?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Alginate beads incorporating zinc oxide (ZnO) nanoparticles were shown as an alternative to treat water contaminated with sedge dye by combining photocatalytic activity with adsorption. Dry and fresh alginate beads were prepared by changing ZnO contents in the range of 0.5, 1, and 2% (w/w) and then characterized for their morphology as well as dye removal. The dye removal efficiency was increased by increasing the active surface area of the alginate beads and ZnO photocatalytic activity. Under ultraviolet irradiation for 24 h, the dye removal efficiency reached 64% in the case of 2% ZnO loading. The use of alginate beads dried at 80 °C before ZnO loading increased the maximum value to 78%. With more than 20% removal during the first 2 h through the adsorption and diffusion, the dye removal of alginate beads incorporating 1 and 2% ZnO was modeled as a function of time according to the Weber-Morris intra-particle diffusion equation. The dye adsorption per mass of the adsorbent having 0 and 0.5% ZnO loading fitted the Elovich equation better.
1. Introduction
Currently, polluted wastewater is released from a variety of sectors, including the food industry (Aderiye, Iteke, Akinyeye, & Oluwole, Citation2021), textile manufacturing (Redha, Yusuf, Amin, & Bououdina, Citation2020; Redha, Citation2020), and handicraft production (Murcia Mesa et al., Citation2021). These effluents must be treated before being discharged into the drain. Many standard treatments used for industrial effluents are costly and ineffective in some situations. Cationic dyes released from dyeing processes into the environment can be allergenic (Shaban, Abukhadra, Shahien, & Ibrahim, Citation2018), carcinogenic (Shanker, Rani, & Jassal, Citation2017), toxic (Nigiz, Citation2019a) to the water ecosystem, and health. They impart undesirable color to the water, preventing sunlight penetration into the bottom of water reservoirs. Wastewater from local handicrafts dyeing is also a significant cause of environmental degradation. One example is Krajood (Lepironia articulata), or grey sedge handicraft dyeing by the Thale Noi community enterprise in Phatthalung Province of Thailand (Wunbua, Nakhapakorn, & Jirakajohnkool, Citation2012). Without proper treatment before discharging into canals, the effluent interrupts the photosynthetic activity in the water ecosystem and slows down the growth of the biotic community. Also, the water supply becomes unsuitable for consumption. Therefore, it is essential to remediate the synthetic dye from the wastewater properly and safely.
There are several techniques applied for dye removal, including chemical precipitation (Zare et al., Citation2021), ion exchange (Kaur & Jindal, Citation2019), membrane-based separation (Ben Dassi et al., Citation2021), redox method (Ait-Touchente et al., Citation2020a), biological treatment (Ali, Citation2010), catalytic degradation (Mudhoo et al., Citation2020; Ait-Touchente et al., Citation2020b; Kamran, Bhatti, Iqbal, Jamil, & Zahid, Citation2019), and adsorption (Nigiz, Citation2019a; Nigiz, Citation2019b; Liu, Zhang, Tang, & Zhu, Citation2021; Zare et al., Citation2021). Each technique has its limitations and advantages. The adsorption technique is considered to be highly convenient (Nigiz, Citation2019a), adjustable (Nigiz, Citation2019b), cost-effective (Afroze, Sen, & Ang, Citation2016), and environmentally friendly route (Shanker et al., Citation2017) for the removal of dye molecules. The dye removal efficiencies were seen to be improved for water remediation when different techniques were employed (Mudhoo et al., Citation2019; Jain et al., Citation2020; Mudhoo et al., Citation2020; Zare et al., Citation2021).
Activated carbon (Ghaedi, Nasab, Khodadoust, Rajabi, & Azizian, Citation2014), zeolite (Tian, Guan, Gao, Wen, & Ren, Citation2016), silica (Tahir, Bhatti, Hussain, Bhatti, & Asghar, Citation2020), bentonite (Shaban et al., Citation2018), kaolin (Musa et al., Citation2020), cellulose (Tu et al., Citation2017), chitosan (Kaur & Jindal, Citation2019; Noreen, Bhatti, Iqbal, Fazli, & Sarim, Citation2020a), and alginate (Nigiz, Citation2019b; Biftu & Ravindhranath, Citation2020; Bhatti et al., Citation2020; Liu et al., Citation2021) may be cited as examples of different adsorbents that can be used to remove dye molecules from water. With the environmental concerns, the adsorbents should be biodegradable. For example, cellulose and lignin biosorbents from camphor stem can remove green and violet dyes from wastewater (Moawed, Kiwaan, & Elbaraay, Citation2019). Developing adsorbents based on nanoparticles seems to be one of the recent trends in water treatment. Nanoparticles exhibit high dye removal efficiency due to their high surface area, quantum confinement effect, and other morphological properties (Ravulapalli & Ravindhranath, Citation2019). One of the main disadvantages of nanoparticles, when used as adsorbents, is the low percolation of water through them because of the presence of micro or nano-sized voids. This can be avoided by immobilizing the nanoparticles in beads (Biftu & Ravindhranath, Citation2020).
