzymatic methods except for fasting glucose, and insulin (Allain et al. Citation1974). LDL-cholesterol was calculated with the Friedewald equation (Friedewald et al. Citation1972). Insulin was measured in serum using immulite automated analyzer (Siemens, Erlangen, Germany). As a measure of insulin resistance the HOMA IR was used. HOMA-IR was calculated by the formula (glucose (mmol/L) * insulin (mU/L))/22.5 (Matthews et al. Citation1985). Concentrations of hsCRP were measured in serum at the Laboratory of the Division of Human Nutrition, Wageningen University by the V-PLEX Plus Human Biomarker 40-Plex Kit (Meso Scale Diagnostics, Rockville, Maryland). Leptin and adiponectin concentrations were measured at the Laboratory of Translational Immunology of the University Medical Center Utrecht using a validated multiplex immunoassay (Schipper et al. Citation2010). Written informed consent was obtained from the parents/guardians of all participants.
The medical ethical committee of the Wageningen University (Medische Ethische Toetsingscommissie van Wageningen University, METC-WU) approved the study protocol (METC-WU 10/08, ABR-no. NL 32376.081.10).
Statistical analysis
Analyses were performed by using IBM SPSS statistics for Windows version 22 (IBM Corp, Armork, NY). In case of > 25% of values of a variable below the lower detection limit we excluded the variable from further analysis. This was the case for leptin. In case of ≤ 25% of values below the lower detection limit we used 50% of this limit as imputed value in subsequent data analysis. Baseline characteristics are presented as mean ± standard deviation in case of normal distribution, otherwise values are presented as median with interquartile range (IQR). Because of non-normal distribution differences between groups were tested using the Mann-Whitney U test and Kruskal-Wallis test depending on the number of groups. For categorical variables a Chi-Square test was used. To test differences in variables over time the Wilcoxon matched pairs signed rank sum test was used. Spearman’s rho was calculated to assess correlations. Multivariate analysis was performed using a multiple linear regression model with forced entry. Variables with p < 0.2 in bivariate analyses were included in the multiple linear regression model. The level of significance was set at p < 0.05.
Results
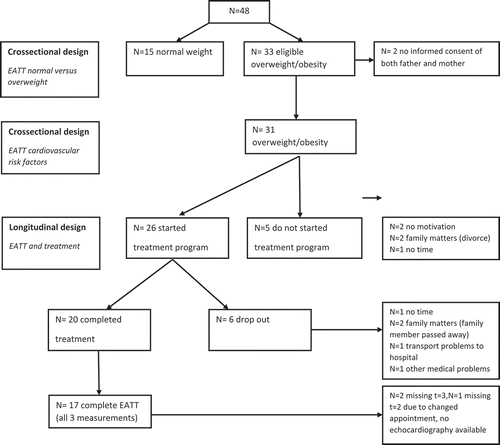
A total of 48 children (25 obese, 8 overweight and 15 normal weight) were included in the study. The median age of all children (17 boys and 31 girls) was 5.1 years (IQR 4.3–6.9 years). Of the 33 overweight or obese children, 26 children participated in the treatment program. Of these 26 children 20 children completed the treatment program; in 17 out of these 20 children complete echo views were available at baseline and during follow-up (). Patients who dropped-out during treatment (n = 6) did not differ according to age and BMI z-score, but had significantly higher systolic blood pressure and HOMA IR (data not shown) than the patients who did not dropped out.
The obese, overweight and normal weight children were not significantly different regarding height, but they differed regarding sex, age, weight, BMI, and BMI z-score (). In obese children EATT was significantly higher than in overweight children, respectively 1.57 versus 0.87 mm (p = 0.001 ). In the total study population EATT was significantly associated with BMI z-score (). This remained after adjusting for age and sex. This multiple regression analysis also showed that EATT was independently related to sex, with girls have higher EATT than boys. The positive association of EATT with age was weak when taking sex and BMI z-score into account.
Table 1. Baseline characteristics of children aged 3–8 year with normal weight, overweight and obesity.
Table 2. Results of single and multiple regression analysis of EATT on age, sex and BMI z-score in participating normal weight, overweight and obese children (n = 48).
Within the children with overweight or obesity, the association between EATT cardiovascular and metabolic risk factors was investigated (). An inverse association of EATT with adiponectin and HDL cholesterol was observed. These associations were independent of sex and BMI z-score (partial r = −0.50, p = 0.009 for adiponectin and r = −0.41, p = 0.036 for HDL-cholesterol)
Table 3. Correlation between EATT and cardiovascular risk factors in 31 children age 3–8 yr with overweight (n = 6) or obesity (n = 25).
Multidisciplinary treatment of the 17 obese children included in the treatment program decreased BMI z-score by −0.4 (SD 0.57) and increased HDL cholesterol (+ 0.16 mmol/L, SD 0.26). However, EATT did not change during treatment (+ 0.09 mm, SD 0.17) and neither did adiponectin ().
Table 4. Longitudinal data of obese young children participating in AanTafel! (n = 17).
