Abstract
This research aims to beneficiate the clay deposits abundant in the Al Baha district to produce ceramic filters for “point-of-use” household water treatment technology to remove dissolved solids from water that is no longer valid for drinking purposes to meet the needs of the community through a safe household and industrial water supply after further treatment purposes. In addition, it helps to reduce the expenses of citizens earmarked to buy drinking water. A sample of laterite, clay, and kaolin from Al Baha district was thermally treated at 800 °C, acid treated with HCl, analyzed by XRD, FTIR, and SEM, and tested for treatment of household water. The temperature, pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured. In the application step for point-of-use water treatment of household water, the treated materials were introduced in calcined kaolin filters and commercial polypropylene filters. Based on the results of household water treatment with raw and treated clay materials, the raw clay materials, has poor adsorption capacity. The thermal treatment of clay samples dehydrates the clay minerals into sintered aluminosilicates and enhances the conversion of poorly crystallized iron oxide to α-Fe2O3 without improving the adsorption capacity of clay materials. However, the acid treatment of the clay samples removes iron-bearing minerals leaving behind amorphous aluminosilicate matter characterized with improved adsorption capacity. It was concluded that the acid treatment of clay material by concentrated HCl acid completely leaches iron oxide phases and broke down and disintegrated the clay minerals into the amorphous matter with enhanced the adsorption capacity. The combination of selected acid-treated laterite, clay, and charcoal for the household water treatment filter at the point of use in the calcined kaolin filter and the polypropylene filter reduced the TDS content by 40–42%.
1. Introduction
Drinking water must be free from contaminants to promote a healthy lifestyle that is free from the illnesses associated with waterborne diseases (Bain et al., Citation2012). Every human being has the right to be provided with sufficient, safe, and affordable water (Addae & Adu, Citation2020). Nevertheless, surface water, such as rivers and lakes, is exposed to contamination by microbial pathogens. The municipal water was treated in the treatment facility to emphasize its validity for drinking purposes according to the specified standards. After that, it is usually stored for periods in storage tanks before being pumped to distribution networks (Elasaad, Bilton, Kelley, Duayhe, & Dubowsky, Citation2015). Transportation of the treated water through network pipelines and storing in underground concrete, metallic and plastic household tanks, leads to a risk of recontamination by microbial pathogens and heavy metals (Gundry et al., Citation2006). Consequently, household water is no longer suitable for drinking purposes, which requires citizens to allocate an additional budget item to purchase drinking water. The point-of-use household water treatment technology by filtration aids should be applied to retreat drinking water that has been subjected to microbial and heavy metal contamination. A good example of point-of-use household water treatment technology by filtration aids is a ceramic clay filter that utilizes adsorption/absorption, molecular sieving and ion exchange mechanisms (Lim, Gomes, & Ab Kadir, Citation2013) and provides the capabilities for removing water contaminants such as microbes (Sengco & Anderson, Citation2004), chemicals (Nwuzor, Chukwuneke, Nwanonenyi, Obasi, & Ihekweme, Citation2018), and heavy metals (Pranoto, Inayati, & Firmansyah, Citation2018) from contaminated water.
Simple and low-cost ceramic filter products have become popular among users because of their superior ability to improve the efficiency of fecal coliform and turbidity removal from household water (Sobsey, Stauber, Casanova, Brown, & Elliott, Citation2008). Ceramic water filters made from clay and combustible organic material (sawdust or flour). The point-of-use household water treatment process can be more cost-effective in the long run than centralized water treatment technology (Clasen, Nadakatti, & Menon, Citation2006). The technique has the potential to fill the service gap where piped water of the distribution network systems is not possible or do not deliver safe water, as is the case in rural and remote areas in Saudi Arabia, which potentially causes substantial positive health impacts (Sobsey, Citation2006). Numerous recent studies have demonstrated that the combination of pond sand and ceramic filter technology would be a reliable and sustainable treatment option to address the challenge of poor access to potable water in rural areas (Akosile et al., Citation2020; Das, Kirtunia, & Shaibur, Citation2018; Ihekweme et al., Citation2020; Sheikh & Islam, Citation2017). Many researchers have focused on the developing ceramic filters used in water purification. They concluded that the ceramic filter is efficient in removing 100% of the turbidity and pathogens, as its pore size was found to be less than 40 nm (Suribabu, Sudarsan, & Nithiyanantham, Citation2020). The use of dip slides is recommended for the detection and monitoring of the microbial contamination, to assure the quality of drinking water and to assess the specified lifetime of their filter systems (Lange, Materne, & Grüner, Citation2016). Filter cleaning and disinfection with boiling water is an important factor that is required to improve water quality and promote the filter performance (Meierhofer, Bänziger, Deppeler, Kunwar, & Bhatta, Citation2018). The performance of the ceramic filter could be improved when the filters are coated or impregnated with silver nanoparticle to provide antimicrobial activity (Kallman, Oyanedel-Craver, & Smith, Citation2011; Mwabi, Mamba, & Momba, Citation2012; Rayner, Citation2009). Silver nanoparticles represent a small fraction of the total cost of the filter and extend the filter average life (Rayner, Citation2009).
