?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Activated carbons (ACs) were prepared from Leucaena leucocephala biomass. This is an agricultural solid waste by-product, as well as easily grown species available abundantly in Southeast Asia. In this work, activated carbon derived from Leucaena leucocephala biomass was prepared for sequestration of cadmium from aqueous solutions. The activated carbon was produced by a NaOH chemical activation process using NaOH:char ratios (w/w) of 1:1, 2:1 and 3:1 at 800 °C. The activated carbon’s BET surface areas were determined as 185, 595, and 776 m2 g−1 for the NaOH:char ratios of 1:1, 2:1, and 3:1, respectively. At initial concentration of 30 mg/l, maximum cadmium adsorption was achieved within 40 min. The adsorbent also showed good sorption of cadmium at pH 7.0 with solution temperature of 30 °C. Isothermic studies showed the adsorption process best fitted to Langmuir isotherm which indicates monolayer adsorption had happened while kinetic studies showed physisorption process was favorable, determined by pseudo-first order kinetic model. Maximum adsorption capacity was found to be 70.423 mg/g for NaOH:char ratios of 3:1.
1. Introduction
Heavy metals receive great concern due to their potential risk as common environmental pollutants. Heavy metal contamination of water is not only harmful to aquatic life but also adversely affects human health. Heavy metal contamination comes from electronic, metal smelting and automotive industries (Ahmad Khan, Ibrahim, & Subramaniam, Citation2004; Bailey, Olin, Bricka, & Adrian, Citation1999). One of the heavy metals involved is cadmium. Cadmium (Cd) is a metal identified for its high degree of toxicity. The presence of Cd as an impurity in zinc of galvanized pipes or Cd-containing solders in fittings, water coolers, water heaters and taps leads to contamination of drinking water. Long-term consumption leads to Cd accumulation in the human body especially in the kidneys where dysfunctioning of the organ results in a worst case scenario (Bernard, Citation2008).
The various methods that are used for water treatment have been developed to be applied for heavy metal removal (Ali et al., Citation2018). These methods include filtration, screening, oxidation, sedimentation, distillation, evaporation, reverse osmosis, electro-chemical, ion exchange and adsorption (Basheer, Citation2018). Comparatively, adsorption is one of the applicable methods because of convenience, ease of operation or production and simplicity of design (Yaacoubi, Zidani, Mouflih, Gourai, & Sebti, Citation2014).
Adsorption using activated carbon is one of the ways to eliminate this problem. However, the high cost of producing activated carbon is a limiting factor. The properties of normally activated carbon are still not sufficient to ensure efficient heavy metal uptake from waste water. Therefore, chemical activation of the activated carbon needs to be employed to further increase the effectiveness of water treatment. Phosphoric acid, sulfuric acid, zinc chloride, sodium hydroxide and potassium hydroxide are among the dehydrating agents usually used in chemical activation (Regti, Laamari, Stiriba, & El Haddad, Citation2017). Recent research has been conducted by Devi et al. (Citation2017) using nano particles or other products resulting from the development of nanotechnology as an effective adsorbent for removal of cadmium. Other adsorbents used for sequestration of the cadmium ion from water are given in .
Table 1. Some adsorbents used for removal of cadmium ion from water.
Activated carbon is a popular choice of adsorbent for contaminants removal, especially heavy metal and dye from wastewater (Babel & Kurniawan, Citation2003). Various researches have been conducted, aiming to reduce the cost of activated carbon production without sacrificing the efficiency of the material in the removal of heavy metals from waste water. Low-cost adsorbents are a very popular material and receive much attention for adsorption of pollutants such as heavy metals and dyes (Sadegh, Mazloumbilandi, & Chahardouri, Citation2017). Therefore, this work on Leucaena leucocephala as a low-cost material for activated carbon production, has been processed by chemical means to obtain highest possible adsorption capacity.
Leucaena leucocephala, which is also known locally as the Petai Belalang tree is native to southern Mexico and northern Central America and is widespread in the Asian tropics and Malaysia. Parts of the Leucaena leucocephala plant are commonly used for animal food and as a vegetable for human consumption. Leucaena leucocephala is widely grown all over Malaysia and can be easily cultivated. The abundance of this plant offers a new opportunity to utilize its biomass in many fields. It is a fast-growing species reaching 10 m in height after 24 months of cultivation. The wood volume produced after 24 months of planting can rise to 91.3 m3 per hectare (MacDicken & Brewbaker, Citation1988; Rasat et al., Citation2016). Utilization of Leucaena leucocephala can reduce production cost as it is a wild tree and can be grown without specific silviculture techniques.