Sodium alginate is a natural polysaccharide synthesized from brown seaweed which can act as a cation remover (Perez-Ameneiro et al., Citation2015; Kumar, Vijayakumar, & Tamilarasan, Citation2019; Liu et al., Citation2021; Subhan, Alam, Shah, Ali, & Farooq, Citation2021). The adsorbent used to reduce pollutants in an eco-friendly manner is developed based on the attachment of this biomaterial to metal ions (Kumar et al., Citation2019). To transform the alginate commonly used as a thickening agent into an adsorbent, the gelation or cross-linking was initiated by the cationic agents, e.g. Ca+2 (Li, Lu, Li, Tong, & Ye, Citation2017). Application of specifically used alginate-based adsorbent for cationic dye removal has been reported in many studies (Djebri, Boutahala, Chelali, Boukhalfa, & Zeroual, Citation2016; Shao et al., Citation2018; Kumar et al., Citation2019; Nigiz, Citation2019a; Nigiz, Citation2019b). The enthalpy and entropy changes indicate that the adsorption of methylene blue (MB) dye by calcium alginate (Isik & Ugraskan, Citation2021), and black dye by sodium alginate (Nishtar, Jonnalagadda, Balachandran, & Nair, Citation2007) are spontaneous and endothermic. Composite adsorbents such as titanium dioxide-alginate beads (Nouri et al., Citation2020), crosslinked polyanetholesulfonic acid and sodium alginate beads with magnetic zeolite (Metin, Doğan, & Can, Citation2020), yttrium-alginate beads (Li & Yin, Citation2020), and clinoptilolite-doped alginate beads (Nigiz, Citation2019a), have been developed and tested in some applications.
Interestingly, sodium alginate beads encapsulating zinc oxide (ZnO) nanoparticles were found to be efficient composite materials for water remediation by bacteria disinfection (Motshekga, Ray, & Maity, Citation2018). ZnO is a multifunctional metal oxide with biocompatibility, chemical stability, low cost, ease of mass production, and combination with polymers (Ait-Touchente et al., Citation2020c). ZnO in the form of the nanoparticle can be synthesized using various methods as reviewed by Sirelkhatim et al. (Citation2015). Because of increased surface area and photocatalytic activity (Bibi et al., Citation2020; Ben Dassi et al., Citation2021), nanostructured ZnO is a promising additive to enhance dye removal from wastewater. The thermodynamic studies reveal non-spontaneous, exothermic adsorption by ZnO (Noreen et al., Citation2020b), but the adsorption process in the case of zeolite incorporating ZnO is pontaneous and endothermic (Elfeky, Youssef, & Elzaref, Citation2020). In the present study, alginate beads incorporating ZnO were synthesized for eliminating dye molecules from the wastewater, which is vital for protecting the ecological environment. The dye removal efficiencies of fresh and dry beads with varying ZnO loadings were compared by treating dye solutions widely used in producing Thale Noi grey sedge handicrafts.
2. Materials and methods
2.1. Materials
Sodium alginate (C6H7O6Na) and calcium chloride dihydrate (CaCl2•2H2O) were purchased from L.B. Science Ltd. Partnership, and KemAus, respectively. ZnO nanopowders, from Loba Chemie Pvt. Ltd., have the particle size of the order of 100 nm as characterized by scanning electron microscopy (not shown here). Sedge dye was obtained from Phua Kiam Seen Co. Ltd. which is commonly used by Thale Noi community enterprise. All solutions were prepared by using deionized water (DI).
2.2. Experiment design
ZnO of 0, 0.5, 1, and 2% (w/w) loadings incorporated into alginate beads by adapting the method by Motshekga et al. (Citation2018) are respectively referred to as Alg-Pure, Alg-ZnO_0.5, Alg-ZnO_1, Alg-ZnO_2. Sodium alginate and ZnO nanopowders were thoroughly dissolved in 100 mL DI water. The obtained solution was loaded into a syringe and then added dropwise into the 0.2 M calcium chloride solution. As a result of the cross-link with Ca2+, the alginate beads were formed and cured. After 24 h, the beads were removed and rinsed with DI water three times. To compare fresh and dry alginate beads, the other set was prepared using the same method but dried in a hot-air oven (Binder FED 115, Germany) at 80 °C before its use. Dry alginate beads were weighed, and the water loss was evaluated from the following equation:
(1)
(1)
where
and
represent the masses of fresh and dry alginate beads, respectively.
All alginate beads were scanned by a digital camera and an optical microscope (Olympus SZ40). Their diameters were averaged from 50 measurements by a micrometer (436-25MM, LS Starrett Company). To investigate the dye removal, sedge dye was diluted to 50 mg/100 ml, and the beaker of dye solution was filled with 10 g alginate beads. The beaker was placed in a box under a UV-C lamp (Mitsumaru) with 254 nm irradiation at room temperature. The absorption at 447 nm of the solution sample was measured using a UV-Vis spectrophotometer (Shimadzu, UV-1700 Pharma Spec) to determine the reduction in dye concentration up to 24 h of treatment. The dye removal efficiency at the contact time of h was determined using the following equation:
(2)
(2)
where
and
correspond to the initial and after
h of the treatment concentrations, respectively.
The dye removal per unit mass of the modified adsorbent at the contact time of (
) is defined as:
(3)
(3)
The time dependencies of dye removal are fitted to two kinetics models, namely Elovich equation in (Equation4(4)
(4) ) and Weber-Morris intra-particle diffusion equation in (Equation5
(5)
(5) ) (Juang & Chen, Citation1997; Qiu et al., Citation2009; Simonin & Boute, Citation2016).
(4)
(4)
where
is the initial adsorption rate, and
is the adsorption rate constant.
(5)
(5)
where
is the intra-particle diffusion rate constant.