The aim of this research article is to beneficiate the clay deposits abundant in the Al Baha district for “point-of-use” household water treatment technology. Via the production of ceramic filters used for the removal of the dissolved solids of water that are no longer valid for the drinking purposes to meet the needs of the community through a safe household and industrial water supply after further treatment purposes. In addition, it helps to reduce the expenses of citizens earmarked to buy drinking water.
2. Materials and experimental methods
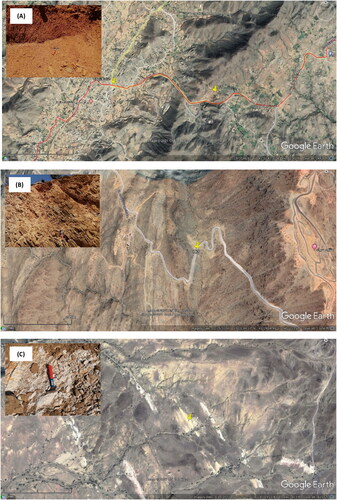
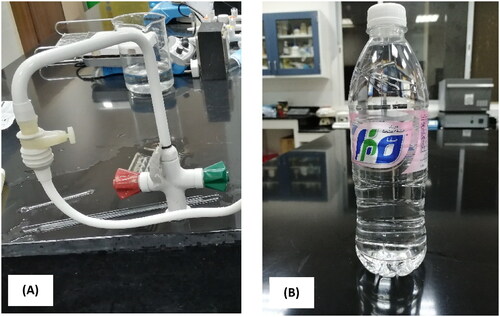
A sample of laterite (C1) and a sample of clay (C2) were provided from different locations in Ghamid Al Zanad, Al Baha district, KSA (19°35’49.13” N–41°32’42.93” E and 19°31’44.98” N–41°29’12.02” E). Kaolin (K) was provided from southeastern Nawan, Al Baha, KSA (19°29’10.27” N and 41°15’20.27” E). Laterite, clay and kaolin were ground to a fineness of less than 150 microns. illustrates the location of laterite, clay and kaolin samples. Clay and kaolin were used in this study based on previous extensive experiments, in three forms: raw clay and kaolin (C1, C2, and K), clay and kaolin calcined at 800 °C (CC1, CC2, and CK), and clay and kaolin treated with hydrochloric acid (AC1, AC2, and AK). Clay and kaolin were calcined at 800 °C for two hours and cooled in a muffle furnace. The resulting calcined materials were ground to a fineness of less than 150 microns. Clay and kaolin were treated with hydrochloric acid according to the following procedure. One hundred grams of raw clay and kaolin were treated with 200 mL of hydrochloric acid solution (1:1) heated at the boiling point for half an hour. The yellow acid solution, which contains iron-(III) chloride, was separated by filtration. The whitish acid treated clay or kaolin was washed with distilled water until the filtrate gave no precipitate with silver nitrate solution and then dried at 100 °C. Charcoal activated pharma grade (CAS 7440-44-0) was used in its original form (C) and after treatment with hydrochloric acid (AC). The charcoal was acid-treated in the same way as clay and kaolin. Two water samples were used in this study, as shown in . The first sample is household water (Wd), and the second sample is a commercial drinking water (Wr). Each water sample was packed into a 20-liter polyethylene bottle. As shown in , household water treatment was performed by adding 5 g of treated clay to 250 ml of water in a 500 ml round flask, stirring for 30 minutes at a speed of 1100 rpm (), and then filtering to separate the treated water (). The temperature, pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured (). The temperature and pH of the water was measured with a pH 212 Microprocessor pH meter (HANNA Instruments). The EC and TDS of the water were measured using a calibrated electrical conductivity meter EC 215 HANNA.
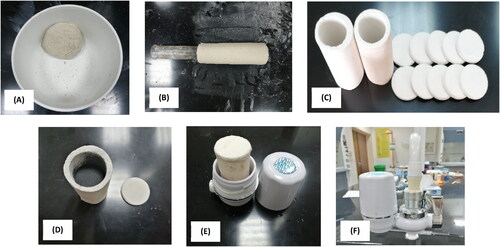
In the application step for point-of-use water treatment of household water by the treated materials, two filter models were used to hold the treated materials. The first model is the calcined kaolin filter (CKF), which consists of a hollow calcined kaolin ceramic cylinder, open on both sides, with a height of 9 cm, thickness of 0.4 cm, and inner diameter of 3.75 cm, as well as two disks closing the cylinder cylinders, with a diameter of 3.75 cm and height 0.4 cm. illustrates the preparation of the ceramic filter. Ground kaolin was mixed with 5% fine sawdust (pore forming agent) and was mixed with water until a cohesive, easy-to-shape paste was formed (). The paste was formed in the form of a cylinder hollow from the inside (). The cylinder was dried in air for two days in a drying oven at 100 °C for 12 hours, then the cylinder was burned in a firing oven at 950 °C for 2 hours (), and the resulting ceramic filter was cooled inside the oven. The ceramic filter was washed with boiling water for 1 hour to dissolve any soluble materials affecting the processing. After drying, the filter was filled with the treated materials (), and the filter cover was adhered by silicone adhesive. The filter was placed in the unit designated for it (). Household water treatment was carried out at the point-of-use by connecting the filter to the water supply (). The second model is a commercial polypropylene filter (PPF) as shown in . The hollow polypropylene pipe was filled with the treated materials (), and the filter was closed tightly () and connected to the household water supply (). A sample of treated water was taken, and the temperature, pH, EC, and TDS were measured. A third filter that was used for comparison of the results which is the commercial for household water treatment filter (CF) ().