Studies on the adsorption behavior of the adsorbent materials towards heavy metals are important to evaluate the optimum condition, thus increasing the efficiency of the water treatment process. Due to lack of information on activated carbon prepared from Leucaena leucocephala biomass, this work explores the potential of this new raw material together with its performance in the water purification process.
2. Experimental
2.1. Material preparation
Samples from wild Leucaena leucocephala were collected around Selangor, Malaysia. The samples were left to air dry until the moisture content became less than 10%. The dried sample was cut into a smaller size before being ground to produce particles between 1 and 2 µm size. The wood particles were kept in a closed container for further use (Rafatullah, Sulaiman, Hashim, & Amini, Citation2011).
2.2. Activated carbon preparation
Carbonization was carried out using a pyrolyzer to produce a large quantity of charcoal. Carbonized Leucaena leucocephala biomass particles were activated by a chemical process where the carbonized sample was impregnated with NaOH at the NaOH:carbonized sample ratios of 3:1, 2:1 and 1:1. Approximately 10 g of carbonized sample were homogenized with 30 g of NaOH in 100 mL distilled water in a vertical stainless steel reactor under continuous magnetic stirring for 2 h before drying in an oven at 105 °C for 24 h (Cazetta et al., Citation2011). After heating for 24 h, the sample was placed in the muffle furnace for the activation process. The sample was heated at 800 °C under a nitrogen flow of 150 cm3/min for 90 min during the process.
2.3. Surface area analysis
Surface area analysis was carried out using Micromeritics (ASAP 2010) analyzer. The activated carbon was degassed at 300 °C before nitrogen adsorption was carried out at –196 °C. Surface area was calculated using Brunauer–Emmett–Teller (BET) analysis software provided with the instrument {Sudaryanto, 2006 #4745}.
2.4. Scanning electron microscope (SEM) observation
The raw Leucaena leucocephala and activated carbon samples were sieved to obtain 35–60 µm sized fractions. The particles were sprinkled onto the tub with carbon glue before gold-plating with a vacuum electroplating machine for about 60 s. The samples were then analyzed using an SEM (JSM-IT100 JEOL Co.) microscope at 150x and 550x magnifications.
2.5. Batch adsorption studies
Approximately 0.5 g of the prepared activated carbon was mixed with 100 ml of the aqueous solutions of cadmium inside a conical flask, continuously shaken using a temperature-controlled water bath shaker for a pre-determined period. The mixtures were filtered with filter paper to separate the treated cadmium solution from the adsorbent. The metal ion concentration was determined by inductively coupled plasma optical emission spectrometry, ICP-OES at Forest Research Institute of Malaysia. All the experiments were performed in triplicate to reduce errors (Sulaiman, Amini, Rafatullah, Hashim, & Ahmad, Citation2010).
2.6. Isothermal studies
2.6.1. Langmuir isotherm
The Langmuir isotherm model suggested an ideal monolayer adsorption onto a homogenous surface. Three temperatures of the reaction were used, predetermined at 30, 40, and 50 °C. The following Equation 1 was used:
(1)
(1)
where qe is the amount of adsorbate adsorbed at equilibrium per gram of sample (mg/g), qm is the saturated amount of adsorbate adsorbed in mg/g, Ce is the equilibrium concentration of cadmium in mg/l, and Ka is the Langmuir adsorption constant in l/mg. A 1/qe versus 1/Ce plot was made, showing a straight line with a slope of 1/Kaqm and an intercept of 1/qm Ka was then calculated by the formula (Lee, Amini, Sulaiman, Mohamed, & Boon, Citation2018).
2.6.2. Freundlich isotherm
Freundlich isotherm assumes that heterogenous and multilayer adsorption occurred on the adsorbent surface (Çetinkaya, Targan, & Nüket Tirtom, Citation2018), calculated using EquationEquation 2(2)
(2) :
(2)
(2)
where KF is Freundlich equilibrium constant, n is an empirical constant and others as stated in EquationEquation 1(1)
(1) . Thus, a plot of ln qe vs. ln Ce should provide a straight line with a slope of (1/n) and an intercept of lnKF
2.7. Kinetic studies
Adsorption kinetics which investigate the rate and mechanism of the adsorption process pseudo-first-order kinetic model and pseudo-second order kinetics model.