3. Results and discussion
3.1. Characterization of ZnO-alginate beads
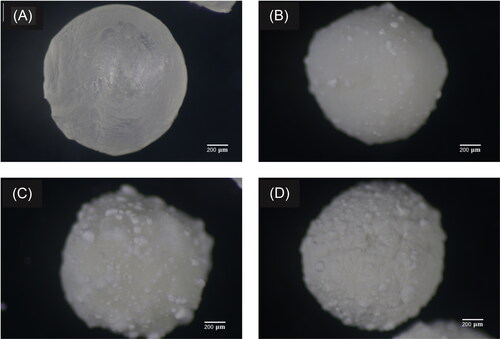
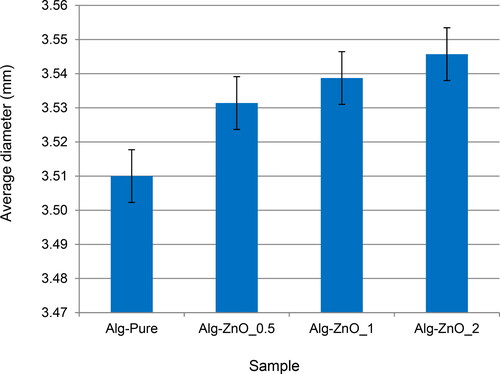
As shown in , fresh alginate beads are rather uniform, and their colors change from translucent light blue to white after loading with ZnO. An inset of shows a magnified image of a spherical pristine bead. The alginate surface becomes increasingly rough by ZnO coating, as shown by the insets of . shows the increase in diameter with increasing ZnO loading. The average diameter of coated beads reaches the maximum of 3.545 mm, which is slightly larger than the value of 3.510 mm in the case of pristine beads.
Figure 1. Images of fresh alginate beads with (A) 0, (B) 0.5, (C) 1, (D) 2% (w/w) ZnO loading. Insets show magnified optical micrographs.
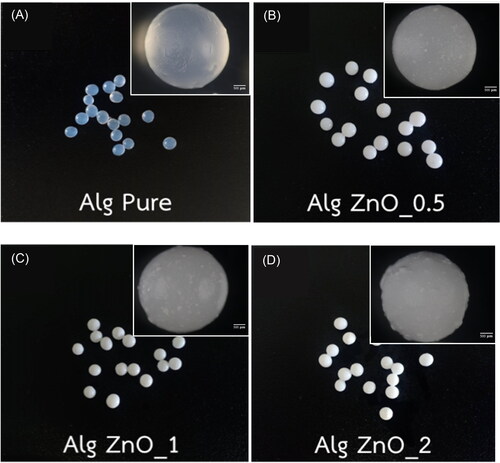
Figure 2. Average diameters of fresh alginate beads with 0% (Alg-Pure), 0.5% (Alg-ZnO_0.5), 1% (Alg-ZnO_1), and 2% (Alg-ZnO_2) ZnO loading.
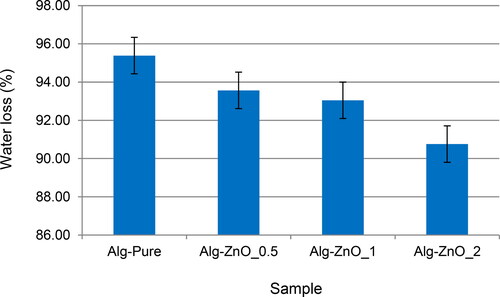
Optical micrographs of dry alginate beads are shown in . The beads increasingly deviate from the sphere, and the roughness is increased by the formation of the ZnO clusters on the surface. The sizes of dry beads are less than half of the fresh alginate. These substantial reductions correspond to the water loss shown in . The water loss is 95% in the pristine beads, decreasing with increasing ZnO loading. Depending on the ZnO mass and coating, this value reaches a minimum of around 90% in the case of 2% ZnO.
3.2. Dye removal by ZnO-alginate beads
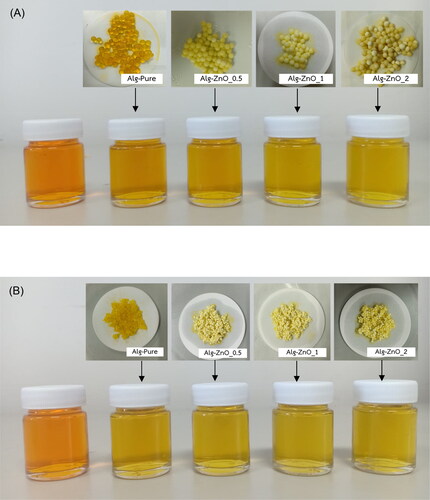
After 24 h of treatments, the alginate beads are changed to yellow, as depicted by photographs in . The ZnO-alginate beads are opaque, whereas the pristine beads are translucent. also shows the decolorization of dye solutions after 24 h. The color is substantially changed from that of the initial solution verifying the removal of the dye. The adsorption is combined with the photocatalytic activity of ZnO on the surface of alginate beads. The dye degradation mechanism is initiated by generating electron-hole pairs from ZnO under UV irradiation. Combining with water, the hydroxyl free radicals are obtained and proceed to eliminate the dye molecules during the reaction (Bibi et al., Citation2020; Elfeky et al., Citation2020). However, the variation with the ZnO loading and the difference between the fresh and dry beads cannot be spotted through visual inspection. The dye removal is further confirmed by using UV-Vis spectrophotometry.
Figure 5. Visual decolorization of sedge dye solutions before and after 24 h treatment by (A) fresh and (B) dry alginate beads with varying ZnO loading.
