Abstract
Spinel ferrites nanoparticles (SNPs) have been extensively studied, synthesized and used in many applications including wastewater treatment, biosensors and as photocatalysts. The studies of the effect of these SNPs at cellular and molecular levels and their influence in the environment are scarce. Thus, in the current study, different metal ferrite SNPs were synthesized via sol-gel-combustion route and were characterized by X-ray Diffraction (XRD), Scanning Electron Microscope (SEM) with Energy Dispersive X-ray (EDX) and Vibrating Sample Magnetometry (VSM) analysis techniques. The effect of these SNPs on the growth of the microalgae Picochlorum sp. was then investigated. The results showed that the synthetic method used to prepare the metal ferrite SNPs leads to highly crystalline SNPs with average crystallites size between 33–36 nm except for ZnFe2O4 NPs which have crystallite size of 52 nm. The results of XRD and EDS confirmed the formation of SNPs. VSM analysis of CoFe2O4 showed the highest magnetic energy (U) and saturation magnetization (Ms) of 30870 erg/g and 60 emu/g, respectively. Although, the microalgae culture of Picochlorum sp. treated with different SNPs showed significant difference in viable cells concentration at 48 h and 72 h of incubation compared to control samples, the growth pattern of both treated and untreated samples were seen similar. This could indicate that SNPs may reduce the growth of the microalgae but will not cause severe inhibition when used at the proper concentration. This promotes their potential use for many applications.
1. Introduction
Spinel ferrite nanoparticles (SNPs) have general formula of MFe2O4, where M is a metal cation with (2+) charge such as Mn, Fe, Cu, Ni and Zn. These NPs have unique magnetic and electrical properties and thus have been utilized in many applications such as biomedical (Ahamed et al., Citation2011), biosensors (Sandu, Presmanes, Alphonse, & Tailhades, Citation2006), high frequency components (Praveena, Chen, Liu, Sadhana, & Murthy, Citation2016), photocatalytic activity (Sharma, Bansal, & Singhal, Citation2015; Valero-Luna, Palomares-Sanchéz, & Ruíz, Citation2016; Yean-Ling, Steven, Hwai-Chyuan, & Wen-Tong, Citation2016) and degradation of organic Azo dyes (Oladipo, Ifebajo, & Gazi, Citation2019) and removal of tetracycline antibiotic from aqueous solutions (Ifebajo, Oladipo, & Gazi, Citation2019). The SNPs have been synthesized using various methods including microemulsion (Ganure, Dhale, Katkar, & Lohar, Citation2017), hydrothermal (Wang, Citation2006), solvothermal (Li et al., Citation2015; Ni, Lin, Xiaoling, Xinqing, & Hongliang, Citation2015), co-precipitation (Pereira et al., Citation2012), sol-gel (Jasso-Terán et al., Citation2017) mechanical milling (Marinca, Chicinaş, Isnard, & Neamţu, Citation2016) and chemical combustion (Pérez, Gomez-Polo, Larumbe, Pérez-Landazabal, & Sagredo, Citation2012). Due to the increase in the production of designed nanoparticles, human exposure to SNPs and their potential for adverse health effects have been of great concern. Thus, there are number of publications focused on studying the toxicity and the effects of these SNPs on different human cells and living organisms. For example, NiFe2O4 SPs caused a decrease in cell viability in some types of human cells (Ahamed, Akhtar, Alhadlaq, Khan, & Alrokayan, Citation2015; Ahamed et al., Citation2011) and their cytotoxicity increases as the size of the particle increases (Yin, Too, & Chow, Citation2005). ZnFe2O4 SPs induced cytotoxicity and oxidative stress in some human cells in the dosage range of 10–40 µgmL−1 (Alhadlaq, Akhtar, & Ahamed, Citation2015). On the other hand, CoFe2O4 SPs showed no significant toxicity effect on human cancer line cells at the concentration of 0.095–0.95 µgmL−1 and cell viability increases in parallel with the concentration of the NPs (Kim, Kim, Kim, Shim, & Lee, Citation2007; Pašukonienė et al., Citation2014). Considering the effect of SNPs on aquatic organisms, Sebastian, Vijayalakshmy, Lakshmi, Saramma, and Mohammed (Citation2014) observed a decrease in the content of chlorophyll of Chlorella pyrenoidosa algae on exposure to high concentration (> 0.5 µM) of ZnFe2O4 SNPs. López-Moreno et al. (Citation2016) studied the effect of CoFe2O4 on the growth of Lycopersicon lycopersicum (tomato plants) and observed that when the concentration of NPs was increased, root growth and the uptake of Fe, Co, Mg and Ca in the plant tissues slightly increased. It has been found that the size of Fe3O4 SNPs had a great influence on the growth of Picochlorum sp. and increasing particle size lowered the toxicity and the negative effect was only observed in the early growth stages (Hazeem et al., Citation2015).
