?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this literature review, the removal of heavy metals from aqueous media by an environmentally friendly method, known as biosorption has been discussed. Biosorption can be referred to as an alternative to the common unsustainable industrial methods currently used. Removal of heavy metals from aqueous media by biosorption can take place by the aid of different types of biomass including algae, fungi, bacteria and plants. The mechanism(s) of biosorption can vary accordingly, mechanisms include physical adsorption, ion exchange, complexation, precipitation and transport across the cells. The efficiency of removal of heavy metals by a specific biosorbent at specified conditions can be compared by the calculated biosorption capacity of the respected biosorbent. Several factors can influence the biosorption capacity of different biosorbents, that mainly includes water pH, temperature, contact time, biomass dosage and initial heavy metal concentration. This literature review focuses on the types of biosorbents, mechanisms of biosorption and factors affecting biosorption capacity. In addition, biosorption, as a method which has the potential of competing with common industrial methods has been critically analysed.
1. Introduction
With the growth of the industrial world, environmental challenges have also increased. A common type of pollution the world is currently struggling with is water pollution. Water pollution can be due to many different causes such as agriculture, sewage and wastewater, oil pollution and radioactive substances. Wastewater, especially which is discarded by industries, can contain a wide range of heavy metals. Heavy metals are considered as one of the common causes of water contaminates. Heavy metals are non-degradable and accumulate in the environment. Heavy metals in streams, lakes and seas contaminate water in which marine organisms live, for example fish which is consumed by humans, not only the aquatic life becomes threated, but the human health also becomes in danger as those heavy metals bioaccumulate through the food chain. The major water contaminants are arsenic, cadmium, chromium, copper, lead, nickel and zinc (Jaishankar, Tseten, Anbalagan, Mathew, & Beeregowda, Citation2014).
Heavy metals are removed from industrial wastewater by conventional methods such as reverse osmosis, electrodialysis, ultrafiltration, industrial ion exchange process and chemical precipitation (Sao, Citation2014). These common industrial methods of heavy metal removal have several disadvantages such as being expensive, requiring high amounts of energy, not appropriate for removal of heavy metals at low concentrations (1-50 mg/L), requirement of high amounts of reagents, causing secondary pollution through the production of chemical sludge and disposal of floc residues making these methods non-sustainable in the long run (Wei et al., Citation2016). An alternative environmentally favourable method of heavy metal removal can be through biosorption methods. Biosorption methods are considered sustainable and more efficient in removal of heavy metals from aqueous media.
In this literature review, biosorption process will be discussed with an evaluation of different types of biosorbents, the mechanisms involved in biosorption and factors affecting the biosorption capacity of biosorbents.
2.1. Biosorption – a deeper insight
Biosorption is a biological physico-chemical process in which a biological material, such as plant biomass or microorganisms, is used to absorb or adsorb a target species, such as metal ions or dyes (Gadd, Citation2009). In a biosorption process, two phases are present, a solid phase which it is referred as the biosorbent (e.g., plants, bacteria and fungi) and a liquid phase which is usually aqueous and contains the metal ions which are known as the sorbate (Abbas Ali, Mohamed Sihabudeen, & Zahir Hussain, Citation2016). Algae, bacteria, fungi and plants are the most common biosorbents used for the removal of heavy metals from aqueous media. The main biosorption site of the biological sorbents is their cell wall (Tsezos, Remoundaki, & Hatzikioseyian, Citation2014). The cell walls of different biosorbents contain a range of different functional groups that contribute in biosorption process, the possible functional groups which are capable of biosorbing heavy metals are hydroxyl (found in alcohols and carbohydrates), carboxyl (found in fatty acids, proteins and organic acids), amino (found in proteins and nucleic acids), ester (found in lipids), sulfhydryl (found in cysteine (amino acid) and proteins), carbonyl (can be terminal such as in aldehydes and polysaccharides, or internal such as in ketones and polysaccharides too) and phosphate groups (found in DNA, RNA and tissue plasminogen activator) (Javanbakht, Alavi, & Zilouei, Citation2014). The functional groups of a biosorbent contributing in the removal of a specific metal can be studied and analysed using several analytical methods such as titration, infrared spectroscopy (IR), raman spectroscopy, X-ray photoelectron spectroscopy (XPS), energy-dispersive X-ray spectroscopy (EDS) and X-ray absorption fine structure spectroscopy (Javanbakht et al., Citation2014).
The potential of biosorption of a metal ion by a biosorbent is evaluated by the biosorption capacity value and equilibrium time for the biosorption process. Biosorption capacity is calculated according to the following formula:
where qe is the biosorption capacity (in mg of metal/g of biosorbent), C0 and Ce are the initial and equilibrium concentration of metal ion solution (in mg/L) respectively, V is the volume of metal ion solution (in L), and W is the amount of biosorbent material dose used (in g) (Wei et al., Citation2016). The higher the biosorption capacity, the higher the amount of heavy metal ion(s) a biosorbent can biosorb. Under optimum pH of media and temperature, the maximum biosorption capacity can be achieved. Optimising factors such as contact time of biosorbent and metal ion solution, and the dosage of biosorbent can further enhance the biosorption capacity, nevertheless pH and temperature are the main influential factors. While choosing a biosorbent for the removal of a specific metal ion, it is highly significant to consider the biosorption capacity. It is recommended to use biosorbents with higher biosorption capacities, as this would suggest that higher amount of sorbate can be biosorbed, biosorbents with low biosorption capacity will ultimately require their replacement. However, other aspects should be also considered while choosing the biosorbent, such as the degree of sustainability with respect to any undesirable effects of using the biosorbent on the environment, the speed of biosorption of target species with respect to the removal efficiency, the possibility of regenerating the biosorbent and reusing it.
2.1.1. Algae as biosorbents
Algae are divided into two main types: micro-algae and macro-algae. Macro-algae, which are also known as seaweeds, are multi cellular plants that grow in fresh or salt water, they are classified based on their pigmentation, which includes three groups: brown, red and green algae (Anastopoulos & Kyzas, Citation2015). On the other hand, micro-algae are photosynthetic unicellular plants that also grow in fresh or salt water, they are classified based on their pigmentation, arrangement(s) of their photosynthetic membranes or other morphological features (Anastopoulos & Kyzas, Citation2015). The four groups of micro-algae are diatoms, green algae, golden algae and blue-green algae (cyanobacteria) (Anastopoulos & Kyzas, Citation2015). The high biosorption ability of algae is due to the composition of their cell wall that is comprised on chitin, polysaccharides, proteins and lipids which contain vital functional groups aiding in biosorption (Davis, Volesky, & Mucci, Citation2003). Functional groups containing oxygen, nitrogen, sulphur and phosphorus were found to be the main contributors in biosorption of heavy metals by algae (Ivánová, Kaduková, Kavuličová, & Horváthová, Citation2012).