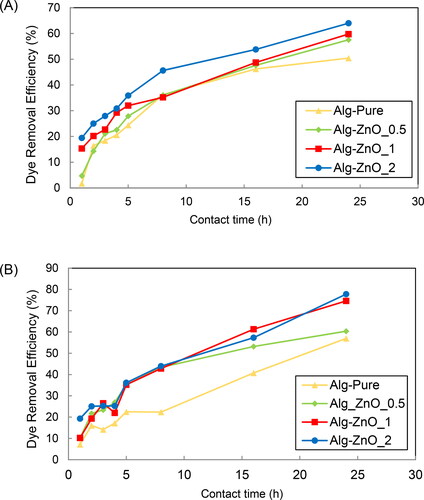
The decolorization is related to the changes in dyes characterized by fast Fourier transform infrared spectroscopy and mass spectrometry in some published articles (Pirok et al., Citation2019; Ikram, Zahoor, Khan, & Khayam, Citation2020; Khayam, Zahoor, Khan, & Shah, Citation2020). Without such characterizations in this study, the decolorization of dye solutions is assessed in terms of dye removal efficiency by ZnO-alginate beads. The adsorption process occurs rapidly in the first 2 h of all treatments using either ZnO-alginate or pristine beads. The dye removal efficiency of more than 10% at the beginning indicates the main role of diffusion and adsorption in the initial stage. With additional adsorption and photocatalysis of ZnO, the effect of ZnO loading is shown by comparing the plots in . The ZnO loading by 0.5% does not significantly increase the dye removal within 10 h. The dye removal efficiency is much enhanced by the further increase of ZnO to 1 and 2%, exceeding 20% in the first few hours and reaching 60% and 64% after 24 h, respectively.
The dye removal efficiency is highly enhanced by using dry alginate beads in the treatment. The value reaches 57% after 24 h of contact time with pristine beads. It is further increased up to 60%, 75%, and 78% with the ZnO loadings of 0.5%, 1%, and 2%, respectively. These findings suggest that the increase in roughness and surface area can be closely related to the arrangement and amount of ZnO species inside the alginate beads Citation(Caglar, Afsin, Tabak, & Eren, 2009). However, an excessive ZnO loading has an adverse effect due to agglomeration and reduced surfaces for adsorption, as observed by loading 4% ZnO in zeolite, whereas the optimum loading for MB dye removal was 3% ZnO (Elfeky et al., Citation2020). Besides the characteristics of ZnO and ZnO nanocomposites, the efficiency of water treatment was also dependent on the pH, temperature, shaking speed, and initial concentration of the solution (Mohammed & Kareem, Citation2019; Elfeky et al., Citation2020). Whereas the optimum pH for ZnO in removing MB dye is 7-8 (Elfeky et al., Citation2020), Noreen et al. (Citation2020b) reported the highest removal efficiency for direct sky blue dye when the lowest pH of 2 was used. According to a review article by Mudhoo et al. (Citation2020), the removal efficiencies of various dyes using ZnO composites are varied between 80 and 100%. The dye removal efficiency in this study is also lower than the values generally attained by the biodegradation method using fungi and bacteria, as reviewed by Ali (Citation2010).
3.3. Adsorption kinetics by ZnO-alginate beads
The values corresponding to the changes in are relatively low because of the inclusion of masses of alginate beads. The change of
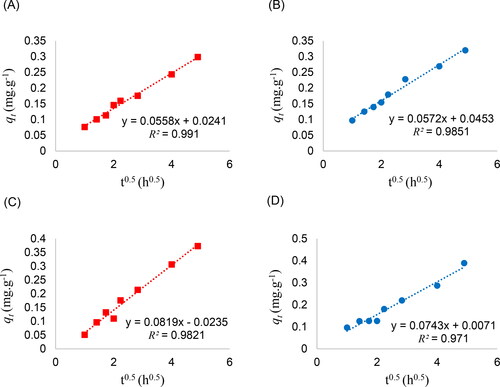
with time is not consistent with the Lagergren pseudo-first-order and pseudo-second-order equations because of the additional effect of photocatalytic activity. The deviation is especially significant in the initial period, and the dye removal by dry beads does not reach equilibrium within 24 h. However, the modified adsorption kinetics throughout the contact time tend to follow the Elovich and Weber-Morris intra-particle diffusion models. Straight lines are obtained from the graph fitting with the Elovich equation in and the intra-particle diffusion equation in .
Figure 7. Linear fitting of of fresh alginate beads with (A) 0, (B) 0.5% ZnO loading, and dry alginate beads with (C) 0, and (D) 0.5% ZnO loading according to Elovich equation.
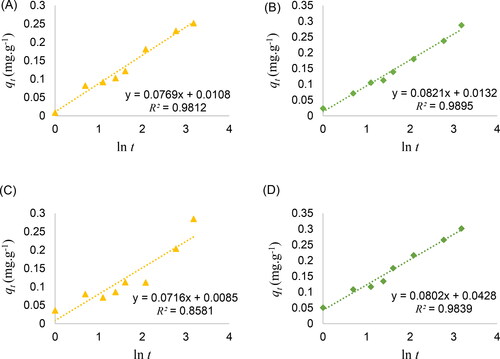
Figure 8. Linear fitting of of fresh alginate beads with (A) 1, (B) 2% ZnO loading, and dry alginate beads with (C) 1, and (D) 2% ZnO loading according to Weber-Morris intra-particle diffusion equation.
The adsorption reaction model using the Elovich equation assumes the heterogeneous energy distribution over the solid surfaces (Peers, Citation1965). The Elovich equation was originally used to describe the chemisorption of gases onto solid surfaces and has increasingly been implemented in the case of the removal of pollutants from aqueous solutions (Wu, Tseng, & Juang, Citation2009; Qiu et al., Citation2009). Its application to dye removal is less frequent than the Lagergren pseudo-first-order and pseudo-second-order equations, but the Elovich model is apparently suitable for the adsorbents with the additional ZnO photocatalysis in this study. The from the linear plots of
against
following the Elovich equation ranges from 0.9508 to 0.9895, as listed in . show the plots with higher
in the case of 0 and 0.5% ZnO. The
is substantially reduced for dry beads and becomes as low as 0.8581 in the case of pristine beads in . The best fit is still obtained in the case of 0.5% ZnO in . The slope equal to
exhibits a considerable variation between the fresh and dry beads in the case of 1 and 2% ZnO. The
parameter corresponds to the extent of surface coverage and activation energy for chemisorption. In this model, the rate of adsorption decreases with time due to the increase in surface coverage in that the interaction between the adsorbed species and the desorption rate are neglected (Wu et al., Citation2009; Qiu et al., Citation2009). For both fresh and dry beads, the
parameter derived from the y-intercept of the fitting is found to increase with the ZnO loading.