In the present paper, various metal ferrite nanoparticles were synthesized via sol-gel combustion method and the structures of these SNPs were characterized by XRD, SEM with (EDX) which is used to identify the elements in the sample quantitatively. The magnetization properties of the nanoparticles were followed by VSM. The effect of these SNPs on the growth of the marine microalgae Picochlorum sp. were investigated. The effect of SNPs was tested using 10 mgL−1 during exponential growth phase. The concentration was selected according to recent publications using different NPs and their effect on microalgae, in which the overall findings indicated that the NPs generally at low concentrations do not have a negative impact on the growth of microalgae. For examples, Li et al. (Citation2006) found that Cu+2 and Zn+2 salts were not toxic to the marine microalgae Pavlova viridis (3012) at low concentrations (0 and 3 mgL−1) and Zn+2 salt was used at concertation’s ranged between 0 and 32.5 mgL−1. Additionally, ZnFe2O4 was found to enhance algal growth at low concentration (0.5 µM (0.12 mgL−1)) using the microalgae Chlorella pyrenoides and at higher concentration (1 to 2 µM (0.24–0.48 mgL−1)), the growth was found to be hindered (Sebastian et al., Citation2014). Ahmad, Yao, Zhou, and Liu (Citation2015) treated the microalgae Chlorella vulgaris with CoFe2O4 at concentrations ranged between 6.3 to 100 µM (1.48 to 23.5 mgL−1) and found that the NPs didn’t generate a negative effect on microalgae at low concentration 6.3 µM (1.48 mgL−1). Hazeem et al. (Citation2015) found that the magnetic Fe3O4 NPs inhibited cell growth of the microalgae Picochlorum sp. using 20 nm NPs, whereas the 40 nm Fe3O4 NPs and the bulk material of Fe3O4 were found to promote algal growth. Furthermore, they revealed that the 20 nm Fe3O4 NPs did not show significant difference between the treated and untreated samples, in the lag and decline phases at different concentrations (50–1600 mgL−1).Therefore, the authors of the current study and according to previous published work and literature search decided to evaluate the effect of the synthesized SNPs at low concentration for comparison with existing literature.
2. Method and materials
All chemicals and solvents were of analytical grade and they were purchased from Sigma-Aldrich and used without further purification.
2.1. Chemical synthesis of spinel ferrites nanoparticles (sol-gel combustion method)
The SNPs were synthesized using sol-gel combustion method reported by Pérez et al. (Citation2012). An equimolar mixture of M(NO3)2. XH2O, where M = Cr, Co, Cu, Ni, Zn, and Fe(NO3)3.9H2O salts and citric acid (1 g, 0.0025 mol) in 150 mL distilled water was stirred for 20 min. The pH of the mixture was adjusted to 7 by adding concentrated ammonium hydroxide (NH4OH). Then the mixture was heated at 135˚C until formation of gel and then was burned to obtain the dark brown to black powder. The powder product was kept for characterization.