2.7.1. Pseudo-first-order kinetic model
Pseudo-first-order kinetic model was derived from EquationEquation 3(3)
(3) :
(3)
(3)
Into linear Equation 4:
(4)
(4)
where qe and qt are the amount of ions adsorbed (mg/g) at the equilibrium and at time t, respectively. K1 represents the rate constant of pseudo-first order reaction. A plot of log (qe – qt) against t was plotted to obtain K1 from the slope and qe from the intercept.
2.7.2. Pseudo- second order kinetics model
While the pseudo- second order kinetics model is expressed by EquationEquation 5(5)
(5) :
(5)
(5)
Integration of Equation 5 generates linearized EquationEquation 6(6)
(6) :
(6)
(6)
where K2 is the rate constant of pseudo-second order reaction. K2 and qe can be determined from the intercept and slope of a linear plot of t/qt versus t, respectively (Borah et al., Citation2018).
3. Results and discussion
3.1. Surface area of activated carbon
The BET surface areas of Leucaena leucocephala activated carbon were measured to be 185, 595 and 776 m2g−1 for the NaOH:carbonized sample ratios of 1:1, 2:1 and 3:1, respectively. While increasing surface area results in higher adsorption capacity, it also contributes to the reduction in bulk density and physical strength of activated carbons. For harsh cyclic adsorption applications where high volumetric capacity is needed, this characteristic could be potentially a disadvantage (Lee, Byamba-Ochir, Shim, Balathanigaimani, & Moon, Citation2015). Hence, the activation process should be controlled correctly to obtain an excellent adsorbent with suitable capacity and bulk density for specific applications. The total pore volume and mesopore fraction were known to have positive relationship with the increment of mass ratio of the activating agent: raw material until a certain optimal point.
Among the most important parameters that determine the adsorption capacity of an adsorbent are the pore structure, the specific surface area and the total number of micropores and mesopores (Carrott & Carrott, Citation2007; Caglar et al., Citation2013). The International Union of Pure and Applied Chemistry (IUPAC) classifies ultramicropores for pore width less than 0.7 nm, supermicropores for pore width between 0.7 nm and 2 nm, mesopores for pore width between 2 nm and 50 nm, and macropores for pore width bigger than 50 nm. Activation process type and parameters affect the total number of micropores and mesopores, which leads to significant changes in the surface area and porosity, and directly influences the adsorption rate of the adsorbate.
The internal surface of activated carbon was mainly contributed by the micropores which comprise the main portion of the total surface area, while mesopores, macropores and the non-porous surface represent the external surface of the sample. Micropores usually act as adsorption sites, whereas the mesopores and macropores give rise to channels for the adsorbate to reach the micropores. The enlarged radii of the mesopores and macropores, on the other hand, allow the multilayer adsorption to occur.
3.2. Surface morphology analysis
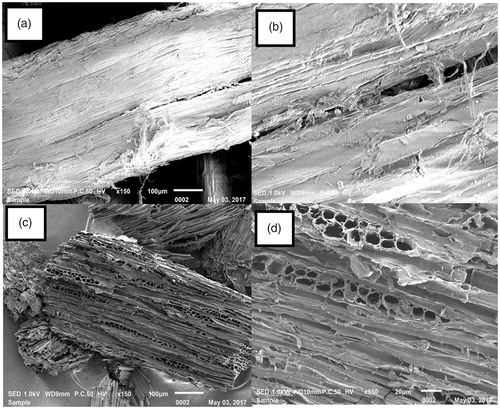
shows 150x (a) and 550x (b) magnified SEM photos of the activated carbon between the raw material of Leucaena leucocephala and activated carbon. It may be seen in that the raw material sample had some tiny holes between activated carbon samples. The SEM images of activated carbon obtained from Leucaena leucocephala () demonstrate that activated carbon possessed higher amounts of tiny and large holes than the raw material samples which can be explained by the emergence of the pores in the interlayer spacings of the samples during the activation process through heating (Caglar, Afsin, Tabak, & Eren, Citation2009).