Discussion
The present study showed that EATT in young obese children is increased compared to EATT in normally weight children and is independently associated with BMI z-score. Among overweight and obese children EATT is negatively associated with adiponectin, also when adjusted. EATT did not change during multidisciplinary treatment, despite a reduction of BMI z-score.
As far as we know, this is the first study comparing EATT, measured by echocardiography, in young normal weight children with EATT in young obese children. The three most used imaging techniques to measure EATT are echocardiography, magnetic resonance imaging (MRI) and computer tomography (CT). An advantage of measuring EATT by transthoracic echography is that it is non-invasive, fast, cheap, radiation free and relatively easy applicable in young children. A limitation of measuring EATT by transthoracic echography is the question of whether thickness measurements from a single location truly reflect the total EATT burden, while MRI and CT create volumetric measurements in addition to thickness measurements (Douglass et al. Citation2017).
In adults, epicardial fat can be routinely detected by non-contrast CT performed for enabling risk assessment through coronary calcium artery calcification (CAC). Several studies have demonstrated a strong association between EAT and coronary plaque burden while others describe an association of EAT with overall plaque burden (Greif et al. Citation2009; Ito et al. Citation2013). EAT seems to complement information derived from the CAC scores to predict coronary events in adults. Wu et al. showed that right ventricular free-wall EATT had a strong correlation with EAT volume (r = 0.49) suggesting that right ventricular free-wall EATT can indeed be used as a proxy for EAT volume (Wu et al. Citation2013).
Epidemiological studies demonstrate the association of EAT with cardiovascular disease. In asymptomatic patients EAT is independently related to major adverse cardiac events. The association of increased EAT volume with cardiovascular disease remains significant even after adjustment for body mass index, and traditional cardiovascular risk factors in the adult population (Cheng et al. Citation2010; Mahabadi et al. Citation2013). Current evidence indeed supports the hypothesis that in obese persons EAT may become an adverse lipotoxic and proinflammatory organ that contributes to the in the development of coronary atherosclerosis (Echavarria-Pinto et al. Citation2013).
Increased carotid intima-media thickness (CIMT) and increased EAT has been found in children and adolescents with obesity (Slyper Citation2004; Cabrera-Rego et al. Citation2014). In our study we did not measure CIMT due to the young age of our children. CIMT is a marker to assess preclinical atherosclerosis and is associated with future cardiovascular events (van den Oord et al. Citation2013). Increased CIMT is inversely related to adipokine levels and HDL-cholesterol (Gilardini et al. Citation2011; Stroescu et al. Citation2017). Goeller et al. recently showed that EAT volume was inversely related to adipokines, HDL-cholesterol and angiotensin in adults (Goeller et al. Citation2018). In our study adiponectin was measured in overweight and obese children. An inverse relation with EATT was found, showing that also in young children EAT may contribute to cardio- metabolic risk.
We did not find a change of EATT during effective treatment based on the change of BMI z-score in young overweight or obese children. In adults, weight loss following dietary intervention was significantly associated with reductions in EATT of 0.8 to 4.0 mm (Rabkin and Campbell Citation2015). A possible explanation could be that EATT in young children is relatively thin, even in obese children as shown in our study. In combination with the small sample size, changes in EATT due to a decrease in BMI z-score are probably outside the limits of detection in our study. Another reason could be that the inter-observer and intra-observer variability were considerable, again possibly due to the thin EATT.
Conclusion
Our study showed that even in young obese children EATT was increased compared to normal weight children, and as EATT was inversely related to adiponectin levels and HDL-cholesterol this contributes to an unfavorable cardiovascular risk profile. Despite some limitations, transthoracic echocardiographic measurement is an easy tool to measure EAT in young children. It is non-invasive, fast, cheap, radiation free and relatively easy applicable and might serve as a simple tool for cardio-metabolic risk stratification in children and adolescents.
Authorship contribution
All authors have participated in the concept and design; analysis and interpretation of data; drafting or revising of the manuscript
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahrens W, Moreno LA, Marild S, Molnar D, Siani A, De Henauw S, Bohmann J, Gunther K, Hadjigeorgiou C, Iacoviello L, et al. 2014. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes (Lond). 38(Suppl 2):S4–14.
- Akyol B, Boyraz M, Aysoy C. 2013. Relationship of epicardial adipose tissue thickness with early indicators of atherosclerosis and cardiac functional changes in obese adolescents with metabolic syndrome. J Clin Res Pediatr Endocrinol. 5(3):156–163.
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. 1974. Enzymatic determination of total serum cholesterol. Clin Chem. 20(4):470–475.
- Cabrera-Rego JO, Iacobellis G, Castillo-Herrera JA, Valiente-Mustelier J, Gandarilla-Sarmientos JC, Marin-Julia SM, Navarrete-Cabrera J. 2014. Epicardial fat thickness correlates with carotid intima-media thickness, arterial stiffness, and cardiac geometry in children and adolescents. Pediatr Cardiol. 35(3):450–456.
- Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, et al. 2010. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 3(4):352–360.
- Choi J, Joseph L, Pilote L. 2013. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 14(3):232–244.