Table 1. Parameters from the fitting of dye removal with Elovich and intra-particle diffusion kinetics models.
From the intra-particle diffusion equation fitting, exemplifies the plots in the case of 1 and 2% ZnO, and the parameters in all cases are compared in . The slopes of linear variations of with
correspond to the
Both fresh and dry alginate beads with 0 and 0.5% ZnO exhibit comparable
around 0.6 mg.g−1.h−0.5. By contrast, the
value reaches up to 0.74–0.82 mg.g−1.h−0.5 in the case of dry alginate beads with the ZnO loading of 1 and 2%. The y-intercept has the minimum value consistent with EquationEq. (5)
(5)
(5) . The non-zero values imply the contribution of the boundary layer. The larger the y-intercept, the greater is the boundary layer effect (Inyinbor, Adekola, & Olatunji, Citation2017). The adsorption is not controlled by only the intra-particle diffusion but the liquid film diffusion also takes part. It is common to observe both mechanisms in the adsorption process (Oyelude, Awudza, & Twumasi, Citation2017). Furthermore, the liquid film diffusion is pronounced in microporous adsorbents (Qiu et al., Citation2009; Inglezakis, Balsamo, & Montagnaro, Citation2020). The
obtained from the fitting reaches the values of 0.9910 and 0.9851 for 1% and 2% ZnO-alginate beads, respectively. Referring to the values of
given in , the adsorption mechanism of 1 and 2% ZnO-alginate beads is best modeled by the intra-particle diffusion whereas that of alginate beads with lower ZnO loading follows the Elovich equation.
Elfeky et al. (Citation2020) and Noreen et al. (Citation2020a) also fitted the adsorption kinetics by ZnO nanoparticles and their composites with the intra-particle diffusion equation. The was higher than the value from the Lagergren pseudo-first-order equation but still lower than that of the pseudo-second-order equation. With the additional contribution from 1 to 2% ZnO photocatalysis in this study, the dye removal is better described by the adsorption diffusion model. Higher values of
in are consistent with the diffusion through porous adsorbents of 1 and 2% ZnO and fresh beads. By contrast, the adsorption reaction model is more suitable to describe alginate beads with 0 and 0.5% ZnO as confirmed by the best fitting with the Elovich equation.
4. Conclusion
Alginate beads and ZnO nanoparticles were combined to treat water contaminated by sedge dye. By treating dye solutions under UV irradiation of 254 nm for 24 h, the dye removal efficiency was increased with increasing photocatalytic ZnO loading. The dry alginate bead exhibited a higher dye removal efficiency than that of the fresh one which has the same ZnO loading because of the higher surface area and the lower mass. The dye removal efficiency was maximized at 78% with dry 2% ZnO-alginate beads, whereas the highest value of 64% was attained with fresh beads. For 1–2% ZnO-alginate beads, the time-dependent dye removal was well fitted and described by the intra-particle diffusion model with a minor contribution of the liquid film diffusion. On the other hand, the dye removal per unit mass of 0.5% ZnO-alginate beads was proportional to the square root of contact time, consistent with the Elovich equation.
Before implementing ZnO-alginate beads in dye removal from wastewater in the handicraft productions and so on, further research is recommended on treatment optimization. Varying the solid mass to liquid volume, stirring, temperature, and pH can improve the dye removal efficiency of dry alginate beads ZnO-alginate beads. From the literature, the optimum pH has a diverse trend depending on the type of adsorbent and dye involved. The thermodynamic study and additional chemical characterizations could improve the understanding of combined effects of adsorption and photocatalytic activity by ZnO-alginate beads.