3.3. Batch adsorption studies
3.3.1. Effect of contact time on the cadmium adsorption
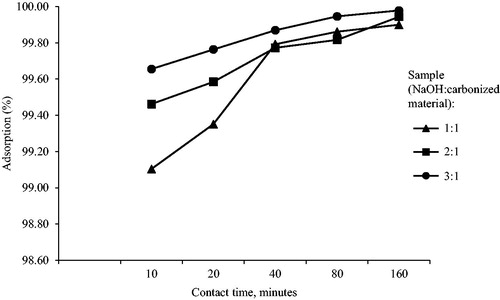
Effects of contact time, initial concentration, pH and temperature on the removal of cadmium by the activated carbon sample prepared from Leucaena leucocephala were studied. shows the removal of cadmium at the activation temperature of 800 °C for NaOH:carbonized sample ratios of 1:1, 2:1 and 3:1 after different contact times between 10 min and160 min with the initial Cd2+ concentration of 30 mg/l. Approximately 0.5 g/l of adsorbent dosage was used at pH 7.0 of the solution. Adsorption equilibrium was established within 40 min of treatment. At 160 min of contact time, the maximum amounts of adsorption of the samples were found as 99.86%, 99.82% and 99.97% for NaOH:carbonized sample ratios of 1:1, 2:1 and 3:1, respectively. The higher the ratio of NaOH:carbonized sample used for chemical activation, the higher the adsorption efficiency for cadmium removal as reflected from the larger surface area obtained by the higher NaOH:carbonized sample ratios in BET surface area analysis. No significant increment on the amount of removal of solute from the solutions was recorded beyond the maximum point where the system reaches equilibrium.
3.3.2. Effect of initial adsorbate concentration on the cadmium adsorption
The effect of initial concentration on the removal of cadmium by Leucaena leucocephala activated carbon was investigated at different sorbate solution concentrations (10, 20, 30, 40 and 50 mg/L) using 0.5 g adsorbent dose at pH 7.0. The percentage of cadmium removal decreased with the increase in the initial concentration of the solution. The surface area and the number of available active sites were relatively high compared to the adsorbate ions to be retained at low initial concentration. Thus, the cadmium ions were easily adsorbed and removed. On the other hand, the total number of available adsorption sites is smaller than the amount of adsorbate ions when the initial solution concentration is higher, thus decreasing the percentage of cadmium removal. The adsorption percentage for all samples was comparably high at relatively low concentrations of 10 mg/L and 20 mg/L as shown in . At the concentrations of 30 mg/L, 40 mg/L and 50 mg/L, NaOH:carbonized sample for the ratio 3:1 showed better performance than for the ratios of 2:1 and 1:1. These results clearly indicate that the maximum adsorption percentage of metal ions by activated carbon was affected by the initial metal ion concentration. Higher initial concentration gives rise to lower adsorption rate due to the higher adsorbate: adsorption site ratio.
3.3.3. Effect of pH on the cadmium adsorption
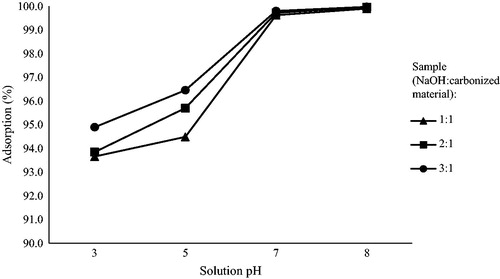
Rao, Rao, Seshaiah, Choudary, and Wang (Citation2008) stated that the most crucial parameter controlling the adsorption process which affects the binding of cadmium ions by surface functional groups was the pH value. previously only indicated the cadmium removal at the same pH and initial concentration. Experimental studies were conducted for 40 min of contact time with 40 mg/l initial cadmium ion concentration and 0.5 g/l of the adsorbent dose at a different pH range. The high pH value of the solution leads to increase in the negative charge on the adsorbent surface (Gupta & Sharma, Citation2002; Krishnan & Anirudhan, Citation2003). Therefore, the electrostatic interactions between positive charged Cd2+ ions and the layer of negatively charged adsorbent material, cause a more powerful affinity, thus increasing the amount of Cd2+ ions adsorbed onto the adsorbent (Banerjee & Chattopadhyaya, Citation2017). In contrast, where the pH was lowered to pH 5 and pH 3, the percentage of Cd2+adsorption was decreasing, which may be ascribed to the competition between H3O+ and Cd2+ ions in acidic media (Zheng et al., Citation2017).