3. Results
shows XRF analysis of clay and kaolin. Clay sample C1 is a loose rusty red laterite, characterized by the presence of a high content of iron oxide (11.2 wt.%), a large proportion of fine particles, and a soapy feel. The Fe2O3 content is high in sample C1 probably due to the products of weathering and alteration of the basalt rocks. Sample C2 is a yellow-brown clay consisting of semicohesive layers containing a high percentage of silica (65.25 wt.%) and a small amount of iron oxide (3.70 wt.%). The kaolin sample is white, hard, soapy-texture stone, rich in mineral kaolinite and contains a small percentage of iron oxide (1.79 wt.%). Clay sample C2 contains higher amounts of CaO, MgO, Na2O, and K2O than C1 and K.
Table 1. XRF analysis of clay and kaolin.
illustrates the XRD patterns of clay, calcined clay, acid treated clay, kaolin, and calcined kaolin. Kaolinite, montmorillonite, and illite are the main clay minerals represented in all samples. However, quartz, dolomite, albite, and hematite are the main non-clay minerals present (Rampe, Kraft, & Sharp, Citation2013). Laterite sample C1 contains quartz and small proportions of kaolinite, montmorillonite, and illite minerals. The laterite sample C1, contains a high percentage of iron oxide, as is evident from the XRF analysis (). Although, the absence of iron oxide phases in the XRD analysis confirms that iron oxide presents in an amorphous or poorly crystallized form. Commonly, the presence of dissolved silica in laterite-forming solutions may prevent the growth of iron oxide crystals formed and lead to a low degree of crystallization of iron oxide minerals (Pokrovski, Schott, Farges, & Hazemann, Citation2003). The acid treatment of the laterite sample C1 removes iron-bearing minerals (hematite) and leaves behind part of the kaolin, quartz, and albite in treated sample AC1. Peaks of the rhombohedral hematite phase (α-Fe2O3) were observed after calcination of the laterite sample (CC1). This is due to the conversion of the poorly crystallized iron oxide form (γ-Fe2O3) to α-Fe2O3 (Ulfa et al., Citation2021).
Figure 6. XRD patterns of clay (C1, C2), calcined clay (CC1, CC2), acid treated clay (AC1, AC2), kaolin (K) and calcined kaolin (CK).
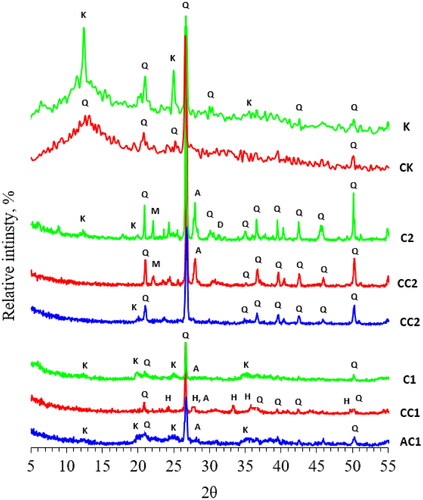
The two broad peaks at 20–25 °C and 35–40 °C are due to the formation of amorphous aluminosilicate structures after the acid treatment of the laterite sample (AC1) (Zhang et al., Citation2016). This result is consistent with lowering the quartz content both in the laterite and clay samples after acid treatment (AC1 and AC2). The hematite disappears in the acid treated sample AC1 due to the leaching of iron oxide by hydrochloric acid. The clay sample C2 contains quartz, montmorillonite, albite, and dolomite minerals, with no indication for the presence either of kaolinite or illite minerals. Additionally, no indication of any crystalline iron oxide phases could be detected either before (C2) or after calcination (CC2). As mentioned in , this is due to the presence of a small amount of amorphous iron oxide in sample C2, which could not be detected after calcination of the sample (CC2). The dolomite mineral was removed after acid leaching in sample AC2. The acid treatment of the clay sample C2 removes iron-bearing minerals (hematite), dolomite and albite but leaves behind residual kaolin and quartz in treated sample AC2. Kaolin sample K is composed of kaolinite and quartz. The calcined kaolin (CK) does not contain kaolinite mineral due to the dehydration of the hydroxyl groups from the kaolinite crystals leaving behind metakaolin consisting of amorphous anhydrous aluminum silicate and quartz.