2.2. Characterization of SNPs
The crystal structure, phase composition and average particle size of the newly prepared spinel ferrites nanoparticles were determined using Rigaku-Ultima IV x ray diffractometer equipped with Cu- radiation (λ=1.542 A˚) in the range of 20° to 80° with an accelerating voltage of 40 kV and counting rate of 1°/min. The morphology and the chemical composition of the samples were identified using EVO LS 10 Scanning Electron Microscope (SEM) with EDX. The magnetic properties measurements were carried out at room temperature using PMC MicroMag 3900 model VSM in the field range of +10 kOe to −10k Oe.
2.3. Effect of SNPs on the growth of microalgae Picochlorum sp
Picochlorum sp. (Trebouxiophyceae, Chlorophyta) was obtained from the National Mariculture Centre, Ministry of Municipalities and Urban Planning, Kingdom of Bahrain. The microalgae were cultured in sterilized seawater and Walne’s medium at initial pH value of 8. For the growth inhibition test, the microalgae were used in the exponential growth phase. The culture was incubated in each of the SNPs at a concentration of 10 mgL−1. Each treatment was run in triplicates and the incubation test was conducted under controlled laboratory conditions (18 °C, 100 µmol photons/m2.sec and 12:12 h light: dark cycle). Viable cell concertation was measured using MuseTM cell analyzer (Millipore, USA) at 24, 48 and 72 h after incubation to elucidate the effects of SNPs on the growth of microalgae. The data is presented as mean and standard deviation (SD). Sigma Plot 13 was used to plot the graph between viable cell concentration and time. One-way ANOVA was used to determine whether there exists any significant difference between control and treated samples at any time.
3. Results and discussion
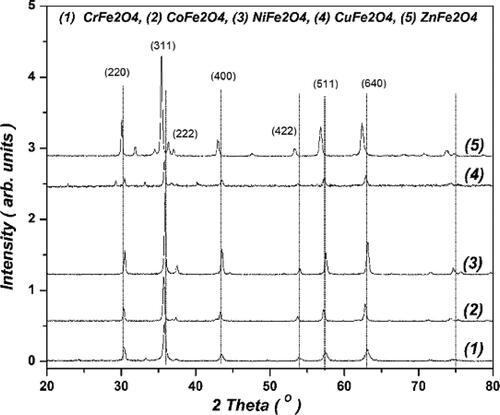
The structures of the prepared SNPs were identified by XRD and SEM with EDX. The results of XRD analysis are shown in and . XRD is usually used to analyze the crystal structure, phase equilibrium, and crystallite size. Debye-Scherrer equation was used for calculating particle size (D = 0.94λ/(β cos θ), where λ is the wavelength of the x-ray and β is the line broadening at half maximum intensity. The average crystallite sizes of FeCr2O4, CoFe2O4, NiFe2O4, CuFe2O4 and ZnFe2O4 were determined as 34, 33, 36, 35 and 52 nm, respectively. The diffraction patterns of all the powders confirmed the formation of the corresponding spinel metal ferrite (MFe2O4), except for CrFe2O4 which is unstable and thus the weight percentage was difficult to estimate () and the peak pattern most probably corresponded to FeCr2O4. In addition, the diffraction peaks of all the samples were sharp which indicate highly crystalline nature of the prepared samples. Some traces of metal oxides phases are present in the prepared samples in which the determination of the charge of distribution has been of general interest (Afsin & Roberts, Citation1994).
Table 1. Crystal structure and the magnetic parameters of the prepared nanoparticles.