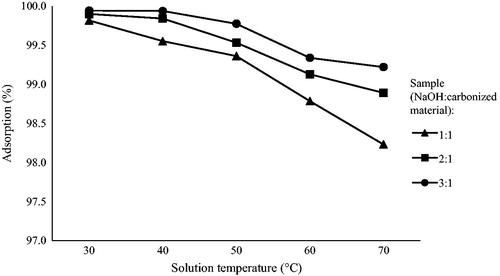
3.3.4. Effect of temperature on the cadmium adsorption
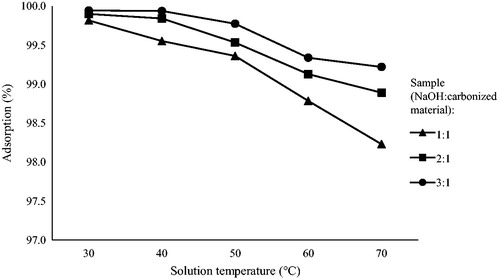
The effect of temperature on the removal of cadmium by the prepared activated carbon is shown in . The experiment was carried out for the contact time of 40 min, the initial cadmium ion concentration of 40 mg/l using 0.5 g/l of the adsorbent dose at different temperatures. shows the highest adsorption percentage for all samples was at 30 °C. Adsorption percentages dropped for all samples as the temperature was increased from 40 °C to 50 °C, 60 °C, and 70 °C. Therefore, it can be concluded that the adsorption percentage of cadmium decreases in contrast with the temperature rise and 30 °C may be considered as the equilibrium temperature of the system. This result seems to be in conformity with the previous study by Kumar, Ramakrishnan, Kirupha, and Sivanesan (Citation2010), reporting the decrease of adsorption rate from 86.87 to 76.25% for 50 mg/L of the cadmium ion concentration within the temperature range from 25 °C to 55 °C.
3.4. Isothermal studies
3.4.1. Langmuir isotherm
shows 1/qe vs 1/Ce plot of Langmuir isotherm for Cd(II) ion adsorption for the sample NaOH:carbonized sample ratios of 3:1 (a), 2:1 (b) and 1:1 (c). The value of the correlation coefficient for the NaOH:carbonized sample ratios of 1:1 are 0.9671, 0.9542 and 0.9655 at 30 °C, 40 °C and 50 °C, respectively. NaOH:carbonized sample ratios of 2:1 and 3:1 showed the same high values of correlation coefficient with the lowest value of 0.9542 recorded. The high R2 values showed the adsorption process follows Langmuir isotherm which suggested a monolayer type of adsorption.
Figure 6. 1/qe vs 1/Ce plot of Langmuir isotherm of Cd(II) ion adsorption for NaOH:carbonized sample ratios of 3:1 (a), 2:1 (b) and 1:1 (c).
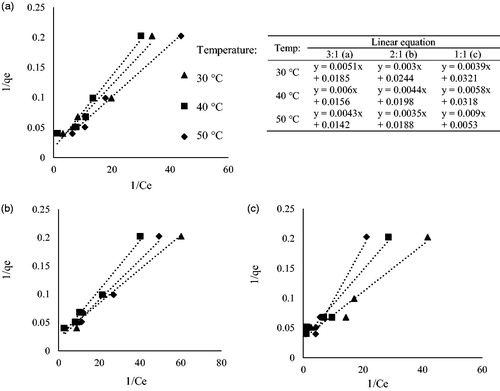
shows the calculated values and constant of the Langmuir equation. The qm, which is the measure of the adsorption capacity to form a monolayer, is recorded to be as high as 70.423 mg/g at 50 °C. Constant KL, which denotes adsorption energy, for the corresponding adsorption capacity, ranged between 0.589 and 3.627 l/mg. The maximum monolayer adsorption capacity, qm (mg/g) was found to increase with the increment of temperature and increment of NaOH:carbonized sample ratio as obtained by Karthikeyan, Rajgopal, and Miranda (Citation2005). Comparison of the adsorption capacity of the Leucaena leucocephala activated carbon compared to other adsorbents for cadmium removal from water in showed that this adsorbent performs above average with the maximum adsorption reaching 70.423 mg/g.
Table 2. Isothermic and kinetics models for the adsorption of Cd2+ by Leucaena leucocephala based activated carbon.
Table 3. Comparison of different adsorption capacity for different cadmium adsorbent.