Considering macro-algae, brown algae have cell walls mainly composed of cellulose, alginic acid, polymers (such as mannuronic and guluronic acids) which are complexed with light metals (such as sodium, potassium and calcium), and polysaccharides (e.g., fucoidan) (He & Chen, Citation2014). Alginate and fucoidan have the potential of metal binding by ion exchange, the main biosorption binding sites in alginate contain carboxyl groups and secondly sulphate groups (Ivánová et al., Citation2012). The main content of green algae cell walls are proteins, which include functional groups such as amino, carboxyl, hydroxyl and sulphate that contribute in metal biosorption (Ivánová et al., Citation2012). On the other hand, red algae have cell walls mainly comprised of cellulose, nevertheless, their biosorption capacity lies in the presence of sulphated polysaccharides made up from galactans (He & Chen, Citation2014). Micro-algae slightly differ in their cell wall composition, as they mainly contain polysaccharides, proteins and lipids, these components contain carboxyl, hydroxyl, phosphate and sulphate groups that provide an overall negative net charge on the cell surface, favouring the binding of metal cations through counter ion interactions (Anastopoulos & Kyzas, Citation2015).
Out of all types of algae and other biosorbents, brown algae has shown the most significant biosorption capacities towards different metal ions (Akbari, Hallajisani, Keshtkar, Shahbeig, & Ali Ghorbanian, Citation2015). Moreover, using brown algae has additional advantages as it is available in large quantities, has a high surface area to volume ratio and high efficiency in dilute effluents, has the possibility of regenerating and recovering biosorbed metal, and produces less sludge (Akbari et al., Citation2015). Overall, algae is the most common biosorbent used compared to other biosorbent due to its high availability, relative cheap processing costs and high performance (Al-Homaidan, Al-Houri, Al-Hazzani, Elgaaly, & Moubayed, Citation2014).
A research evaluated the removal of cadmium(II) and lead(II) from aqueous solution by blue green alga Anabaena sphaerica (Abdel -Aty, Ammar, Abdel Ghafar, & Ali, Citation2013). The results suggested that this alga has a relatively high biosorption capacities for the removal of cadmium(II) and lead(II), calculated to be 111.1 mg/g and 121.95 mg/g, respectively (Abdel -Aty et al., Citation2013). The Fourier transform infrared (FTIR) analysis of algal surface functional groups, suggested that the amino, carboxyl, hydroxyl, and carbonyl groups were responsible for biosorption of those ions (Abdel -Aty et al., Citation2013). Another research studied the ability of chemically modified Cystoseira indica in the biosorption of uranium(VI) oxide and lead(II) cations (Moghaddam, Fatemi, & Keshtkar, Citation2013). In addition, Sargassum biomass was found capable of biosorption of cadmium and lead cations by partial or complete esterification of carboxylic sites present on its cell wall (Abdi & Kazemi, Citation2015).
2.1.2. Bacteria as biosorbents
Bacteria are classified as gram-positive bacteria or gram-negative bacteria, the most significant difference between these two types is in their cell wall composition and thickness (Zyoud et al., Citation2019). The cell wall of gram-positive bacteria have thicker peptidoglycan layers which are connected by amino acid bridges (Zyoud et al., Citation2019)(Abdi & Kazemi, Citation2015). Gram-positive bacteria have greater potential of removal of heavy metal cations due to the significant electronegative charge density they hold due to the presence of teichoic and teichuronic acids (polyalcohol) which are linked by phosphodiester bonds, attached to peptidoglycan of the cell wall (Tsezos et al., Citation2014; Abdi & Kazemi, Citation2015).
Functional groups containing oxygen, nitrogen, sulphur or phosphorus are the common contributors of heavy metal biosorption in bacteria (Javanbakht et al., Citation2014). A research studying biosorption of lead(II) ions by Aeromonas hydrophila suggested, with the aid of FTIR spectral data, that the carboxyl, hydroxyl and amine groups of the biosorbent surface contributed in removal of lead(II) ions from water (Javanbakht et al., Citation2014). A very recent research studied the biosorption of uranium(VI) onto Bacillus amyloliquefaciens, the functional groups contributing in removal of uranium(VI) were analysed by FTIR and XPS (Liu et al., Citation2019). After biosorption of uranium(VI) by the bacterium, a new peak appeared in the FTIR spectra of the biosorbent as a result of anti-symmetric vibration of [O = U=O]2+ group (Liu et al., Citation2019). The XPS results also suggested the formation of chemical binding between uranium and oxygen-containing functional groups (Liu et al., Citation2019). According to this study, oxygen-containing functional groups were the main site of biosorption of uranium(VI).
2.1.3. Fungi as biosorbents
Fungi started to receive more attention as a biosorbent recently due to the rich cell wall content, which suggests the presence of a wide range functional groups which be involved in metal removal (Benila Smily & Sumithra, Citation2017). The fungal cell wall is comprised of chitin, cellulose, β-glucan, α-glucan, chitosans, polyuranides, glucoproteins, lipids, inorganic salts and pigments (Dhankhar & Hooda, Citation2011). Comparing the usage of fungi as biosorbents with algae and plants, fungal biomass is easy cultivated in large scales and it is easily grown by simple fermentation techniques with overall low costs (Dhankhar & Hooda, Citation2011). A research studied the functional groups of Mucor rouxii contributing in biosorption of lead, cadmium, nickel and zinc (Yan & Viraraghavan, Citation2008). It was found, by the aid of FTIR, that amine and phosphate groups were the main contributors in the biosorption process, while role of lipid fraction was not vital (Yan & Viraraghavan, Citation2008).
2.1.4. Plants as biosorbents
Plants, as agricultural waste materials and food industries discarded material have been used as biosorbents, it is a form of reusing and recycling those waste materials thus no significant costs are associated with using plant materials. The potential of plant biosorbents are mainly due to the presence of carboxylic and phenolic functional groups in the cellulosic matrix or components associated with cellulose such as lignin and hemicellulose (Abdi & Kazemi, Citation2015). Aloe vera wastes were used as biosorbent for the removal of uranium and cadmium from water, it was found that the carboxyl, carbonyl and hydroxyl groups facilitated metal binding (Noli, Kapashi, & Kapnisti, Citation2019).