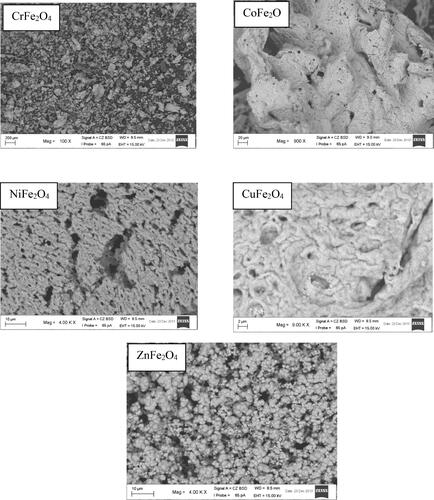
SEM was used to determine the external morphology, shape and chemical composition of a crystalline material. represents the SEM images of the prepared SNPs. The images revealed irregular shapes except for ZnFe2O4 NPs which have non uniform spherical shape.
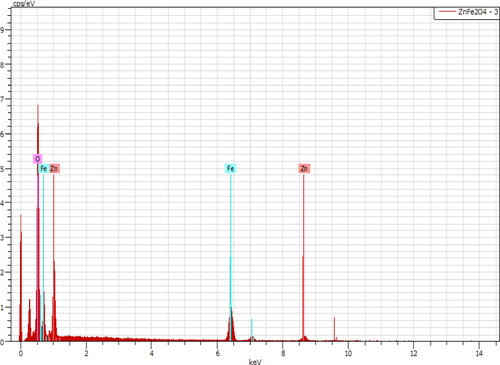
Energy dispersive X-ray (EDX) results confirmed that the ratio of the transition metal atoms in each system is consistent with the nominal stoichiometry. The atomic ratio of Co/Fe, Ni/Fe, Cu/Fe and Zn/Fe for CoFe2O4, NiFe2O4, Zn Fe2O4 and CuFe2O4 was approximately 1:2. An ERX of ZnFe2O4 NPs is shown in and .
Table 2. EDX analysis of ZnFe2O4 NPs.
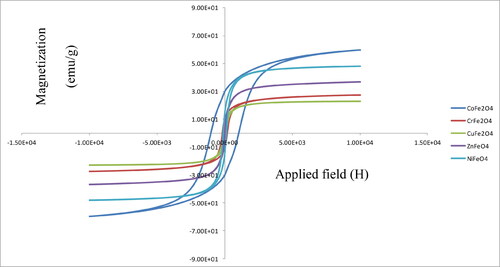
Vibrating Sample Magnetometer (VSM) is used to measure the magnetic properties of magnetic materials. The magnetic properties of the prepared samples were measured at room temperature between +10 kOe −10 kOe. The M-H relationships of the studied ferrites were demonstrated in . In order to compare magnetic energy of the studied ferrites, magnetic energy U (erg/g) was used.
CoFe2O4 NPs has high hysteresis loss (), which means it is hard metal ferrite. The hard ferrites are usually characterized by high coercivity and magnetic energy. CoFe2O4 SPs exhibited ferromagnetic behavior with coercivity (Hc) and saturation magnetization (Ms) of about 1029 Oe and 60 emu/g, respectively. These results agree with the data reported by Matsuda, Nakanishi, Kaneko, and Osaka (Citation2015). NiFe2O4 is soft metal ferrite and exhibited saturation magnetization and coercivity of 47 emu/g and 120 Oe, respectively, which are in conformity with the literature values (Pérez et al., Citation2012). ZnFe2O4 had the lowest magnetic energy with the saturation magnetization of 44 emu/g (Alhadlaq et al., Citation2015). It was found that coercivity values (Hc) of the prepared SNPs increased as the crystallite size decreased. For example, the coercivity values are found about 1029, 119 and 40 Oe for CoFe2O4, NiFe2O4 and ZnFe2O4 with the corresponding crystallite sizes of 33, 35 and 52, respectively. Such experimental result was known previously for different magnetic materials and alloys (Adler & Pfeiffer, Citation1974; Bertotti, Fiorillo, & Pasquale, Citation1991; Degauque, Astie, Porteseil, & Vergne, Citation1982; Landgraf, Da Silveira, & Rodrigues, Citation2011), which shows the influence of grain size (GS) upon the magnetic properties of one group of compounds.