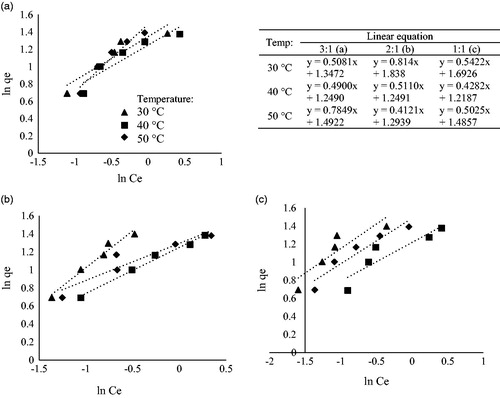
3.4.2. Freundlich isotherm
shows the plots of linearized equations by Freundlich for the adsorption of cadmium at 800 °C activation temperature. The Freundlich model is applicable for non-ideal sorption on heterogeneous surfaces and multilayer sorption processes. also shows the difference of R2 value for both Langmuir and Freundlich isotherm. The R2 value for this activation temperature shows that the Langmuir model exhibits a higher R2 value than the Freundlich model which suggests that the adsorption was a better fit to the Langmuir isotherm. It was stated by Annadurai, Ling, and Lee (Citation2008) that the value of the exponent 1/n indicates the favorability and capacity of the adsorbent/adsorbate system. Most of the samples showed n > 1 which indicates favorable adsorption conditions where in most cases the exponent between 1 < n < 10 shows beneficial adsorption.
3.5. Kinetic studies
3.5.1. Pseudo-first-order kinetic model and pseudo-second order kinetics model
shows log (qe-qt) vs. t linear plot of pseudo-first-order (a) and t/qt vs. t linear plot of pseudo-second-order (b) kinetic model of Cd(II) ion adsorption using Leucaena leucephala activated carbon. The R2 values showed the adsorption process followed the pseudo-firstorder kinetic model, as shown in . The adsorption process was severely fitted to pseudo-second order kinetics model where the R2 value goes as low as 0.0004. The pseudo-first-order kinetic model assumed that the adsorption process depends only on the two factors which are the number of metal ions present in the aqueous solution and the available binding sites on the adsorbent at any particular period, which explain the physisorption nature of the adsorption process. Meanwhile, the pseudo-second-order kinetic model assumed that the rate controlling step of an adsorption process is the chemical adsorption (Manirethan, Raval, Rajan, Thaira, & Balakrishnan, Citation2018).
Figure 8. log (qe-qt) vs t linear plot of pseudo first order (a) and t/qt vs t linear plot of pseudo second order (b) kinetic model of Cd(II) ion adsorption using Leucaena leucephala activated carbon.
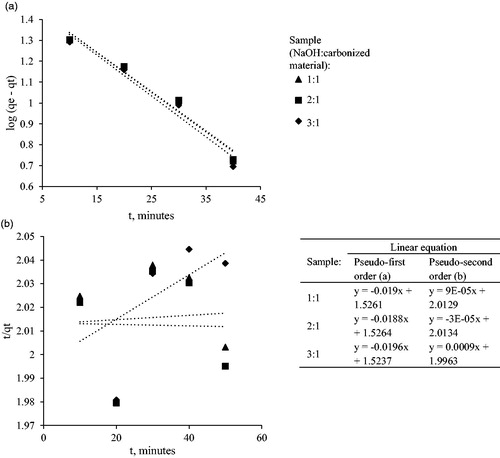
Table 4. Kinetic models for Cd(II) ion adsorption using Leucaena leucephala activated carbon.
4. Conclusions
Three activated carbon (AC) samples with different characteristics were prepared using chemical activation using the NaOH:carbonized sample ratios of 1:1, 2:1 and 3:1 in the present study. Each sample exhibited different adsorption percentage during batch adsorption studies. Considering the effect of contact time on Cd2+ removal, the highest adsorption percentage was observed for the ratio of 3:1. The adsorption equilibrium was reached within 40 min at the initial Cd2+ concentration of 30 mg/l. The concentration, pH, and temperature also gave rise to the highest adsorption rate for the NaOH:carbonized sample ratio of 3:1. The higher the initial concentration, the lower the level of adsorption performance. The higher adsorption equilibrium was established at pH 7.0 and reaction temperature of 30 °C is the optimum. Isothermic studies showed the adsorption process best fitted to Langmuir isotherm which indicates monolayer adsorption had happened while kinetic studies showed a physisorption process was favorable. It can be concluded based on the present results that the AC sample obtained from Leucaena leucocephala biomass at the NaOH:carbonized sample ratio of 3:1 could be the best to be used efficiently for removal of cadmium from contaminated water.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmad Khan, N., Ibrahim, S., & Subramaniam, P. (2004). Elimination of heavy metals from wastewater using agricultural wastes as adsorbents. Malaysian Journal of Science, 23, 43–51.
- Ali, I., Alharbi, O. M. L., Alothman, Z. A., Badjah, A. Y., Alwarthan, A., & Basheer, A. A. (2018). Artificial neural network modelling of amido black dye sorption on iron composite nano material: kinetics and thermodynamics studies. Journal of Molecular Liquids, 250, 1–8.