2.2. Biosorption of selected heavy metals
2.2.1. Biosorption of lead
Lead is a heavy transition metal. The approximated concentration ranges of discharged lead(II) ions in the environment from battery industry is 5-66 mg/L, from mining industry is 0.02-2.5 mg/L and that from the oil industry is 125-150 mg/L (Taşar, Kaya, & Özer, Citation2014). Lead is considered lethal to animals and plants (Nadeem, Manzoor, Iqbal, & Nisar, Citation2016). It causes several illness for humans, such as anaemia, brain damage, mental deficiency, kidney damage, encephalopathy, anorexia, cognitive impairment, behavioural issues and vomiting (Nadeem et al., Citation2016)(Morosanu, Teodosiu, Paduraru, Ibanescu, & Tofan, Citation2017). Lead(II) ions have high affinity to thio, oxo and phosphate groups that are present in some enzymes and biomolecules in living organisms (Morosanu et al., Citation2017). In humans, binding of lead to those enzymes can change the cellular membrane permeability of organs and haemoglobin synthesis, it can also bioaccumulate in bones (its half-time is over 20 years) (Morosanu et al., Citation2017). Thus, it is highly important to remove lead from wastewater before discharging it in the environment. Several studies were conducted evaluating the capacity of different biosorbents for the removal of lead(II) ions from aqueous media, selected studies are presented in , comparing the biosorption capacity of each.
Table 1. Comparison between biosorption capacity of different biosorbents for removal of lead(II) ions from aqueous media.
2.2.2. Biosorption of chromium
Chromium, a toxic, carcinogenic, mutagenic and teratogenic heavy metal, which usually exists as trivalent and hexavalent forms in aqueous environment (Rezaei, Citation2016). Chromium is one of the main pollutants in surface water and groundwater, especially its hexavalent form due its high mobility and solubility in aqueous environment, the hexavalent form is also about 500 times more toxic than trivalent form (Mishra, Dubey, & Shinghal, Citation2015). In humans, it can be a cause of allergic reaction, respiratory disorders, weakness of immunity system and kidney, liver and gastric damage (Netzahuatl-Muñoz, Del Carmen Cristiani-Urbina, & Cristiani-Urbina, Citation2015). The main industrial sources of chromium are electroplating, textile and metal finishing industries, iron and steel foundries, inorganic chemical plants and tanneries (Mishra et al., Citation2015)(Netzahuatl-Muñoz et al., Citation2015). It is highly important that industries remove chromium from wastewater before discharging it in the environment. Several studies were conducted evaluating the capacity of different biosorbents for the removal of different forms of chromium from aqueous media, selected studies are presented in , comparing the biosorption capacity of each.
Table 2. Comparison between biosorption capacity of different biosorbents for removal of chromium ions from aqueous media.
2.2.3. Biosorption of cadmium
Cadmium accumulates in photosynthetic plants and fish, which can then be easily transferred to humans (Al-Homaidan, Alabdullatif, Al-Hazzani, Al-Ghanayem, & Alabbad, Citation2015). Cadmium has the potential to bind with cysteine, glutamate, histidine and aspartate ligands in the human body causing iron deficiency (Jaishankar et al., Citation2014). When cadmium binds to cysteine-rich proteins such as metallothionein in the liver, the complex formed can cause hepatotoxicity and upon circulation and transport to the kidney it can accumulate in the renal tissue causing nephrotoxicity (Jaishankar et al., Citation2014). The polluting sources of cadmium are electroplating, smelting, paint pigments, batteries, fertilizers, mining and alloy industries (Ghoneim et al., Citation2014). compares the biosorption capacity of different biosorbents studied for the removal of cadmium ions.
Table 3. Comparison between biosorption capacity of different biosorbents for removal of cadmium(II) from aqueous media.
2.3. Mechanisms of biosorption
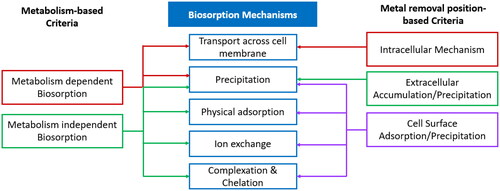
Heavy metals are taken up by the biosorbent through several mechanisms, different literatures suggested different mechanisms based on the type of biosorbent and classification criteria. Besides, most mechanisms are not fully understood in detail due to complexations in the biological nature of the biosorbents. The mechanisms are either classified according to (a) the dependence on cell metabolism or (b) the location within the cell where the metal is removed which is divided into three types: extra cellular accumulation/precipitation, cell surface sorption/precipitation and intracellular accumulation (Ahalya, Ramachandra, & Kanamadi, Citation2003). Very recently, the biosorption based on cellular metabolism has been referred to as bioaccumulation rather than a type of biosorption (Bilal et al., Citation2018). The mechanisms of cell metabolism dependent biosorption or bioaccumulation are transport across cell membrane and precipitation, while the mechanisms of cell metabolism independent biosorption are precipitation, physical adsorption, ion exchange and complexation () (Perpetuo, Souza, & Nascimento, Citation2011). Looking at the second criteria, extra cellular accumulation/precipitation is achieved by transport across cell membrane; while cell surface sorption/precipitation is achieved through ion exchange, complexation, physical adsorption and precipitation; finally, intracellular accumulation is achieved by precipitation () (Perpetuo et al., Citation2011). Simple diffusion is also a basic mechanism observed in most types of biosorption (Sao, Citation2014).
Several factors can contribute in controlling the mechanisms, those include the chemical, stereochemical and coordination characteristics of the metal of interest which involves the ion mass, ionic radius and the oxidation state of the metal ion (Kanamarlapudi, Chintalpudi, & Muddada, Citation2018). The properties of the biosorbent of choice also affect the mechanism of biosorption, properties such as the number of reactive binding sites, accessibility and availability of binding sites, type of binding site and the affinity between the binding site of the biosorbent and metal ion of interest (Abdi & Kazemi, Citation2015). There are other factors which are based on the environmental conditions that biosorption is taking place such as pH, temperature and complexity of solution containing the metal ion (Kanamarlapudi et al., Citation2018). In some cases, more than one mechanism may take place in a multi-step process mechanism, biosorption of uranium by Aloe vera waste involved physical adsorption, ion exchange, complexation and precipitation (Noli et al., Citation2019).