The effect of the synthesized SNPs on the growth of Picochlorum sp. is depicted in . Control samples showed an increase in viable cell concentration during 72 h of incubation time from 14.3 × 103 cells/mL (±2.8) to 32.23 × 103 cells/mL (±3.2). There was no significant difference between the control and treated samples at 24 h; however, a significant difference (p < 0.05) was observed between the control and treated samples at 48 and 72 h.
Figure 5. Changes in viable cell concentration in control and treated samples at 24, 48 and 72 h. (SNPs were used at 10 mgL−1, pH 8).
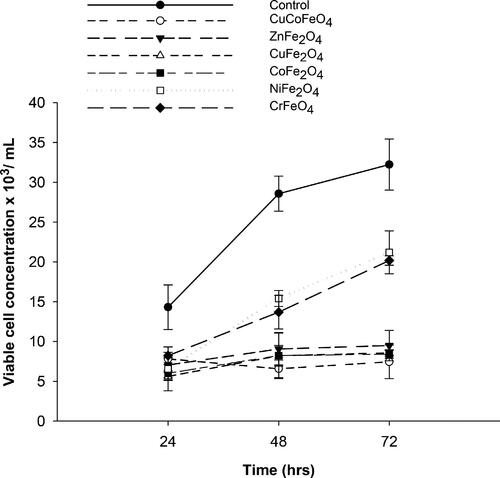
The microalgae treated with different SNPs showed similar trend of growth, but the number of viable cells were lower than the control samples, which implicated that the SNPs could reduce the growth of microalgae. Complete inhibition of cell growth was not recorded with any of the tested SNPs which make them potential materials for many environmental, industrial and medical applications.
The results of this study agree with the previous literature. For instance, the effect of zinc ferrite NPs on the growth of the freshwater Chlorella pyrenoidosa was investigated and it was found that the effect changes depending on the concentration of the NPs. Algal growth can be promoted when proper concentrations of NPs are used (Sebastian et al., Citation2014). López-Moreno et al. (Citation2016) demonstrated that Lycopersicon lycopericum (tomato plants) did not exhibit any negative effect when exposed to cobalt ferrite NPs (CoFe2O4). Tomato plants were able to tolerate the NPs concentration up to 1000 mgL−1 without noticeable toxicity. Hazeem et al. (Citation2015) found that magnetic Fe3O4 NPs promoted algal growth during late growth stages (stationary and decline phases). Kim et al. (Citation2007) revealed that CoFe2O4 was not toxic and the tissues injected (ICR mice cells) showed no notable changes in comparison to the control samples. Ahmad et al. (Citation2015) found that CoFe2O4 nanobeads at 6.3 µM did not show any significant inhibition to microalgae growth or chlorophyll a content using Chlorella vulgaris, though, concentrations above 12.5 µM caused oxidative stress and mechanical damage to the algae.
Contrary to the current study, Ahamed et al. (Citation2011) found that nickel ferrite NPs induced dose- dependent cytotoxicity in human lung epithelial (A549 cells) through ROS generation and oxidative stress. Additionally, Ahamed et al. (Citation2015) found that nickel ferrite also induces dose- dependent cytotoxicity in liver HepG2 and breast MCF-7 cell lines through many assays including oxidative stress. Matsuda et al. (Citation2015) found that CoFe2O4 exhibited higher coercivity than Fe3O4 NPs of the same diameter (10 nm). This higher coercivity led to more heating efficiency of NPs under an AC magnetic field and consequently caused cell death of human breast cancer MCF-7 cells (Matsuda et al., Citation2015).
In some other studies, some dissimilarities were noticed. Pašukonienė et al. (Citation2014) have observed different responses in different cell lines (human pancreatic and ovarian cancer cells) on the treatment with superparamagnetic cobalt ferrite NPs (Co- SPOINS). They found that both cell lines accumulated Co- SPOINS, but they displayed different responses. A2780 cells (ovarian cancer cell line) found to be more sensitive to exposure to the NPs. However, the general conclusion of their study indicated that Co- SPOINs were not toxic when a safe concentration is used. A summary of the different responses of different cell lines to SNPs is illustrated in .