- Andelescu, A., Nistor, M. A., Muntean, S. G., & Rădulescu-Grad, M. E. (2018). Adsorption studies on copper, cadmium, and zinc ion removal from aqueous solution using magnetite/carbon nanocomposites. Separation Science and Technology, 1–13.
- Annadurai, G., Ling, L. Y., & Lee, J.-F. (2008). Adsorption of reactive dye from an aqueous solution by chitosan: Isotherm, kinetic and thermodynamic analysis. Journal of Hazardous Materials, 152(1), 337–346.
- Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: A review. Journal of Hazardous Materials, 97(1–3), 219–243.
- Bailey, S. E., Olin, T. J., Bricka, R. M., & Adrian, D. D. (1999). A review of potentially low-cost sorbents for heavy metals. Water Research, 33(11), 2469–2479.
- Banerjee, S., & Chattopadhyaya, M. C. (2017). Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arabian Journal of Chemistry, 10, S1629–S1638.
- Basheer, A. A. (2018). New generation nano-adsorbents for the removal of emerging contaminants in water. Journal of Molecular Liquids, 261, 583–593.
- Basu, M., Guha, A. K., & Ray, L. (2018). Adsorption of cadmium on cucumber peel: Kinetics, isotherm and co-ion effect. Indian Chemical Engineer, 60(2), 179–195.
- Bernard, A. (2008). Cadmium & its adverse effects on human health. The Indian Journal of Medical Research, 128(4),557–564.
- Borah, R., Kumari, D., Gogoi, A., Biswas, S., Goswami, R., Shim, J., … Kumar, M. (2018). Efficacy and field applicability of Burmese grape leaf extract (BGLE) for cadmium removal: An implication of metal removal from natural water. Ecotoxicology and Environmental Safety, 147, 585–593.
- Caglar, B., Afsin, B., Koksal, E., Tabak, A., & Eren, E. (2013). Characterization of Unye bentonite after treatment with sulfuric acid. Química Nova, 36(7), 955–959.
- Caglar, B., Afsin, B., Tabak, A., & Eren, E. (2009). Characterization of the cation-exchanged bentonites by XRPD, ATR, DTA/TG analyses and BET measurement. Chemical Engineering Journal, 149(1–3), 242–248.
- Carrott, P., & Carrott, M. R. (2007). Lignin–from natural adsorbent to activated carbon: A review. Bioresource Technology, 98, 2301–2312.
- Cazetta, A. L., Vargas, A. M. M., Nogami, E. M., Kunita, M. H., Guilherme, M. R., Martins, A. C., … Almeida, V. C. (2011). NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chemical Engineering Journal, 174(1), 117–125.
- Chen, Z., Liu, T., Tang, J., Zheng, Z., Wang, H., Shao, Q., … Feng, T. (2018). Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environmental Science and Pollution Research, 25(12), 11854–11866.
- Çetinkaya, S., Targan, Ş., & Nüket Tirtom, V. (2018). Comparative adsorption of Pb(II) and Cd(II) ions on chestnut shell in aqueous system. Chemistry and Ecology, 34 (7), 640–654.
- Devi, V., Selvaraj, M., Selvam, P., Kumar, A. A., Sankar, S., & Dinakaran, K. (2017). Preparation and characterization of CNSR functionalized Fe3O4 magnetic nanoparticles: An efficient adsorbent for the removal of cadmium ion from water. Journal of Environmental Chemical Engineering, 5(5), 4539–4546.
- Gupta, V. K., & Sharma, S. (2002). Removal of cadmium and zinc from aqueous solutions using red mud. Environmental Science & Technology, 36(16), 3612–3617.
- Jin, X., Wu, X., Zhang, H., Jiang, X., Huang, Z., Liu, Y., … Min, X. (2018). Novel humic acid-based carbon materials: adsorption thermodynamics and kinetics for cadmium(II) ions. Colloid and Polymer Science, 296(3), 537–546.
- Karthikeyan, T., Rajgopal, S., & Miranda, L. S. (2005). Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. Journal of Hazardous Materials, 124(1–3), 192–199.
- Khan, M. A., Khan, S., Ding, X., Khan, A., & Alam, M. (2018). The effects of biochar and rice husk on adsorption and desorption of cadmium on to soils with different water conditions (upland and saturated). Chemosphere, 193, 1120–1126.