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. 2000. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (Clin Res ed). 320(7244):1240–1243.
- Dietz WH. 1994. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 59(5):955–959.
- Douglass E, Greif S, Frishman WH. 2017. Epicardial fat: pathophysiology and clinical significance. Cardiol Rev. 25(5):230–235.
- Echavarria-Pinto M, Hernando L, Alfonso F. 2013. From the epicardial adipose tissue to vulnerable coronary plaques. World J Cardiol. 5(4):68–74.
- Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18(6):499–502.
- Gilardini L, Pasqualinotto L, Di Matteo S, Caffetto K, Croci M, Girola A, Invitti C. 2011. Factors associated with early atherosclerosis and arterial calcifications in young subjects with a benign phenotype of obesity. Obesity (Silver Spring, Md). 19(8):1684–1689.
- Goeller M, Achenbach S, Marwan M, Doris MK, Cadet S, Commandeur F, Chen X, Slomka PJ, Gransar H, Cao JJ, et al. 2018. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 12(1):67–73.
- Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M, et al. 2009. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 29(5):781–786.
- Han JC, Lawlor DA, Kimm SY. 2010. Childhood obesity. Lancet. 375(9727):1737–1748.
- Haslam DW, James WP. 2005. Obesity. Lancet. 366(9492):1197–1209.
- Iacobellis G. 2015. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 11(6):363–371.
- Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F. 2003. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 11(2):304–310.
- Iacobellis G, Bianco AC. 2011. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 22(11):450–457.
- Ito T, Suzuki Y, Ehara M, Matsuo H, Teramoto T, Terashima M, Nasu K, Kinoshita Y, Tsuchikane E, Suzuki T, et al. 2013. Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int J Cardiol. 167(6):2852–2858.
- Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, Moebus S, Jockel KH, Erbel R, et al. 2013. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf recall study. J Am Coll Cardiol. 61(13):1388–1395.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28(7):412–419.
- Montero D, Walther G, Perez-Martin A, Roche E, Vinet A. 2012. Endothelial dysfunction, inflammation, and oxidative stress in obese children and adolescents: markers and effect of lifestyle intervention. Obes Rev. 13(5):441–455.
- Mossberg HO. 1989. 40-year follow-up of overweight children. Lancet. 2(8661):491–493.
- Rabkin SW. 2014. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 12(1):31–42.
- Rabkin SW, Campbell H. 2015. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev. 16(05):406–415.
- Sacks HS, Fain JN. 2011. Human epicardial fat: what is new and what is missing? Clin Exp Pharmacol P. 38(12):879–887.
- Schipper HS, de Jager W, van Dijk ME, Meerding J, Zelissen PM, Adan RA, Prakken BJ, Kalkhoven E. 2010. A multiplex immunoassay for human adipokine profiling. Clin Chem. 56(8):1320–1328.
- Schusterova I, Leenen FH, Jurko A, Sabol F, Takacova J. 2014. Epicardial adipose tissue and cardiometabolic risk factors in overweight and obese children and adolescents. Pediatr Obes. 9(1):63–70.
- Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, Fukuda D, Soeki T, Kitagawa T, Takanashi S, et al. 2013. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 33(5):1077–1084.
- Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. 2008. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 9(5):474–488.
- Slyper AH. 2004. Clinical review 168: what vascular ultrasound testing has revealed about pediatric atherogenesis, and a potential clinical role for ultrasound in pediatric risk assessment. J Clin Endocrinol Metab. 89(7):3089–3095.
- Stroescu R, Bizerea T, Doros G, Marazan M, Lesovici M, Marginean O. 2017. Correlation between adipokines and carotid intima media thickness in a group of obese Romanian children: is small for gestational age status an independent factor for cardiovascular risk? Arch Endocrinol Metab. 61(1):14–20.
- Talma H, Schönbeck Y, Bakker B, Hirasing RA, van Buuren S. 2010. Groeidiagrammen 2010: handleiding bij het meten en wegen van kinderen en het invullen van groeidiagrammen [Growth diagrams 2010: manual for measuring and weighing of children and the use of growth diagrams]. Leiden: TNO Kwaliteit van Leven.
- van den Oord SC, Sijbrands EJ, Ten Kate GL, van Klaveren D, van Domburg RT, van der Steen AF, Schinkel AF. 2013. Carotid intima-media thickness for cardiovascular risk assessment: systematic review and meta-analysis. Atherosclerosis. 228(1):1–11.
- van Hoek E, Bouwman LI, Koelen MA, Lutt MA, Feskens EJ, Janse AJ. 2016. Development of a Dutch intervention for obese young children. Health Promot Int. 32(4):624-635.
- Weiss R. 2011. Childhood metabolic syndrome: must we define it to deal with it? Diabetes Care. 34(Suppl 2):S171–176.
- Wu FZ, Huang YL, Wang YC, Lin HS, Chen CS, Ju YJ, Chiou KR, Cheng CC, Wu MT. 2013. Impact of location of epicardial adipose tissue, measured by coronary artery calcium-scoring computed tomography on obstructive coronary artery disease. Am J Cardiol. 112(7):943–949.