Table 3. A summary of the different effects of SNPs on different cell lines.
4. Conclusion
Various metal ferrite SNPs of high crystalline structures were synthesized in high purity via sol-gel-combustion method. The structures of the SNP samples were confirmed by XRD and SEM techniques. The average crystallite size of the prepared samples was determined between 33–36 nm, except for ZnFe2O4 which was about 52 nm. The highest saturated magnetization was about 60 emu/g for CoFe2O4. There were no significant differences in cell growth on exposure to different spinel nanoparticles at 24 h of incubation. However, a significant difference between control samples and treated samples were noticed at 48 and 72 h. The growth pattern of the treated samples was similar to the control samples but with lower viable cell concentration, indicating that the SNPs may reduce the growth of microalgae at certain concentration, but do not induce complete inhibition. SNPs of different doses, different organisms and cell lines could be investigated to draw a more detailed picture on the effect of SNPs and their potential uses in many environmental, industrial and medical applications and to understand their ultimate impact on environment.
Acknowledgments
The authors gratefully acknowledge College of Science, University of Bahrain for providing chemicals and various instrumental facilities. Authors thank Dr. Mohamed Bououdina from University of Bahrain for XRD analysis. A sincere thank go to Dr. Akel Dakhel from University of Bahrain for proofreading of the manuscript earnestly.
References
- Adler, E., & Pfeiffer, H. (1974). The influence of grain size and impurities on the magnetic properties of soft magnetic alloy 47.5% NiFe. IEEE Transactions on Magnetics, 10(2), 172–174. doi:10.1109/TMAG.1974.1058314
- Afsin, B., & Roberts, M. W. (1994). Formation of an oxy-chloride overlayer at a Bi (0001) surface. Spectroscopy Letters, 27(1), 139–146. doi:10.1080/00387019408002513
- Ahamed, M., Akhtar, M. J., Alhadlaq, H. A., & Alshamsan, A. (2016). Copper ferrite nanoparticle- induced cytotoxicity and oxidative stress in human breast cancer MCF-7 cells. Colloids and Surfaces B: Biointerfaces, 142, 46–54. doi:10.1016/j.colsurfb.2016.02.043
- Ahamed, M., Akhtar, M. J., Alhadlaq, H. A., Khan, M. A., & Alrokayan, S. A. (2015). Comparative cytotoxic response of nickel ferrite nanoparticles in human liver HepG2 and breast MFC-7 cancer cells. Chemosphere, 135, 278–288. doi:10.1016/j.chemosphere.2015.03.079
- Ahamed, M., Akhtar, M. J., Siddiqui, M. A., Ahmad, J., Musarrat, J., Al-Khedhairy, A. A., … Alrokayan, S. A. (2011). Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology, 283(2–3), 101–108. doi:10.1016/j.tox.2011.02.010
- Ahmad, F., Yao, H., Zhou, Y., & Liu, X. (2015). Toxicity of cobalt ferrite (CoFe2O4) nanobeads in Chlorella vulgaris: Interaction, adaptation and oxidative stress. Chemosphere, 139, 479–485. doi:10.1016/j.chemosphere.2015.08.008
- Alhadlaq, H. A., Akhtar, M. J., & Ahamed, M. (2015). Zinc ferrite nanoparticle-induced cytotoxicity and oxidative stress in different human cells. Cell & Bioscience, 5, 55. doi:10.1186/s13578-015-0046-6
- Bertotti, G., Fiorillo, F., & Pasquale, M. (1991). Reversible and irreversible magnetization in soft iron- based polycrystalline materials. Journal of Applied Physics, 69(8), 5930–5932. doi:10.1063/1.347819
- Degauque, J., Astie, B., Porteseil, J. L., & Vergne, R. (1982). Influence of the grain size on the magnetic and magnetomechanical properties of high-purity iron. Journal of Magnetism and Magnetic Materials, 26(1–3), 261–262. doi:10.1016/0304-8853(82)90166-4
- Ganure, K. A., Dhale, L. A., Katkar, V. T., & Lohar, K. S. (2017). Synthesis and characterization of lanthanum-doped Ni-Co-Zn spinel ferrites nanoparticles via normal micro-emulsion method. International Journal of Nanotechnology and Applications, 11(2), 189–195.