Acknowledgements
The authors are grateful for the facility support by the Faculty of Science, Thaksin University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aderiye, B. I., Iteke, U. N., Akinyeye, R. O., & Oluwole, O. A. (2021). Monitoring degradation of restaurant wastewater by Lysinibacillus sphaericus C3-41. Arab Journal of Basic and Applied Sciences, 28(1), 319–329. doi:10.1080/25765299.2021.1969741
- Afroze, S., Sen, T. K., & Ang, H. M. (2016). Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using Eucalyptus sheathiana bark biomass. Research on Chemical Intermediates, 42(3), 2343–2364. doi:10.1007/s11164-015-2153-8
- Ait-Touchente, Z., Falah, S., Scavetta, E., Chehimi, M. M., Touzani, R., Tonelli, D., & Taleb, A. (2020a). Different electrochemical sensor designs based on diazonium salts and gold nanoparticles for pico molar detection of metals. Molecules, 25(17), 3903. doi:10.3390/molecules25173903
- Ait-Touchente, Z., Khalil, A. M., Simsek, S., Boufi, S., Ferreira, L. F. V., Vilar, M. R., … Chehimi, M. M. (2020b). Ultrasonic effect on the photocatalytic degradation of Rhodamine 6G (Rh6G) dye by cotton fabrics loaded with TiO2. Cellulose, 27(2), 1085–1097. doi:10.1007/s10570-019-02817-y
- Ait-Touchente, Z., Sakhraoui, H. E. E. Y., Fourati, N., Zerrouki, C., Maouche, N., Yaakoubi, N., … Chehimi, M. M. (2020c). High performance zinc oxide nanorod-doped ion imprinted polypyrrole for the selective electrosensing of mercury II ions. Applied Sciences, 10(19), 7010. doi:10.3390/app10197010
- Ali, H. (2010). Biodegradation of synthetic dyes: A review. Water, Air, & Soil Pollution, 213(1-4), 251–273. doi:10.1007/s11270-010-0382-4
- Bhatti, H. N., Safa, Y., Yakout, S. M., Shair, O. H., Iqbal, M., & Nazir, A. (2020). Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: Adsorption/desorption, kinetics and recycling studies. International Journal of Biological Macromolecules, 150, 861–870. doi:10.1016/j.ijbiomac.2020.02.093
- Ben Dassi, R., Chamam, B., Méricq, J. P., Faur, C., El Mir, L., Trabelsi, I., & Heran, M. (2021). Novel polyvinylidene fuoride/lead‑doped zinc oxide adsorptive membranes for enhancement of the removal of reactive textile dye. International Journal of Environmental Science and Technology, 18(9), 2793–2804. doi:10.1007/s13762-020-03026-y
- Bibi, I., Kamal, S., Abbas, Z., Atta, S., Majid, F., Jilani, K., … Abbas, A. (2020). A new approach of photocatalytic degradation of remazol brilliant blue by environment friendly fabricated zinc oxide nanoparticle. International Journal of Environmental Science and Technology, 17(3), 1765–1772. doi:10.1007/s13762-019-02586-y
- Biftu, W. K., & Ravindhranath, K. (2020). Synthesis of nanoZrO2 via simple new green routes and its effective application as adsorbent in phosphate remediation of water with or without immobilization in Al-alginate beads. Water Science and Technology , 81(12), 2617–2633. doi:10.2166/wst.2020.318
- Caglar, B., Afsin, B., Tabak, A., & Eren, E. (2009). Characterization of the cation-exchanged bentonites by XRPD, ATR, DTA/TG analyses and BET measurement. Chemical Engineering Journal, 149(1-3), 242–248. doi:10.1016/j.cej.2008.10.028
- Djebri, N., Boutahala, M., Chelali, N. E., Boukhalfa, N., & Zeroual, L. (2016). Enhanced removal of cationic dye by calcium alginate/organobentonite beads: Modeling, kinetics, equilibriums, thermodynamic and reusability studies. International Journal of Biological Macromolecules, 92, 1277–1287. doi:10.1016/j.ijbiomac.2016.08.013
- Elfeky, A. S., Youssef, H. F., & Elzaref, A. S. (2020). Adsorption of dye from wastewater onto ZnO nanoparticles-loaded zeolite: Kinetic, thermodynamic and isotherm studies. Zeitschrift Für Physikalische Chemie, 234(2), 255–278. doi:10.1515/zpch-2018-1342
- Ghaedi, M., Nasab, A. G., Khodadoust, S., Rajabi, M., & Azizian, S. (2014). Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. Journal of Industrial and Engineering Chemistry, 20(4), 2317–2324. doi:10.1016/j.jiec.2013.10.007
- Ikram, M., Zahoor, M., Khan, E., & Khayam, S. M. U. (2020). Biodegradation of Novacron Turqueiose (Reactive Blue 21) by Pseudomonas aeruginosa. Journal of the Chemical Society of Pakistan, 42(5), 737–745. doi:10.52568/000677/JCSP/42.05.2020
- Inglezakis, V. J., Balsamo, M., & Montagnaro, F. (2020). Liquid − solid mass transfer in adsorption systems: An overlooked resistance? Industrial & Engineering Chemistry Research, 59(50), 22007–22016. doi:10.1021/acs.iecr.0c05032
- Inyinbor, A. A., Adekola, F. A., & Olatunji, G. A. (2017). Kinetics and isothermal modeling of liquid phase adsorption of rhodamine B onto urea modified Raphia hookerie epicarp. Applied Water Science, 7(6), 3257–3266. doi:10.1007/s13201-016-0471-7
- Isik, B., & Ugraskan, V. (2021). Adsorption of methylene blue on sodium alginate–flax seed ash beads: Isotherm, kinetic and thermodynamic studies. International Journal of Biological Macromolecules, 167, 1156–1167. doi:10.1016/j.ijbiomac.2020.11.070
- Jain, M., Mudhoo, A., Ramasamy, D. L., Usman, M., Zhu, R., Kumar, G., … Sillanpää, M. (2020). Adsorption, degradation, and mineralization of emerging pollutants (pharmaceuticals and agrochemicals) by nanostructures: A comprehensive review. Environmental Science and Pollution Research International, 27(28), 34862–34905. doi:10.1007/s11356-020-09635-x
- Juang, R. S., & Chen, M. L. (1997). Application of the Elovich equation to the kinetics of metal sorption with solvent-impregnated resins. Industrial & Engineering Chemistry Research, 36(3), 813–820. doi:10.1021/ie960351f
- Kamran, U., Bhatti, H. N., Iqbal, M., Jamil, S., & Zahid, M. (2019). Biogenic synthesis, characterization and investigation of photocatalytic and antimicrobial activity of manganese nanoparticles synthesized from Cinnamomum verum bark extract. Journal of Molecular Structure, 1179, 532–539. doi:10.1016/j.molstruc.2018.11.006
- Kaur, K., & Jindal, R. (2019). Comparative study on the behaviour of chitosan-gelatin based hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydrate Polymers, 207, 398–410. doi:10.1016/j.carbpol.2018.12.002
- Khayam, S. M. U., Zahoor, M., Khan, E., & Shah, M. (2020). Reduction of keto group in drimarene blue by aspergillus Niger: A predominant reason for subsequent decolorization. Fresenius Environmental Bulletin, 29(3), 1397–1410.