- Krishnan, K. A., & Anirudhan, T. (2003). Removal of cadmium (II) from aqueous solutions by steam-activated sulphurised carbon prepared from sugar-cane bagasse pith: Kinetics and equilibrium studies. Water SA, 29, 147–156.
- Kumar, P. S., Ramakrishnan, K., Kirupha, S. D., & Sivanesan, S. (2010). Thermodynamic and kinetic studies of cadmium adsorption from aqueous solution onto rice husk. Brazilian Journal of Chemical Engineering, 27(2), 347–355.
- Lee, H.-C., Byamba-Ochir, N., Shim, W.-G., Balathanigaimani, M., & Moon, H. (2015). High-performance super capacitors based on activated anthracite with controlled porosity. Journal of Power Sources, 275, 668–674.
- Lee, Y. C., Amini, M. H. M., Sulaiman, N. S., Mohamed, M., & Boon, J. G. (2018). Batch adsorption and isothermic studies of malachite green dye adsorption using Leucaena leucocephala biomass as potential adsorbent in water treatment. Songklanakarin Journal of Science & Technology, 40(3), 563–569.
- Liu, W., Zhao, C., Wang, S., Niu, L., Wang, Y., Liang, S., & Cui, Z. (2018). Adsorption of cadmium ions from aqueous solutions using nano-montmorillonite: Kinetics, isotherm and mechanism evaluations. Research on Chemical Intermediates, 44(3), 1441–1458.
- MacDicken, K., & Brewbaker, J. (1988). Growth rates of five tropical leguminous fuelwood species. Journal of Tropical Forest Science, 85, 93.
- Manirethan, V., Raval, K., Rajan, R., Thaira, H., & Balakrishnan, R. M. (2018). Kinetic and thermodynamic studies on the adsorption of heavy metals from aqueous solution by melanin nanopigment obtained from marine source: Pseudomonas stutzeri. Journal of Environmental Management, 214, 315–324.
- Obregón-Valencia, D., & Sun-Kou, M. R. (2014). Comparative cadmium adsorption study on activated carbon prepared from aguaje (Mauritia flexuosa) and olive fruit stones (Olea europaea L.). Journal of Environmental Chemical Engineering, 2(4), 2280–2288.
- Rafatullah, M., Sulaiman, O., Hashim, R., & Amini, M. (2011). Adsorption of Copper (II) ions onto surfactant-modified oil palm leaf powder. Journal of Dispersion Science and Technology, 32(11), 1641–1648.
- Rao, M. M., Rao, G. P. C., Seshaiah, K., Choudary, N. V., & Wang, M. C. (2008). Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Management, 28(5), 849–858.
- Rasat, M. S. M., Wahab, R., Mohamed, M., Ahmad, M. I., Amini, M. H. M., Rahman, W. M. N. W. A., … Yunus, A. A. M. (2016). Preliminary study on properties of small diameter wild Leucaena leucocephala species as potential biomass energy sources. ARPN Journal of Engineering and Applied Sciences, 11, 6128–6137.
- Regti, A., Laamari, M. R., Stiriba, S.-E., & El Haddad, M. (2017). Potential use of activated carbon derived from Persea species under alkaline conditions for removing cationic dye from wastewaters. Journal of the Association of Arab Universities for Basic and Applied Sciences, 24(1), 10–18.
- Sadegh, H., Mazloumbilandi, M., & Chahardouri, M. (2017). Low-cost materials with adsorption performance handbook of ecomaterials. Switzerland AG: Springer.
- Saini, S., Kumar, R., Chawla, J., & Kaur, I. (2017). Punica granatum (pomegranate) carpellary membrane and its modified form used as adsorbent for removal of cadmium (II) ions from aqueous solution. Journal of Water Supply: Research and Technology - Aqua, 67(1), 68–83.
- Sulaiman, O., Amini, M.H.M., Rafatullah, M., Hashim, R., & Ahmad, A. (2010). Adsorption equilibrium and thermodynamic studies of copper (II) ions from aqueous solutions by oil palm leaves. International Journal of Chemical Reactor Engineering, 8, A108.
- Yaacoubi, H., Zidani, O., Mouflih, M., Gourai, M., & Sebti, S. (2014). Removal of cadmium from water using natural phosphate as adsorbent. Procedia Engineering, 83, 386–393.
- Zheng, X., Dou, J., Yuan, J., Qin, W., Hong, X., & Ding, A. (2017). Removal of Cs+ from water and soil by ammonium pillared montmorillonite/Fe3O4 composite. Journal of Environmental Sciences, 56, 12–24.