- Hazeem, L. J., AbdulWaheed, F., Rashdan, S., Bououdina, M., Brunet, L., Slomianny, C., … Elmeselmani, W. A. (2015). Effect of magnetic iron oxide (Fe3O4) nanoparticles on the growth and photosynthetic pigment content of Picochlorum sp. Environmental Science and Pollution Research, 15(22), 11728–11739. doi:10.1007/s11356-015-4370-5
- Ifebajo, A. O., Oladipo, A. A., & Gazi, M. (2019). Efficient removal of tetracycline by CoO/CuFe2O4 derived from layered double hydroxides. Environmental Chemistry Letters, 17(1), 487–494. doi:10.1007/s10311-018-0781-0
- Jasso-Terán, R. A., Cortés-Hernández, D. A., Sánchez-Fuentes, H. J., Reyes-Rodríguez, P. Y., de-León-Prado, L. E., Escobedo-Bocardo, J. C., & Robles, J. M. A. (2017). Synthesis, characterization and hemolysis studies of Zn(1 − x) CaxFe2O4 ferrites synthesized by sol-gel for hyperthermia treatment applications. Journal of Magnetism and Magnetic Materials, 427, 241–244. doi:10.1016/j.jmmm.2016.10.099
- Kim, D. H., Kim, K. N., Kim, K. M., Shim, I. B., & Lee, Y. K. (2007). In vitro & in vivo toxicity of CoFe2O4 for application to magnetic hyperthermia. NSTI-Nanotech, 2, 748–751.
- Landgraf, F. J. G., Da Silveira, J. R. F., & Rodrigues, D. Jr (2011). Determining the effect of grain size and maximum induction upon coercive field of electrical steels. Journal of Magnetism and Magnetic Materials, 323(18–19), 2335–2339. doi:10.1016/j.jmmm.2011.03.034
- Li, M., Hu, C., Zhu, Q., Chen, L., Kong, Z., & Liu, Z. (2006). Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere, 62(4), 565–572. doi:10.1016/j.chemosphere.2005.06.029
- Li, Z., Gao, K., Han, G., Wang, R., Li, H., Zhao, X. S., & Guo, P. (2015). Solvothermal synthesis of MnFe2O4 colloidal nanocrystal assemblies and their magnetic and electrocatalytic properties. New Journal of Chemistry, 39(1), 361–368. doi:10.1039/C4NJ01466A
- López-Moreno, M. L., Avilés, L. L., Pérez, N. G., Irizarry, B. A., Perales, O., Cedeno-Mattei, Y., & Román, F. (2016). Effect of cobalt ferrite (CoFe2O4) nanoparticles on the growth and development of Lycopersicon lycopersicum (tomato plants). Science of the Total Environment, 550, 45–52. doi:10.1016/j.scitotenv.2016.01.063
- Marinca, T. F., Chicinaş, I., Isnard, O., & Neamţu, B. V. (2016). Nanocrystalline/nanosized manganese substituted nickel ferrites—Ni1_xMnxFe2O4 obtained by ceramic-mechanical milling route. Ceramics International, 42(4), 4754–4763. doi:10.1016/j.ceramint.2015.11.155
- Matsuda, S., Nakanishi, T., Kaneko, K., & Osaka, T. (2015). Synthesis of cobalt ferrite nanoparticles using spermine and their effect on death in human breast cancer cells under an alternating magnetic field. Electrochimica Acta, 183, 153–159. doi:10.1016/j.electacta.2015.06.108
- Ni, D., Lin, Z., Xiaoling, P., Xinqing, W., & Hongliang, G. (2015). Preparation and characterization of nickel-zinc ferrites by a solvothermal method. Rare Metal Materials and Engineering, 44(9), 2126–2131. doi:10.1016/S1875-5372(16)30010-8