- Kumar, M., Vijayakumar, G., & Tamilarasan, R. (2019). Synthesis, characterization and experimental studies of nano Zn–Al–Fe3O4 blended alginate/Ca beads for the adsorption of Rhodamin B. Journal of Polymers and the Environment, 27(1), 106–117. doi:10.1007/s10924-018-1318-0
- Li, C., Lu, J., Li, S., Tong, Y., & Ye, B. (2017). Synthesis of magnetic microspheres with sodium alginate and activated carbon for removal of methylene blue. Materials, 10(1), 84. doi:10.3390/ma10010084
- Li, B., & Yin, H. (2020). Superior adsorption property of a novel green biosorbent yttrium/alginate gel beads for dyes from aqueous solution. Journal of Polymers and the Environment, 28(8), 2137–2148. doi:10.1007/s10924-020-01757-0
- Liu, Y., Zhang, L., Tang, Y., & Zhu, L. (2021). Study on the preparation and adsorption properties of sodium alginate graft polyacrylic acid/graphite oxide composite hydrogel. Polymer Science Series A, 63, 133–142. doi:10.1134/S0965545X21020061
- Metin, A. Ü., Doğan, D., & Can, M. (2020). Novel magnetic gel beads based on ionically crosslinked sodium alginate and polyanetholesulfonic acid: Synthesis and application for adsorption of cationic dyes. Materials Chemistry and Physics, 256, 123659. doi:10.1016/j.matchemphys.2020.123659
- Moawed, E. A., Kiwaan, H. A., & Elbaraay, A. A. A. (2019). Application of cellulose, lignin and camphor stem as new biosorbents for removal of brilliant green and crystal violet dyes from wastewater. Arab Journal of Basic and Applied Sciences, 26(1), 414–423. doi:10.1080/25765299.2019.1655190
- Mohammed, A. A., & Kareem, S. L. (2019). Adsorption of tetracycline from wastewater by using Pistachio shell coated with ZnO nanoparticles: Equilibrium, kinetic and isotherm studies. Alexandria Engineering Journal, 58(3), 917–928. doi:10.1016/j.aej.2019.08.006
- Motshekga, S. C., Ray, S. S., & Maity, A. (2018). Synthesis and characterization of alginate beads encapsulated zinc oxide nanoparticles for bacteria disinfection in water. Journal of Colloid and Interface Science, 512, 686–692. doi:10.1016/j.jcis.2017.10.098
- Mudhoo, A., Gautam, R. K., Ncibi, M. C., Zhao, F., Garg, V. K., & Sillanpää, M. (2019). Green synthesis, activation and functionalization of adsorbents for dye sequestration. Environmental Chemistry Letters, 17(1), 157–193. doi:10.1007/s10311-018-0784-x
- Mudhoo, A., Paliya, S., Goswami, P., Singh, M., Lofrano, G., Carotenuto, M., … Kumar, S. (2020). Fabrication, functionalization and performance of doped photocatalysts for dye degradation and mineralization: A review. Environmental Chemistry Letters, 18(6), 1825–1903. doi:10.1007/s10311-020-01045-2
- Murcia Mesa, J. J., Hernández Niño, J. S., González, W., Rojas, H., Hidalgo, M. C., & Navío, J. A. (2021). Photocatalytic treatment of stained wastewater coming from handicraft factories: A case study at the pilot plant level. Water, 13(19), 2705. doi:10.3390/w13192705
- Musa, M. S. M., Sulaiman, W. R. W., Majid, Z. A., Majid, Z. A., Idris, A. K., & Rajaei, K. (2020). Henna extract as a potential sacrificial agent in reducing surfactant adsorption on kaolinite: The role of salinity. Journal of King Saud University - Engineering Sciences, 32(8), 543–547. doi:10.1016/j.jksues.2019.06.001
- Nigiz, F. U. (2019a). Synthesis and characterization of clinoptilolite-alginate beads for dye removal from water. Water Practice and Technology, 14(2), 311–318. doi:10.2166/wpt.2019.015
- Nigiz, F. U. (2019b). Synthesis of a novel graphene–kaolin–alginate adsorbent for dye removal, and optimization of the adsorption by response surface methodology. Research on Chemical Intermediates, 45(7), 3739–3753. doi:10.1007/s11164-019-03818-z
- Nishtar, R. A., Jonnalagadda, N. F., Balachandran, R. R., & Nair, U. (2007). Equilibrium and thermodynamic studies on the removal of basic black dye using calcium alginate beads. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 299(1-3), 232–238. doi:10.1016/j.colsurfa.2006.11.045
- Noreen, S., Bhatti, H. N., Iqbal, M., Fazli, F. H., & Sarim, M. (2020a). Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. International Journal of Biological Macromolecules, 147, 439–452. doi:10.1016/j.ijbiomac.2019.12.257
- Noreen, S., Khalid, U., Ibrahim, S. M., Javed, T., Ghani, A., Naz, S., & Iqbal, M. (2020b). ZnO, MgO and FeO adsorption efficiencies for direct sky blue dye: Equilibrium, kinetics and thermodynamics studies. Journal of Materials Research Technology, 9(3), 5581–5893. doi:10.1016/j.jmrt.2020.03.115
- Nouri, L., Hemidouche, S., Boudjemaa, A., Kaouah, F., Sadaoui, Z., & Bachari, K. (2020). Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. International Journal of Biological Macromolecules, 151, 66–84. doi:10.1016/j.ijbiomac.2020.02.159