2.3.1. Physical adsorption
Physical adsorption takes place on the surface of the biosorbent, possibly with the cell walls while dealing with microorganisms, with the aid of electrostatic interactions such as Van der Waals forces. Physical adsorption mechanism is affected by the surface area of the biosorbent and in some cases the pH of solution (Chojnacka, Citation2006) (Bashir et al., Citation2019). In the biosorption of lead by hami melon peels, physical adsorption was only favoured at basic conditions with the contribution carboxylate and hydroxylate groups of the biomass (Bashir et al., Citation2019). A study suggested that chromium(III) ions were biosorbed by grass and wheat straw through physical adsorption mechanism bounded in a monolayer form, based on calculated maximum biosorption capacity and knowledge of ionic radius of the metal (Chojnacka, Citation2006). It has also been reported that the Zoogloea ramigera (bacterium) and Chiarella vulgaris (alga) are capable of biosorbing copper ions by physical adsorption (Perpetuo et al., Citation2011).
2.3.2. Ion exchange
Ion exchange process is the replacement of an ion bounded into the solid phase which is readily-exchangeable with another ion in solution (Gadd, Citation2009). Considering the biosorption in microorganisms such as bacteria and fungi, it has been found that Gandoderma lucidium biosorbs copper ions by ion exchange mechanism; this is possible in microorganisms due to the composition of their cell walls which have polysaccharides that can ion exchange with their counter ions (Perpetuo et al., Citation2011). It has been reported that upon binding of heavy metal ions such as copper(II), cadmium(III) and zinc(II) to alginate of brown algae, an enhanced release of calcium, potassium, magnesium and sodium was observed due to ion exchange mechanism (Abdi & Kazemi, Citation2015)(Ahalya et al., Citation2003). A study reported that rice straws, as an example of non-metabolism dependent biosorbent, were able to biosorb cadmium(II) ions by ion exchange with sodium, potassium, magnesium and calcium (Kanamarlapudi et al., Citation2018). In addition, it was found that pH of solution can affect some biosorption mechanisms such as ion exchange. The biosorption mechanism of lead ions by hami melon peels was achieved by ion exchange at low pH, while at higher pH it was achieved by electrostatic interactions between carboxylate and hydroxylate groups on the biomass and lead ions (Bashir et al., Citation2019). Spirulina, a cyanobacteria, was capable of biosorbing chromium(III), cadmium(II) and copper(II) through ion exchange by identified functional groups including carboxyl, phosphate and hydroxyl groups (Kanamarlapudi et al., Citation2018).
2.3.3. Complexation
Complexation is a process of complex formation by electrostatic attraction or covalent bonds between metal ions and organic molecules acting as ligands with the ability to donate electrons, the advanced form of complexation is chelation in which the organic ligand would bond coordinatively with the metal ion from more than one position at the same time to form a complex of higher stability (Tsezos et al., Citation2014). The affinity of metal ion and organic ligand, which is the most significant factor in complexation, is based on the Hard and soft acids and bases (HSAB) theory, which implies that elements are classified into hard or soft acids (mainly metals) and hard or soft bases (mainly non-metals), hard acids have stronger affinity to hard bases and so the weak acids and bases (Ayers, Citation2005). Lead being a soft acid, would favour binding covalently to organic ligands of the biosorbent containing nitrogen or sulphur which are soft bases; manganese, zinc, cadmium and copper ions which are classified as borderline ions (hard/soft) also have a high affinity to form complexes with nitrogen or sulphur containing organic compounds (Tsezos et al., Citation2014). Nevertheless, complexation is affected by the accessibility and availability of the binding site containing the donor atom (base). Desorption studies using scanning electron microscopy (SEM), transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDX) can confirm if biosorption mechanism is achieved by complexation (Han, Wong, & Tam, Citation2006). Using those methods, the biosorption mechanism of chromium(III) by Chlorella miniate, a green microalgal, was confirmed to be through complexation by carboxyl, phosphonate and amine ligands (Han et al., Citation2006).
2.3.4. Precipitation
Precipitation refers to the formation of insoluble metal in the form of precipitate, which is one of the few mechanisms involved in the metabolism dependent biosorption, nevertheless metabolism independent biosorption can also occur by precipitation. In the metabolism dependent biosorption, precipitation occurs as a response by the microorganisms’ active defence system in the presence in an environment containing toxic metal ions (Perpetuo et al., Citation2011). In the metabolism independent biosorption, precipitation occurs due to chemical reactions between the functional groups of the cell wall of the biosorbent and the metal ion, the reactions may involve oxidation-reduction reactions (Kanamarlapudi et al., Citation2018). A study confirmed the mechanism of the removal of iron(III) ions from aqueous solution by the aid of green tomato husk to be mainly via precipitation and ion exchange (García-Mendieta, Olguín, & Solache-Ríos, Citation2012). Iron(III) ions precipitated in the form of iron(III) hydroxide at pH 6.0 (García-Mendieta et al., Citation2012).
2.3.5. Transport across cell membrane
Transport of heavy metal ions across cell membrane is a mechanism usually observed in microorganisms only. This mechanism comprises of two stages: in the first stage the metal ion binds to binding sites on the cell wall of the microorganism which is referred to as independent binding metabolism, then in the second stage the metal ion is transported across the cell membrane into the cell and this step is referred to as metabolism dependent intracellular uptake (Perpetuo et al., Citation2011). The mechanism mimics the process of uptake of essential ions by the cell, it has been mentioned that cellular metal transport systems are tricked by heavy metal ions having the similar ionic radius and charge of the essential metal ions, nevertheless the process is not clearly understood yet (Perpetuo et al., Citation2011).
2.4. Factors affecting biosorption capacity
2.4.1. pH
The pH under which biosorption takes place is very significant and directly affects the biosorption capacity, and in some cases the mechanism via which biosorption takes place as mentioned earlier. pH affects both the chemistry of the functional groups of biosorbent and the chemistry of metal ions (Wei et al., Citation2016). The biosorption capacity often increases with the increase of pH until it reaches the optimum pH where the maximum biosorption capacity is observed, following that, upon further increase in pH, metals begin to precipitate due to formation of metal hydroxides or hydroxide anionic complexes (Abdi & Kazemi, Citation2015)(Bilal et al., Citation2018). The biosorption of metal ions such as copper, cadmium, nickel, cobalt and zinc are usually reduced at low acidic pH conditions, in which competition occurs between the metal cations and hydronium ions for the binding sites of the biosorbent (Gadd, Citation2009). At low pH, the functional groups of the biosorbent would favour the hydronium ions as the functional groups exist in protonated form and repulsive forces between the functional groups and metal cations would not allow attraction of those two, however as the pH increases, functional groups such as carboxyl, hydroxyl, phosphate groups would start experiencing negative charges (and amino and imidazole groups turning neutral) due to deprotonation, this would increase binding of metal cations and thus increase the biosorption capacity and speed of biosorption (Abdi & Kazemi, Citation2015)(Bilal et al., Citation2018). Although increasing pH further would ensure that all the acidic groups are deprotonated, however the free metal cations can start to precipitate as metal hydroxides, decreasing the efficiency of removal of the metal.