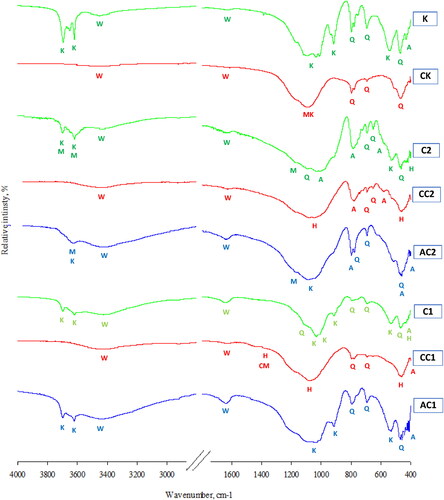
illustrates the FTIR spectra of clay and kaolin as well as their calcined and acid-treated products. The main phases that were identified from the FTIR spectra (Tantawy & Ramadan, Citation2017) based on their characteristic absorption bands were kaolinite (525 cm−1, 909 cm−1, 994 cm−1, 1018 cm−1, 3597 cm−1 and 3687 cm−1 (Eisazadeh, Kassim, & Nur, Citation2012)), montmorillonite (1140 cm−1, 3611 cm−1 and 3687 cm−1 (Ravisankar, Citation2009)), quartz (453 cm−1, 669 cm−1, 781 cm−1 and 1087 cm−1 (Ravisankar, Citation2009)), albite (560 cm−1, 781 cm−1 and 1020 cm−1 (Zhang et al., Citation2019)) and hematite (451 cm−1, 1061 cm−1 and 1395 cm−1 (Ulfa et al. Citation2021)). The additional absorption bands are attributed to absorbed water (1616 cm−1 and 3390 cm−1 (Madejova & Komadel, Citation2001)), amorphous SiO2 groups of metakaolin (1070 cm−1 (Lecomte, Liégeois, Rulmont, Cloots, & Maseri, Citation2003)), and the presence of carbonaceous matter (1394 cm−1 (Sankaran & Ramasamy, Citation2000)). Laterite sample C1 contains the base minerals; Kaolin and quartz, as well as other minor minerals such as hematite and albite. As a result of heat treatment (CC1), kaolinite crystals disintegrate into amorphous metakaolin, as shown by the peaks of absorbed water at 1595 and 3409 cm−1. The percentage of hematite mineral increases, because of the crystallization of amorphous iron oxide (as indicated by the XRD analysis results in ). The minerals quartz and albite are not affected by heat treatment. Hematite dissolves by treating laterite with acid (AC1). The percentage of amorphous matter increases as a result of the acid treatment, as it is clear from peaks of the absorbed water at 1595 and 3409 cm−1. However, quartz and albite minerals are not affected by acid treatment. The presence of carbonaceous matter is detected at 1394 cm−1 due to the incineration of some organic matter. The clay sample (C2) contains montmorillonite, kaolin, and quartz, in addition to other minor minerals such as hematite and albite. As a result of heat treatment (CC2), kaolinite is also transformed into amorphous metakaolin, as shown by the peaks of water absorbed at 1595 and 3409 cm−1. The percentage of hematite increases, because of the crystallization of the amorphous iron oxide. Hematite dissolves and amorphous matter is produced as a result of the acid treatment (AC2), as it is evident that the peaks of absorbed water at 1595 and 3409 cm−1. The kaolin sample (K) contains Kaolin and quartz. The sample does not contain hematite, but little albite. As a result of heat treatment (CK), kaolinite turns into amorphous metakaolin, as shown by the amorphous silica peak at 1073 cm−1. However, quartz and albite are not affected by heat treatment.
Figure 7. FTIR spectra of clay (C1, C2), calcined clay (CC1, CC2), acid treated clay (AC1, AC2), kaolin (K) and calcined kaolin (CK).
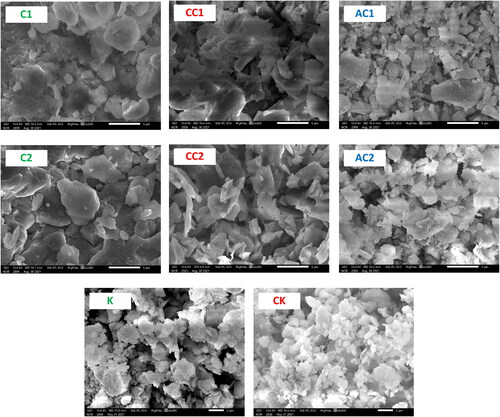
illustrates the SEM micrographs of laterite, clay, and kaolin as well as their calcined and acid treated products. The micrographs show the compactness and adhesion of the untreated laterite, clay, and kaolin particles (C1, C2, and K), and the lack of porosity between the particles. Which reduces the activity of these raw materials towards the adsorption of ions from aqueous solutions in the step of water treatment with these materials. This requires thermal and acid treatments to loosen and disperse the particles, increase the surface area, and enhance the adsorption activity. The thermal treatment of the materials (CC1, CC2, and CK), causes the chemically combined water to escape from the clay minerals and turn them into ceramic minerals. However, the semi-vitrification and sintering of ceramic particles reduce the surface area. This limits the adsorption capacity of the thermally treated products. On the other hand, acid treatment of the materials (AC1 and AC2) led to the removal of iron oxide. The crystallized clay particles are broken down and disintegrated. Some of them are transformed into amorphous matter with a large area and high surface activity that help improve the adsorption properties of the products of acid treatment.