- Oyelude, E. O., Awudza, J. A. M., & Twumasi, S. K. (2017). Equilibrium, kinetic and thermodynamic study of removal of eosin yellow from aqueous solution using teak leaf litter powder. Scientific Reports, 7(1), 12198. doi:10.1038/s41598-017-12424-1
- Peers, A. M. (1965). Elovich adsorption kinetics and the heterogeneous surface. Journal of Catalysis, 4(4), 499–503. doi:10.1016/0021-9517(65)90054-0
- Perez-Ameneiro, M., Bustos, G., Vecino, X., Barbosa-Pereira, L., Cruz, J. M., & Moldes, A. B. (2015). Heterogenous lignocellulosic composites as bio-based adsorbents for wastewater dye removal: A kinetic comparison. Water, Air, & Soil Pollution, 226(5), 133. doi:10.1007/s11270-015-2393-7
- Pirok, B. W.J., Moro, G., Meekel, N., Berbers, S. V.J., Schoenmakers, P. J., & van Bommel, M. R. (2019). Mapping degradation pathways of natural and synthetic dyes with LC-MS: Influence of solvent on degradation mechanisms. Journal of Cultural Heritage, 38, 29–36. doi:10.1016/j.culher.2019.01.003
- Qiu, H., Lv, L., Pan, B. C., Zhang, Q. J., Zhang, W. M., & Zhang, Q. X. (2009). Critical review in adsorption kinetic models. Journal of Zhejiang University-Science A, 10(5), 716–724. doi:10.1631/jzus.A0820524
- Ravulapalli, S., & Ravindhranath, K. (2019). Novel adsorbents possessing cumulative sorption nature evoked from Al2O3 nanoflakes, C. urens seeds active carbon and calcium alginate beads for defluoridation studies. Journal of the Taiwan Institute of Chemical Engineers, 101, 50–63. doi:10.1016/j.jtice.2019.04.034
- Redha, Z. M. (2020). Multi-response optimization of the coagulation process of real textile wastewater using a natural coagulant. Arab Journal of Basic and Applied Sciences, 27(1), 406–422. doi:10.1080/25765299.2020.1833509
- Redha, Z. M., Yusuf, H. A., Amin, R., & Bououdina, M. (2020). The study of photocatalytic degradation of a commercial azo reactive dye in a simple design reusable miniaturized reactor with interchangeable TiO2 nanofilm. Arab Journal of Basic and Applied Sciences, 27(1), 287–298. doi:10.1080/25765299.2020.1800163
- Shaban, M., Abukhadra, M. R., Shahien, M. G., & Ibrahim, S. S. (2018). Novel bentonite/zeolite-NaP composite efficiently removes methylene blue and Congo red dyes. Environmental Chemistry Letters, 16(1), 275–280. doi:10.1007/s10311-017-0658-7
- Shanker, U., Rani, M., & Jassal, V. (2017). Degradation of hazardous organic dyes in water by nanomaterials. Environmental Chemistry Letters, 15(4), 623–642. doi:10.1007/s10311-017-0650-2
- Shao, Z. J., Huang, X. L., Yang, F., Zhao, W. F., Zhou, X. Z., & Zhao, C. S. (2018). Engineering sodium alginate-based cross-linked beads with high removal ability of toxic metal ions and cationic dyes. Carbohydrate Polymers, 187, 85–93. doi:10.1016/j.carbpol.2018.01.092
- Simonin, J. P., & Boute, J. (2016). Intraparticle diffusion-adsorption model to describe liquid/solid adsorption kinetics. Revista Mexicana de Ingeniería Química, 15, 161–173.
- Sirelkhatim, A., Mahmud, S., Seeni, A., Kaus, N. H. M., Ann, L. C., Bakhori, S. K. M., … Mohamad, D. (2015). Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Letters, 7(3), 219–242. doi:10.1007/s40820-015-0040-x
- Subhan, H., Alam, S., Shah, L. A., Ali, M. W., & Farooq, M. (2021). Sodium alginate grafted poly(N-vinyl formamide-co-acrylic acid)-bentonite clay hybrid hydrogel for sorptive removal of methylene green from wastewater. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 611, 125853. doi:10.1016/j.colsurfa.2020.125853
- Tahir, M. A., Bhatti, H. N., Hussain, I., Bhatti, I. A., & Asghar, M. (2020). Sol–gel synthesis of mesoporous silica–iron composite: Kinetics, equilibrium and thermodynamics studies for the adsorption of turquoise-blue X-GB dye. Zeitschrift Für Physikalische Chemie, 234(2), 233–253. doi:10.1515/zpch-2019-1443
- Tian, J., Guan, J., Gao, H., Wen, Y., & Ren, Z. (2016). The adsorption and mass-transfer process of cationic red X-GRL dye on natural zeolite. Water Science and Technology 73(9), 2119–2131. doi:10.2166/wst.2016.055
- Tu, H., Yu, Y., Chen, J., Shi, X., Zhou, J., Deng, H., & Du, Y. (2017). Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: Chitosan and cellulose. Polymer Chemistry, 8(19), 2913–2921. doi:10.1039/C7PY00223H
- Wu, F. C., Tseng, R. L., & Juang, R. S. (2009). Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chemical Engineering Journal, 150(2-3), 366–373. doi:10.1016/j.cej.2009.01.014
- Wunbua, J., Nakhapakorn, K., & Jirakajohnkool, S. (2012). Change detection and identification of land potential for planting Krajood (Lepironia articulata) in Thale Noi, Southern Thailand. Songklanakarin Journal of Science and Technology, 34, 329–336.
- Zare, E. N., Mudhoo, A., Khan, M. A., Otero, M., Bundhoo, Z. M. A., Navarathna, C., … Sillanpää, M. (2021). Water decontamination using bio-based, chemically functionalized, doped, and ionic liquid-enhanced adsorbents: Review. Environmental Chemistry Letters, 19(4), 3075–3114. doi:10.1007/s10311-021-01207-w