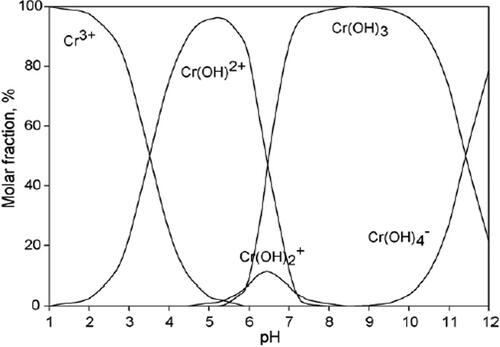
As mentioned earlier, pH does not only affect the functional groups of the biosorbent, it also affects the chemistry of metal ions. pH would affect the form of the metal ion present in aqueous solution and the concentration of that form, resulting in the formation of different metal ion species at different pH levels. In a study considering the biosorption of chromium(III) ions by olive stones, the speciation of chromium(III) ion was analysed () (Blázquez, Hernáinz, Calero, Martín-Lara, & Tenorio, Citation2009). As shown in , it suggests that chromium(III) would be present in five different forms: Cr3+, Cr(OH)2+, Cr(OH)2+, Cr(OH)4- and a solid form Cr(OH)3 as a precipitate. The dominant form of chromium(III) varies according to the pH. Different species would have different affinity to the functional groups of the biosorbent, this would affect the biosorption process. In addition, the solubility and mobility of metal ion species differ and this will also have an impact on biosorption process (Gadd, Citation2009).
Figure 2. Speciation of Cr(III) in different pH at 25˚C and concentration of 10 mg/L, adapted from (Blázquez et al., Citation2009).
2.4.2. Temperature
Temperature affects the surface activity of the biosorbent and thus the biosorption capacity. The effect of temperature on the biosorption process depends on the nature of the process. Increasing the temperature for an exothermic biosorption process would result in a decrease in metal ion removal, the opposite is true for endothermic biosorption processes (Taşar et al., Citation2014). Likewise, decreasing the temperature for an exothermic biosorption process would result in an increase in metal ion removal, the opposite is true for endothermic biosorption processes. Biosorption is not highly affected between changes in temperatures ranging from 20˚C up to 35˚C (Sao, Citation2014)(Perpetuo et al., Citation2011). The biosorption of lead(II) ions by peanut shells decreased with increasing temperature, suggesting the exothermic nature of the process (Taşar et al., Citation2014).
2.4.3. Contact time
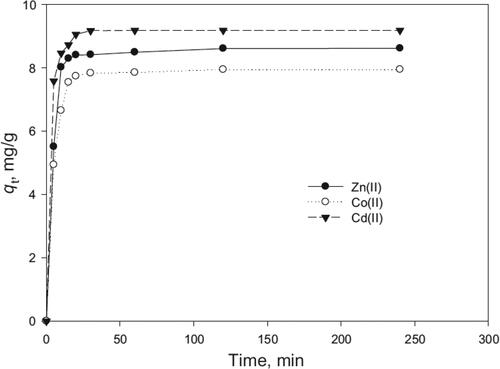
Contact time usually refers to the time allocated for biosorption process to take place. Biosorption capacity is not directly affected by contact time of the biosorbent and sorbate, however it can act as a limiting factor. Under experimental conditions, increasing contact time would allow the biosorbent material to unveil the maximum biosorption capacity. When the biosorbent reaches its maximum biosorption capacity at defined conditions its binding sites become fully saturated, increasing contact time would not have any further effect as shown in (Hajahmadi, Younesi, Bahramifar, Khakpour, & Pirzadeh, Citation2015).
Figure 3. Effect of contact time on biosorption capacity of A. niger for the removal of Zn(II), Co(II) and Cd(II) at pH 7, biomass dosage of 2 g/L and initial ions concentration of 10 mg/L, adapted from (Hajahmadi et al., Citation2015) research.
2.4.4. Biomass dosage
At low biomass dosage an increase in the specific uptake of metal ions has been observed (Sao, Citation2014)(Perpetuo et al., Citation2011). Nevertheless, using low biomass dosage in complex contaminated water would increase competition for binding site of biosorbent and will limit the biosorption capacity of the biosorbent. Increasing the biomass dosage would decrease the competition between metal ions present for binding to the functional groups, specifically when more than one metal ion is present. In most of the published researches, the amount of biomass used was between 0.5 -6.0 g/L.
2.4.5. Initial metal concentration
In a study evaluating the potential of banana peel as a biosorbent for heavy metals, it was found that the metal biosorption increases when the initial metal concentration increases (Ashraf, Citation2011). Increasing initial metal concentration, has a similar effect as increasing contact time. Using low metal concentration would not unveil the true maximum biosorption capacity of the biosorbent, as many metal binding sites may not be occupied. Increasing the metal concentration up to full saturation of binding sites of the biosorbent would reveal the full potential of metal removal. Upon saturation of biosorbent, further increasing the initial metal concentration will not be effective. In most of the published researches, the initial metal concentration used in experimental conditions was 5-200 mg/L.
2.4.6. Other factors
Surface area of the biosorbent material affects the biosorption capacity of the biosorbent, higher surface area would increase biosorption under the same conditions. A study compared the biosorption of lead, copper and zinc ions by (a) dried lettuce leaves and (b) powdered lettuce leaves (in dry form), under all conditions, the metal removal by powdered lettuce leaves was higher than dried leaves (Shartooh, Citation2013).
The presence of more than one metal ion in the same media can influence the removal of one metal ion on another, as one metal ion could have higher affinity to the binding sites of the biosorbent leading to competition on the availability of binding sites (Sao, Citation2014).