Figure 8. SEM micrographs of clay (C1, C2), calcined clay (CC1, CC2), acid treated clay (AC1, AC2), kaolin (K) and calcined kaolin (CK).
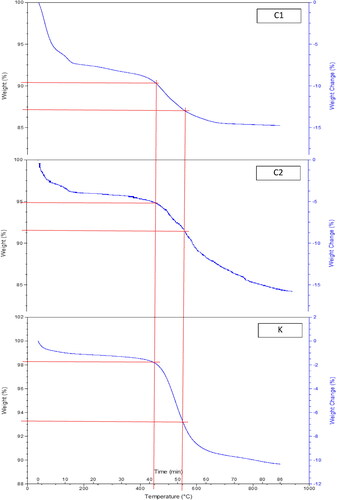
illustrates the TGA of laterite, clay, and kaolin (C1, C2 and K). The total loss in weight, during the thermal decomposition of clay materials is divided into the following stages. Weight loss at 50–180 °C is attributed to the evolution of absorbed water, which is held by van-der-Waals forces in the interlayer of weakly ordered clay minerals (montmorillonite and kaolinite) (Levitskii, Pozniak, & Baranceva, Citation2013). Weight loss at 250–350 °C is attributed to the dehydroxylation of hydrated iron oxides to produce hematite (Levitskii et al., Citation2013). Weight loss at 450–550 °C is attributed to the loss of structural water (hydroxyl groups in the modified gibbsite layers) of the clay minerals (montmorillonite, illite and kaolinite), and transformation into metakaolin (Ngun, Mohamad, Sulaiman, Okada, & Ahmad, Citation2011). Weight loss at 717 °C is attributed to calcination of the carbonate minerals. Weight loss at 900–1000 °C is attributed to a structural reorganization and formation of new crystalline phases. From results of the TGA analysis, it is possible to calculate the approximate percentage of clay minerals in the clay samples. By calculating the weight loss in the temperature range 450–550 °C The descending order of the clay content of clay materials is kaolin K (approximately 5%) > clay sample C2 (approximately 3.5%) > laterite sample C1 (approximately 2.75%).
and illustrate the temperature, pH, EC, and TDS of raw household water before and after treatment as well as after point-of-use treatment by filters, compared with the drinking water. Drinking water has a low content of EC and TDS. Household water contains four times this content because it contains a lot of dissolved salts. Therefore, this household water is not suitable for drinking and is considered harmful from a health point of view. Household water needs to be treated with effective adsorbents to separate most of the dissolved salts. The results of household water treatment with raw clay materials, heat treatment materials, and acid treatment materials are as follows. Raw clay materials are not suitable for direct use in household water treatment. Laterite and clay sample were neutral, while kaolin raised the EC and TDS of household water after treatment, because it contains dissolved salts.
Figure 10. Electrical conductivity and total dissolved solids of raw household water before and after treatment (1–12) as well as after point-of-use treatment by filters (13–14), compared with the drinking water (15).
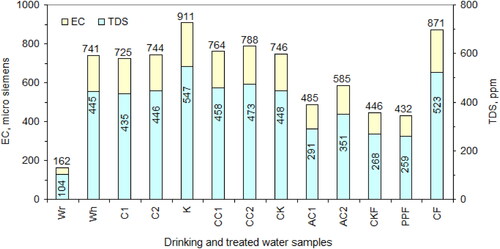
Table 2. The temperature, pH, electrical conductivity, and total dissolved solids of raw household water before and after treatment (1–12) as well as after point-of-use treatment by filters (13–14), compared with the drinking water (15).
Additionally, clay materials that have been thermally treated are not suitable for use in household water treatment. The thermal treatment of laterite and clay sample, led to the formation of a small percentage of the dissolved phases, which slightly increased the EC and the TDS of the household water after treatment. While, the heat thermal treatment of kaolin, transformed a percentage of the soluble phases into insoluble phases that contribute to the formation of metakaolin. Hence, the EC and the TDS of household water decreased after being treated with thermally treated kaolin, compared to raw kaolin. Treatment of samples of laterite and clay with acid, led to the formation of good efficient adsorbents. The EC and TDS of household water after treatment were reduced by approximately 35% and 21%, respectively. To enhance the results of household water treatment, an equal mixture of the acid-treated laterite, acid-treated clay, and acid-treated charcoal was used in the household water treatment filter at the point-of-use. The results of the treatment showed that, the calcined kaolin filter and the polypropylene filter, reduced the EC and the TDS of the household water, by a percentage of approximately 40% and 42%, respectively.