- Oladipo, A. A., Ifebajo, A. O., & Gazi, M. (2019). Magnetic LDH-based CoO–NiFe2O4 catalyst with enhanced performance and recyclability for efficient decolorization of Azo dye via Fenton-like reactions. Applied Catalysis B: Environmental, 243, 243–252. doi:10.1016/j.apcatb.2018.10.050
- Pašukonienė, V., Mlynska, A., Steponkienė, S., Poderys, V., Matulionytė, M., Karabanovas, V., … Rotomskis, R. (2014). Accumulation and biological effects of cobalt ferrite nanoparticles in human pancreatic and ovarian cancer cells. Medicina, 50(4), 237–244. doi:10.1016/j.medici.2014.09.009
- Pereira, C., Pereira, A. M., Fernandes, C., Rocha, M., Mendes, R., Fernández-Garcia, M. P., … Freire, C. (2012). Superparamagnetic MFe2O4 (M = Fe, Co, Mn) nanoparticles: Tuning the particle size and magnetic properties through a novel one-step coprecipitation route. Chemistry of Materials, 24(8), 1496–1504. doi:10.1021/cm300301c
- Pérez, E., Gomez-Polo, C., Larumbe, S., Pérez-Landazabal, J. I., & Sagredo, V. (2012). Structural and magnetic properties of NiFe2O4 and NiFe2O4/SiO2 nanoparticles prepared by Sol-Gel combustion. Revista Mexicana de Física S, 58(2), 104–107.
- Praveena, K., Chen, H. W., Liu, H. L., Sadhana, K., & Murthy, S. R. (2016). Enhanced magnetic domain relaxation frequency and low power losses in Zn2+ substituted manganese ferrites potential for high frequency applications. Journal of Magnetism and Magnetic Materials, 420, 129–142. doi:10.1016/j.jmmm.2016.07.011
- Sandu, I., Presmanes, L., Alphonse, P., & Tailhades, P. (2006). Nanostructured cobalt manganese ferrite thin films for gas sensor application. Thin Solid Films, 495(1–2), 130–133. doi:10.1016/j.tsf.2005.08.318
- Sebastian, R. M., Vijayalakshmy, K. C., Lakshmi, S., Saramma, A. V., & Mohammed, E. M. (2014). Effect of Zinc Ferrite nanoparticles on the growth of Chlorella pyrenoidosa. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 5(5), 1261–1270.
- Sharma, R., Bansal, S., & Singhal, S. (2015). Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M = Cu, Zn, Ni and Co) in the structure. RSC Advances, 5(8), 6006–6018. doi:10.1039/C4RA13692F
- Valero-Luna, C., Palomares-Sanchéz, S. A., & Ruíz, F. (2016). Catalytic activity of the barium hexaferrite with H2O2/visible light irradiation for degradation of methylene blue. Catalysis Today, 266, 110–119. doi:10.1016/j.cattod.2015.08.049
- Wang, J. (2006). Prepare highly crystalline NiFe2O4 nanoparticles with improved magnetic properties. Materials Science and Engineering: B, 127, 81–84. doi:10.1016/j.mseb.2005.09.003
- Yean-Ling, P., Steven, L., Hwai-Chyuan, O., & Wen-Tong, C. (2016). Research progress on iron oxide-based magnetic materials: Synthesis techniques and photocatalytic applications. Ceramics International, 42, 9–34. doi:10.1016/j.ceramint.2015.08.144
- Yin, H., Too, H. P., & Chow, G. M. (2005). The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials, 26(29), 5818–5826. doi:10.1016/j.biomaterials.2005.02.036