Researches have studied the effect of chemical modifications on the biosorption capacity of the biosorbent material. A study evaluating the biosorption of lead(II) ions by untreated and chemically treated olive tree pruning suggested that chemically modified biosorbent samples had a higher biosorption capacity (as numerically outlined in ) (Calero, Pérez, Blázquez, Ronda, & Martín-Lara, Citation2013). The infrared spectra data and potentiometric titrations suggested that the surface of acid treated samples contained more acidic groups than the untreated sample, indicating that the surface of the treated biosorbent became more negative due to the dissociation of weakly acidic oxygen containing functional groups that contribute in biosorption resulting in higher biosorption capacity (Calero et al., Citation2013).
2.5. Desorption
Upon the completion of biosorption, the exhausted biosorbent can be reused by removal of the biosorbed metal ions and safely discarding them, this process is known as desorption and is achieved with the aid of a desorbing agent (Benila Smily & Sumithra, Citation2017). The role of desorbing agent is to desorb the biosorbed species from the biosorbent, and ultimately regenerate the biosorbent for further use. The percentage of desorbed species is calculated according to the following equation:
Desorption Percentage (%) = (Amount of Species Desorbed ÷ Amount of Species Loaded) x 100%
High desorption percentage suggests higher degree of regeneration of biosorbent for further use, this should also be considered while choosing the biosorbent for greater degree of sustainability.
Desorbing agents are commonly divided into three groups: acids (such as hydrochloric acid, sulphuric acid, nitric acid and acetic acid), alkalis (sodium hydroxide, sodium hydrogen carbonate, sodium carbonate and potassium hydroxide) and chelating agents (such as ethylenediaminetetraacetic acid) (Lata, Singh, & Samadder, Citation2015). Acidic desorbing agents have been found to be more efficient than basic and neutral agents in terms of speed and desorption percentage (Lata et al., Citation2015). A good desorbing agent should not alter the chemical and physical properties of the biosorbent.
2.6. Critical analysis of biosorption
Biosorption is economically feasible, easy to operate and use, and shows good adsorption efficacy (Wei et al., Citation2016). Biomass can be obtained from waste and discarded plant products (e.g., peels and stones) or can be obtained from fermentation industries which consider them as waste, thus no costs are associated with their provision (Abdi & Kazemi, Citation2015). Using biosorption methods can be very useful in developing countries with low technological development. Plant biosorbents also have the advantage of minimizing chemical and/or biological sludge (Ashraf, Citation2011). The usage of many types of biosorbents are in compliant with sustainable development as the biosorbent can undergo desorption and be regenerated, besides adding the possibility of recovering the metal for further industrial uses.
Many research papers have studied the removal of one to two metals by biosorption, however the results obtained by these researches may not be applicable in real life situations in which contaminated water as a wide range of heavy metals can be present in contaminated water. In fact, organic pollutants may also be present in water, which can also affect the nature of metal ions and their biosorption process. A recommended phase in biosorption studies can be studying the applications of laboratory findings on real contaminated water samples. For example, water pH, being the most influential factor, would be challenging to optimise in real life situations due to the presence of a wide range of other environmental factors affecting pH, thus biosorption capacities of biosorbents determined for specific pH levels under laboratory conditions may not be true in real life. The water samples experimented usually do not reflect complexity and matrix nature of real contaminated water.
In addition, the main focus of most researches dealing with biosorption is the removal of heavy metals, however removal of toxic organic pollutants has not been studied in depth. In fact, a lot of researches are based on removal of heavy metals in their cationic form and not the anionic (e.g., CrO42-). One of the limitations of biosorption process could be the early saturations of biosorbent, thus biosorbent shall be frequently replaced. The saturated biosorbent shall be desorbed from the biosorbed metal and the recovered metal shall be safely disposed. The efficiency of desorption process and efficiency of recycled/reused biosorbent can be negotiable too. According to Clarivate Analytics, from 1951 up to 13/04/2019, 14,841 papers with “biosorption” as a topic, were published. Unfortunately, the use of biosorbent is still not commercialised by the industries (Gadd, Citation2009). Industries tend to favour using methods including chemical precipitation, ion exchange, oxidation-reduction methods and reverse osmosis (Gadd, Citation2009). Chemical precipitation is highly favoured due to its significant efficiency in the removal of a wide range of heavy metals (it is not selective), nevertheless this method produces high amounts of solid sludge which is not environmentally favourable (Gadd, Citation2009). Another challenge facing implementation of biosorption in industries is that their current methods, such as precipitation and ion exchange, are very well established into their processes and have proven their applicability on large scales. Industries might find it risky and infeasible to replace their current trusted method with new methods.
3. Conclusions
Biosorption methods can be an alternative to the common industrial methods of removal of heavy metals due to being environmentally friendly and sustainable for use. Several types of biosorbent materials can be used such as algae, bacteria, fungi and plants. Biosorption process can take place by several mechanisms including physical adsorption, ion exchange, complexation, precipitation and transport across the cells. The biosorption capacity of biosorbents can be affected by the pH of the environment, temperature, contact time, biomass dosage, initial metal concentration and other factors. A major limitation of the researches conducted in this area, studying the biosorption capacity of different biomass, is that they did not encounter for the complexity of real-life contaminated water.
Disclosure statement
No potential conflict of interest was reported by the author(s).
4. References
- Abbas Ali, A., Mohamed Sihabudeen, M., & Zahir Hussain, A. (2016). Biosorption of Heavy Metals By Pseudomonas Bacteria. International Research Journal of Engineering and Technology, 3(8), 1446–1450.
- Abdel -Aty, A. M., Ammar, N. S., Abdel Ghafar, H. H., & Ali, R. K. (2013). Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. Journal of Advanced Research, 4(4), 367–374. doi:10.1016/j.jare.2012.07.004
- Abdi, O., & Kazemi, M. (2015). A review study of biosorption of heavy metals and comparison between different biosorbents. Journal of Materials and Environmental Science, 6(5), 1386–1399.