4. Discussion
It is clear from the results that the removal of iron oxide was the critical step in improving the adsorption properties of the used clay materials. Iron interferes with the composition of both alumina octahedral and silica tetrahedral sheets of clay minerals (Letaief, Casal, Kbir-Ariguib, Trabelsi-Ayadi, & Ruiz-Hitzky, Citation2002; Amonette, Citation2002) in three forms: solid-phase oxides (oxyhydrate), iron-(III) oxides, and iron-(II) oxides (Melton, Swanner, Behrens, Schmidt, & Kappler, Citation2014; Dong, & Lu, Citation2012). Iron oxide was removed from clay materials by acid treatment by acid leaching. Under acid attack, excess H+ ions promote the breaking of Fe-O bonds connected to alumina octahedral and silica tetrahedral sheets (Wiederhold et al., Citation2006). The extent of iron removal depends on many parameters, such as the acid concentration as well as the type of acid and clay. The dissolution of iron is higher in illitic clay than in kaolinitic clay (Xie et al., Citation2020). The dilute HCl acid may partially remove the poorly crystalline and part of the iron oxide phases from the iron-bearing clay. However, concentrated HCl acid completely leaches iron oxide phases and alters clay minerals (Manceau et al., Citation2000). Previous studies have mentioned that the removal of iron oxides from clay minerals alters the physicochemical properties of clay, such as cation exchange capacity, clay-organic interactions, surface pH, surface area, and swelling behavior (Carriazo, Citation2012; Stucki, Citation2011). Since the laterite sample contains more iron oxide, as shown by the XRF, XRD, and FTIR results ( and and ), there is a greater change in the surface and adsorption properties of laterite resulting from acid treatment. This was confirmed by the results of measuring the properties of treated water ( and ).
Although the maximum result of household water treatment obtained in this study is approximately 42%, the treated water still has a high content of TDS more than twice that of drinking water. These results are satisfactory and close to the results of several previous studies that examined the treatment of drinking water with a ceramic filter. In a previous study, the ceramic filter was reported to be effective in removing turbidity, but it slightly reduced the total hardness, chloride and sulfate contents of the treated water (Suribabu et al., Citation2020). The results of this study are also fully consistent with the results of another study in which the absorption capacity of heavy metal (Cd2+) for clay: andisol (6:4 wt/wt) was reported to be about 18.2 mg/g (Pranoto et al., Citation2018). Therefore, the study needs to conduct more experiments and treatments for the clay materials used and others to narrow this difference to the least possible extent.
5. Conclusions
The main conclusions of this research are:
Laterite has higher iron-content than the other samples. From the results of the TGA analysis, the approximate order of the clay mineral content is kaolin K > clay sample > laterite sample.
The selected clay samples were beneficiated for the production of ceramic filters for “point-of-use” household water treatment technology to remove dissolved solids from water to meet the needs of the community through a safe household and industrial water supply after further treatment purposes.
The raw clay materials are not suitable for use in household water treatment, either because they have poor adsorption capacity or contain dissolved salts that add to the water content of salts. Additionally, thermally treated clay materials are not suitable for use in household water treatment because they have a low adsorption capacity due to the sintering effect.
Acid treatment of clay material by concentrated HCl acid completely leaches iron oxide phases and broke down and disintegrated the clay minerals into the amorphous matter with enhanced the adsorption capacity.
The combination of selected acid-treated laterite, clay, and charcoal for the household water treatment filter at the point of use in the calcined kaolin filter and the polypropylene filter reduced the TDS content by 40–42%.
A series of future studies might be implemented to conduct more treatment options for the available materials to raise the efficiency of their adsorption to be used in reducing the content of household water salts to the least possible extent.
Acknowledgment
The authors are considerable grateful to Al Baha University for financially supporting the present study under research project number 6/1441.
Disclosure statement
The authors proclaim no conflict of benefit.
References
- Addae, E. A., & Adu, D. (2020). Investigating water poverty in sub-Sahara Africa: Addressing the potentials for water resources management, and policy implications. International Journal of Scientific Research in Computer Science, Engineering and Information Technology, 6(6), 57–64. doi:10.32628/CSEIT20664
- Amonette, J. E. (2002). Iron redox chemistry of clays and oxides: Environmental applications (Vol. 10, pp. 89–147). CMS Workshop Lectures. Electrochemical properties of clay, Alanah Fitch, Clay Minerals Society Publisher, Chantilly, USA. doi:10.1346/CMS-WLS-10.3
- Akosile, S. I., Ajibade, F. O., Lasisi, K. H., Ajibade, T. F., Adewumi, J. R., Babatola, J. O., & Oguntuase, A. M. (2020). Performance evaluation of locally produced ceramic filters for household water treatment in Nigeria. Scientific African, 7, e00218. doi:10.1016/j.sciaf.2019.e00218
- Bain, R., Gundry, S., Wright, J., Yang, H., Pedley, S., & Bartram, J. (2012). Accounting for water quality in monitoring access to safe drinking-water as part of the Millennium Development Goals: Lessons from five countries. Bulletin of the World Health Organization, 90(3), 228–235. doi:10.2471/blt.11.094284
- Carriazo, J. G. (2012). Influence of iron removal on the synthesis of pillared clays: A surface study by nitrogen adsorption, XRD and EPR. Applied Clay Science, 67–68, 99–105. doi:10.1016/j.clay.2012.07.010
- Clasen, T., Nadakatti, S., & Menon, S. (2006). Microbiological performance of a water treatment unit designed for household use in developing countries. Tropical Medicine & International Health: TM & IH, 11(9), 1399–1405. doi:10.1111/j.1365-3156.2006.01699.x
- Das, T. K., Kirtunia, R., & Shaibur, M. R. (2018). Application of ceramic filter for improvement of surface water quality. Journal of Innovation and Development Strategy, 12(1), 68–73.