- Ahalya, N., Ramachandra, T. V., & Kanamadi, R. D. (2003). Removal and Recovery of Heavy Metals by Biosorption. Research Journal of Chemistry and Environment, 7(4), 71–79. doi:10.1111/j.1742-4658.2005.04698.x
- Akbari, M., Hallajisani, A., Keshtkar, A. R., Shahbeig, H., & Ali Ghorbanian, S. (2015). Equilibrium and kinetic study and modeling of Cu(II) and Co(II) synergistic biosorption from Cu(II)-Co(II) single and binary mixtures on brown algae C. indica. Journal of Environmental Chemical Engineering, 3(1), 140–149. doi:10.1016/j.jece.2014.11.004
- Al-Homaidan, A. A., Alabdullatif, J. A., Al-Hazzani, A. A., Al-Ghanayem, A. A., & Alabbad, A. F. (2015). Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi Journal of Biological Sciences, 22(6), 795–800. doi:10.1016/j.sjbs.2015.06.010
- Al-Homaidan, A. A., Al-Houri, H. J., Al-Hazzani, A. A., Elgaaly, G., & Moubayed, N. M.S. (2014). Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arabian Journal of Chemistry, 7(1), 57–62. doi:10.1016/j.arabjc.2013.05.022
- Anastopoulos, I., & Kyzas, G. Z. (2015). Progress in batch biosorption of heavy metals onto algae. Journal of Molecular Liquids, 209, 77–86. doi:10.1016/j.molliq.2015.05.023
- Ashraf, M. (2011). Low cost biosorbent banana peel (Musa sapientum) for the removal of heavy metals. Scientific Research and Essays, 6(19), 4055–4064. Retrieved from http://umexpert.um.edu.my/file/publication/0000409471909.pdf.
- Ayers, P. W. (2005). An elementary derivation of the hard/soft-acid/base principle. Journal of Chemical Physics, 122(14). doi:10.1063/1.1897374
- Azhar, W., & Iram, S. (2014). Kinetics, Equilibrium and Thermodynamics Studies on Biosorption of Heavy Metals by Fungal Biomass. International Journal of Engineering Sciences & Emerging Technologies, 7(3), 693–700.
- Bashir, A., Malik, L. A., Ahad, S., Manzoor, T., Bhat, M. A., Dar, G. N., & Pandith, A. H. (2019). Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environmental Chemistry Letters, 17(2), 729–754. doi:10.1007/s10311-018-00828-y
- Benila Smily, J. R. M., & Sumithra, P. A. (2017). Optimization of Chromium Biosorption by Fungal Adsorbent, Trichoderma sp. BSCR02 and its Desorption Studies. HAYATI Journal of Biosciences, 24(2), 65–71. doi:10.1016/j.hjb.2017.08.005
- Bilal, M., Rasheed, T., Sosa-Hernández, J., Raza, A., Nabeel, F., & Iqbal, H. (2018). Biosorption: An interplay between marine algae and potentially toxic elements—A review. Marine Drugs, 16(2), 1–16. doi:10.3390/md16020065
- Blázquez, G., Hernáinz, F., Calero, M., Martín-Lara, M.A., & Tenorio, G. (2009). The effect of pH on the biosorption of Cr (III) and Cr (VI) with olive stone. Chemical Engineering Journal, 148(2-3), 473–479. doi:10.1016/j.cej.2008.09.026
- Calero, M., Pérez, A., Blázquez, G., Ronda, A., & Martín-Lara, M. A. (2013). Characterization of chemically modified biosorbents from olive tree pruning for the biosorption of lead. Ecological Engineering, 58, 344–354. doi:10.1016/j.ecoleng.2013.07.012
- Chojnacka, K. (2006). Biosorption of Cr(III) ions by wheat straw and grass: A systematic characteristics of new biosorbents. Polish Journal of Environmental Studies, 15(6), 845–852.
- Davis, T. A., Volesky, B., & Mucci, A. (2003). A review of the biochemistry of heavy metal biosorption by brown algae. Water Research, 37(18), 4311–4330. doi:10.1016/S0043-1354(03)00293-8
- Dhankhar, R., & Hooda, A. (2011). Fungal biosorption-an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environmental Technology, 32(5), 467–491. doi:10.1080/09593330.2011.572922
- Farhan, S. N., & Khadom, A. A. (2015). Biosorption of heavy metals from aqueous solutions by Saccharomyces Cerevisiae. International Journal of Industrial Chemistry, 6(2), 119–130. doi:10.1007/s40090-015-0038-8
- Fernández-López, J. A., Angosto, J. M., & Avilés, M. D. (2014). Biosorption of Hexavalent Chromium from Aqueous Medium with Opuntia Biomass. The Scientific World Journal, 2014, 1–8. doi:10.1155/2014/670249
- Gadd, G. M. (2009). Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. Journal of Chemical Technology & Biotechnology, 84(1), 13–28. doi:10.1002/jctb.1999
- García-Mendieta, A., Olguín, M. T., & Solache-Ríos, M. (2012). Biosorption properties of green tomato husk (Physalis philadelphica Lam) for iron, manganese and iron-manganese from aqueous systems. Desalination, 284, 167–174. doi:10.1016/j.desal.2011.08.052
- Ghoneim, M. M., El-Desoky, H. S., El-Moselhy, K. M., Amer, A., Abou El-Naga, E. H., Mohamedein, L. I., & Al-Prol, A. E. (2014). Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. The Egyptian Journal of Aquatic Research, 40(3), 235–242. doi:10.1016/j.ejar.2014.08.005
- Hajahmadi, Z., Younesi, H., Bahramifar, N., Khakpour, H., & Pirzadeh, K. (2015). Multicomponent isotherm for biosorption of Zn(II), CO(II) and Cd(II) from ternary mixture onto pretreated dried Aspergillus niger biomass. Water Resources and Industry, 11, 71–80. doi:10.1016/j.wri.2015.07.003
- Han, X., Wong, Y. S., & Tam, N. F. Y. (2006). Surface complexation mechanism and modeling in Cr(III) biosorption by a microalgal isolate, Chlorella miniata. Journal of Colloid and Interface Science, 303(2), 365–371. doi:10.1016/j.jcis.2006.08.028
- Hanif, M. A. (2011). Biosorption of Cr (III) and Cr (VI) by Newly Isolated White Rot Fungi: Batch and Column Studies. Asian Journal of Chemistry, 23(8), 3375–3383.
- He, J., & Chen, J. P. (2014). A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresource Technology, 160, 67–78. doi:10.1016/j.biortech.2014.01.068
- Hou, Y., Cheng, K., Li, Z., Ma, X., Wei, Y., Zhang, L., & Wang, Y. (2015). Biosorption of cadmium and manganese using free cells of Klebsiella sp. isolated from waste water. PLoS One, 10(10), 1–23. doi:10.1371/journal.pone.0140962
- Ivánová, D., Kaduková, J., Kavuličová, J., & Horváthová, H. (2012). Determination of the Functional Groups in Algae Parachlorella Kessleri by Potentiometric Titrations. Nova Biotechnologica et Chimica, 11(2), 93–99. doi:10.2478/v10296-012-0010-3
- Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60–72. doi:10.2478/intox-2014-0009
- Javanbakht, V., Alavi, S. A., & Zilouei, H. (2014). Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Science and Technology, 69(9), 1775–1787. doi:10.2166/wst.2013.718
- Kanamarlapudi, S. L. R. K., Chintalpudi, V. K., & Muddada, S. (2018). Application of Biosorption for Removal of Heavy Metals from Waste Water. School of Enviromental Sciences, 69–116.