- Dong, H., & Lu, A. (2012). Clay-microbe interactions and implications for environmental mitigation. Elements, 8(2), 95–100. doi:10.2113/gselements.8.2.95
- Eisazadeh, A., Kassim, K. A., & Nur, H. (2012). Solid-state NMR and FTIR studies of lime stabilized montmorillonitic and lateritic clays. Applied Clay Science, 67–68, 5–10. doi:10.1016/j.clay.2012.05.006
- Elasaad, H., Bilton, A., Kelley, L., Duayhe, O., & Dubowsky, S. (2015). Field evaluation of a community scale solar powered water purification technology: A case study of a remote Mexican community application. Desalination, 375, 71–80. doi:10.1016/j.desal.2015.08.001
- Gundry, S. W., Wright, J. A., Conroy, R., Du Preez, M., Genthe, B., Moyo, S., … Potgieter, N. (2006). Contamination of drinking water between source and point-of-use in rural households of South Africa and Zimbabwe: Implications for monitoring the Millennium Development Goal for water. Water Practice and Technology, 1(2), 1–9. doi:10.2166/wpt.2006.032
- Ihekweme, G. O., Shondo, J. N., Orisekeh, K. I., Kalu-Uka, G. M., Nwuzor, I. C., & Onwualu, A. P. (2020). Characterization of certain Nigerian clay minerals for water purification and other industrial applications. Heliyon, 6(4), e03783. doi:10.1016/j.heliyon.2020.e03783
- Kallman, E. N., Oyanedel-Craver, V. A., & Smith, J. A. (2011). Ceramic filters impregnated with silver nanoparticles for point-of-use water treatment in rural Guatemala. Journal of Environmental Engineering, 137(6), 407–415. doi:10.1061/(ASCE)EE.1943-7870.0000330
- Lange, J., Materne, T., & Grüner, J. (2016). Do low-cost ceramic water filters improve water security in rural South Africa. Drinking Water Engineering and Science, 9(2), 47–55. doi:10.5194/dwes-9-47-2016
- Lecomte, I., Liégeois, M., Rulmont, A., Cloots, R., & Maseri, F. (2003). Synthesis and characterization of new inorganic polymeric composites based on kaolin or white clay and on ground-granulated blast furnace slag. Journal of Materials Research, 18(11), 2571–2579. doi:10.1557/JMR.2003.0360
- Letaief, S., Casal, B., Kbir-Ariguib, N., Trabelsi-Ayadi, M., & Ruiz-Hitzky, E. (2002). Fe-rich smectites from Gafsa (Tunisia): Characterization and pillaring behavior. Clay Minerals, 37(3), 517–529. doi:10.1180/0009855023730050
- Levitskii, I. A., Pozniak, A. I., & Baranceva, S. E. (2013). Effects of the basaltic tuff additions on the properties, structure and phase composition of the ceramic tiles for interior wall facing. Procedia Engineering, 57, 707–713. doi:10.1016/j.proeng.2013.04.089
- Lim, S. C., Gomes, C., & Ab Kadir, M. Z. A. (2013). Characterizing of bentonite with chemical, physical and electrical perspectives for improvement of electrical grounding systems. International Journal of Electrochemical Science, 8(9), 11429–11447.
- Madejova, J., & Komadel, P. (2001). Baseline studies of the clay minerals society source clays: Infrared methods. Clay Minerals, 49(5), 410–432. doi:10.1346/CCMN.2001.0490508
- Manceau, A., Lanson, B., Drits, V. A., Chateigner, D., Gates, W. P., Wu, J., … Stucki, J. W. (2000). Oxidation-reduction mechanism of iron in dioctahedral smectites. 1. Crystal chemistry of oxidized reference nontronites. American Mineralogist, 85(1), 133–152. doi:10.2138/am-2000-0114
- Meierhofer, R., Bänziger, C., Deppeler, S., Kunwar, B. M., & Bhatta, M. (2018). From water source to tap of ceramic filters-factor that influence water quality between collection and consumption in rural households in Nepal. International Journal of Environmental Research and Public Health, 15(11), 2439. doi:10.3390/ijerph15112439
- Melton, E. D., Swanner, E. D., Behrens, S., Schmidt, C., & Kappler, A. (2014). The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nature Reviews. Microbiology, 12(12), 797–808. doi:10.1038/nrmicro3347
- Mwabi, J. K., Mamba, B. B., & Momba, M. N. B. (2012). Removal of Escherichia coli and faecal coliforms from surface water and groundwater by household water treatment devices/systems: A sustainable solution for improving water quality in rural communities of the Southern African Development Community region. International Journal of Environmental Research and Public Health, 9(1), 139–170. doi:10.3390/ijerph9010139
- Ngun, B. K., Mohamad, H., Sulaiman, S. K., Okada, K., & Ahmad, Z. A. (2011). Some ceramic properties of clays from central Cambodia. Applied Clay Science, 53(1), 33–41. doi:10.1016/j.clay.2011.04.017
- Nwuzor, I. C., Chukwuneke, J. L., Nwanonenyi, S. C., Obasi, H. C., & Ihekweme, G. O. (2018). Modification and physiochemical characterization of kaolin clay for adsorption of pollutants from industrial paint effluent. European Journal of Advanced Engineering Technology, 5(8), 609–620.
- Pokrovski, G. S., Schott, J., Farges, F. O., & Hazemann, J. L. (2003). Iron (III)-silica interactions in aqueous solution: Insights from X-ray absorption fine structure spectroscopy. Geochimica et Cosmochimica Acta, 67(19), 3559–3573. doi:10.1016/S0016-7037(03)00160-1
- Pranoto, P., Inayati, I., & Firmansyah, F. (2018). Effectiveness study of drinking water treatment using clays/andisol adsorbent in Lariat heavy metal cadmium (Cd) and bacterial pathogens. IOP Conference Series Materials Science and Engineering, 349, 012047. doi:10.1088/1757-899X/349/1/012047
- Rampe, E. B., Kraft, M. D., & Sharp, T. G. (2013). Deriving chemical trends from thermal infrared spectra of weathered basalt: Implications for remotely determining chemical trends on Mars. Icarus, 225(1), 749–762. doi:10.1016/j.icarus.2013.05.005
- Ravisankar, R. (2009). Application of spectroscopic technique for the identification of minerals from beach rocks of Tamil Nadu, India. EARFAM, 19, 272–276.
- Rayner, J. (2009). Current practices in manufacturing of ceramic pot filters for water treatment water. M.S. thesis, Engineering and Development Centre (WEDC), Loughborough Univ., Loughborough, UK.
- Sankaran, S., & Ramasamy, K. (2000). Infrared studies on some archaeological samples. Asian Journal of Physics, 9(2), 362–366.
- Sheikh, R., & Islam, S. (2017). Application of ceramic filter for improvement of pond sand filter’s water quality. American Journal of Environmental Protection, 6(3), 62–68. doi:10.11648/j.ajep.20170603.11
- Sobsey, M. D. (2006). Drinking water and health research: A look to the future in the United States and globally. Journal of Water and Health, 4(S1), 17–21. doi:10.2166/wh.2006.0039
- Sobsey, M. D., Stauber, C. E., Casanova, L. M., Brown, J. M., & Elliott, M. A. (2008). Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world. Environmental Science & Technology, 42(12), 4261–4267. doi:10.1021/es702746n
- Sengco, M. R., & Anderson, D. M. (2004). Controlling harmful algal blooms through clay Flocculation. The Journal of Eukaryotic Microbiology, 51(2), 169–172. doi:10.1111/j.1550-7408.2004.tb00541.x
- Suribabu, C. R., Sudarsan, J. S., & Nithiyanantham, S. (2020). Performance and technical valuation of candle‑type ceramic filter for water purification. International Journal of Energy and Water Resources, 4(1), 37–45. doi:10.1007/s42108-019-00043-7
- Stucki, J. W. (2011). A review of the effects of iron redox cycles on smectite properties. Comptes Rendus Geoscience, 343(2–3), 199–209. doi:10.1016/j.crte.2010.10.008
- Tantawy, M. A., & Ramadan, S. A. (2017). Mohamed, Middle Eocene clay from Goset Abu Khashier: Geological assessment and utilization with drinking water treatment sludge in brick manufacture. Applied Clay Science, 138, 114–124. doi:10.1016/j.clay.2017.01.005
- Ulfa, M., Prasetyoko, D., Bahruji, H., & Nugraha, R. E. (2021). Green synthesis of Hexagonal Hematite (α-Fe2O3) flakes using Pluronic F127-Gelatin template for adsorption and photodegradation of ibuprofen. Materials, 14(22), 6779–6796. doi:10.20944/preprints202110.0138.v1
- Xie, T., Lu, S., Zeng, J., Rao, L., Wang, X., Sandar Win, M., … Wang, Q. (2020). Soluble Fe release from iron-bearing clay mineral particles in acid environment and their oxidative potential. The Science of the Total Environment, 726, 138650. doi:10.1016/j.scitotenv.2020.138650
- Wiederhold, J. G., Kraemer, S. M., Teutsch, N., Borer, P. M., Halliday, A. N., & Kretzschmar, R. (2006). Iron isotope fractionation during proton-promoted, ligand-controlled, and reductive dissolution of goethite. Environmental Science & Technology, 40(12), 3787–3793. doi:10.1021/es052228y
- Zhang, C., Yu, Z., Zeng, G., Huang, B., Dong, H., Huang, J., … Zhang, Q. (2016). Phase transformation of crystalline iron oxides and their adsorption abilities for Pb and Cd. Chemical Engineering Journal, 284, 247–259. doi:10.1016/j.cej.2015.08.096
- Zhang, W., Zhang, S., Wang, J., Dong, J., Cheng, B., Xu, L., & Shan, A. (2019). A novel adsorbent albite modified with cetylpyridinium chloride for efficient removal of zearalenone. Toxins, 11, 674. doi:10.3390/toxins11110674