- Lata, S., Singh, P. K., & Samadder, S. R. (2015). Regeneration of adsorbents and recovery of heavy metals: A review. International Journal of Environmental Science and Technology, 12(4), 1461–1478. doi:10.1007/s13762-014-0714-9
- Liu, L., Liu, J., Liu, X., Dai, C., Zhang, Z., Song, W., & Chu, Y. (2019). Kinetic and equilibrium of U(VI) biosorption onto the resistant bacterium Bacillus amyloliquefaciens. Journal of Environmental Radioactivity, 203, 117–124. doi:10.1016/j.jenvrad.2019.03.008
- Mishra, A., Dubey, A., & Shinghal, S. (2015). Biosorption of chromium(VI) from aqueous solutions using waste plant biomass. International Journal of Environmental Science and Technology, 12(4), 1415–1426. doi:10.1007/s13762-014-0516-0
- Moghaddam, M. R., Fatemi, S., & Keshtkar, A. (2013). Adsorption of lead (Pb2+) and uranium (UO22+) cations by brown algae; experimental and thermodynamic modeling. Chemical Engineering Journal, 231, 294–303. doi:10.1016/j.cej.2013.07.037
- Morosanu, I., Teodosiu, C., Paduraru, C., Ibanescu, D., & Tofan, L. (2017). Biosorption of lead ions from aqueous effluents by rapeseed biomass. New Biotechnology, 39, 110–124. doi:10.1016/j.nbt.2016.08.002
- Nadeem, R., Manzoor, Q., Iqbal, M., & Nisar, J. (2016). Biosorption of Pb(II) onto immobilized and native Mangifera indica waste biomass. Journal of Industrial and Engineering Chemistry, 35, 185–194. doi:10.1016/j.jiec.2015.12.030
- Netzahuatl-Muñoz, A. R., Del Carmen Cristiani-Urbina, M., & Cristiani-Urbina, E. (2015). Chromium biosorption from Cr(VI) aqueous solutions by Cupressus lusitanica bark: Kinetics, equilibrium and thermodynamic studies. PLoS One, 10(9), 1–23. doi:10.1371/journal.pone.0137086
- Noli, F., Kapashi, E., & Kapnisti, M. (2019). Biosorption of uranium and cadmium using sorbents based on Aloe vera wastes. Journal of Environmental Chemical Engineering, 7(2), 102985. doi:10.1016/j.jece.2019.102985
- Perpetuo, E. A., Souza, C. B., & Nascimento, C. A. O. (2011). Engineering Bacteria for Bioremediation. Progress in Molecular and Environmental Bioengineering, 605–632.
- Reddy, N. A., Lakshmipathy, R., & Sarada, N. C. (2014). Application of Citrullus lanatus rind as biosorbent for removal of trivalent chromium from aqueous solution. Alexandria Engineering Journal, 53(4), 969–975. doi:10.1016/j.aej.2014.07.006
- Rezaei, H. (2016). Biosorption of chromium by using Spirulina sp. Arabian Journal of Chemistry, 9(6), 846–853. doi:10.1016/j.arabjc.2013.11.008
- Sahmoune, M. N. (2018). Performance of Streptomyces rimosus biomass in biosorption of heavy metals from aqueous solutions. Microchemical Journal, 141(May), 87–95. doi:10.1016/j.microc.2018.05.009
- Sao, K. (2014). A Review on Heavy Metals Uptake by Plants through Biosorption. International Proceedings of Economics Development and Research, 75(17), 78–83. doi:10.7763/IPEDR
- Shartooh, S. M. (2013). Lettuce Leaves as Biosorbent Material to Remove Heavy Metal Ions from Industrial Wastewater. J. Baghdad for Science, 11(3), 1164–1170.
- Sibi, G. (2016). Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. Green Energy & Environment, 1(2), 172–177. doi:10.1016/j.gee.2016.08.002
- Taşar, Ş., Kaya, F., & Özer, A. (2014). Biosorption of lead(II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. Journal of Environmental Chemical Engineering, 2(2), 1018–1026. doi:10.1016/j.jece.2014.03.015
- Tsezos, M., Remoundaki, E., & Hatzikioseyian, A. (2014) Biosorption - Principles and Applications for Metal Immobilization from waste-water streams. Clean Production and Nano Technologies, 23–33. Retrieved from http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.490.1498&rep=rep1&type=pdf.
- Wang, G., Zhang, S., Yao, P., Chen, Y., Xu, X., Li, T., & Gong, G. (2018). Removal of Pb(II) from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent. Arabian Journal of Chemistry, 11(1), 99–110. doi:10.1016/j.arabjc.2015.06.011
- Wei, W., Wang, Q., Li, A., Yang, J., Ma, F., Pi, S., & Wu, D. (2016). Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: Adsorption behavior and mechanism assessment. Scientific Reports, 6(1), 10. pp. doi:10.1038/srep31575
- Yan, G., & Viraraghavan, T. (2008). Mechanism of biosorption of heavy metals by Mucor rouxii. Engineering in Life Sciences, 8(4), 363–371. doi:10.1002/elsc.200820237
- Yusoff, S. N. M., Kamari, A., Putra, W. P., Ishak, C. F., Mohamed, A., Hashim, N., & Isa, I. M. (2014). Removal of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Agricultural Wastes: Adsorption and Characterisation Studies. Journal of Environmental Protection, 05(04), 289–300. doi:10.4236/jep.2014.54032
- Zhou, K., Yang, Z., Liu, Y., & Kong, X. (2015). Kinetics and equilibrium studies on biosorption of Pb(II) from aqueous solution by a novel biosorbent: Cyclosorus interruptus. Journal of Environmental Chemical Engineering, 3(3), 2219–2228. doi:10.1016/j.jece.2015.08.002
- Zyoud, A., Alkowni, R., Yousef, O., Salman, M., Hamdan, S., Helal, M. H., … Hilal, H. S. (2019). Solar light-driven complete mineralization of aqueous gram-positive and gram-negative bacteria with ZnO photocatalyst. Solar Energy, 180, 351–359. doi:10.1016/j.solener.2019.01.034