?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The influence of ferroelectric strontium nanoparticles on the photocatalytic performance of 1 D TiO2 nanotubes (NT) was herein highlighted. The effect of adding various amounts of Sr nanoparticles onto NTs was therefore deeply characterized by means of Raman, UV-vis diffuse reflectance, photoluminescence (PL) and X-ray photoelectron spectroscopies, X-ray diffraction, N2 adsorption-desorption measurements and scanning electron microscopy coupled with energy-dispersive X-ray analysis as well as determining dielectric properties. The different xwt%Sr-NTs with x =(0.2, 0.4, 0.6, 0.8 and 1 wt%) were investigated for the photodegradation of Methylene Blue (MB) under visible light and formic acid under UV-A. Results emphasize that the kinetic study fits well with Langmuir-Hinshelwood (LH) model; for the same surface coverage (θ =0.99), the rate constant kLH increases versus Sr loading with an optimum at 0.8 wt% Sr. Under UV-A light, photodegradation increases versus Sr loading. As evidenced by PL, impedance and XPS measurements, the presence of ferroelectric strontium generates structural defects (Ti3+ and Ov) with strong opportunity to monitor the separation the photogenerated (electron- hole) pairs regardless of the synthesis limitations. Herein, the molar ratio O2-/Ti4+ increases vs strontium loading. Hence, Sr-NTs opens new route for “assisted” photocatalysts.
1. Introduction
Several dyes are employed in different industries such as textiles, food, printing, medicine, plastic, and mainly the paper industry for numerous purposes (Alencar et al., Citation2020). Nevertheless, these activities generate large amounts of wastewater containing toxic dyes, inadequate for human and plants consumption (Pandey et al., Citation2020). Among them, the textile manufactory remains the most methylene blue (MB) consuming industry (Derakhshan et al., Citation2013). The textile wastewater treatment was the topic of numerous literature reports (Bilinska & Gmurek, Citation2021; Wang et al., Citation2020). When low biodegradability is concerned, advanced Oxidation Processes (AOPs) have been used as an alternative method for MB degradation, among them, heterogeneous photocatalysis, in which radiation (UV and / or visible) is used by a semiconductor (photocatalyst) to create free hydroxyl radicals, known to be responsible of the degradation of large spectrum of organic compounds until complete mineralization into CO2 and H2O (Ahmad et al., Citation2015). Many innovative catalysts have been successfully employed to achieve maximum degradation efficiency. Typically, semiconductors and semiconductor heterojunctions like ZnS (Rao et al., Citation2016), TiO2 (Zhu, Hong & Ho, Citation2015), ZnO (Barnes, Molina, Xu, Dobson, & Thompson, Citation2013), carbon-based catalysts such as graphene, graphene oxide, and carbon nitrides (Lu et al., Citation2017; Sarfraz et al., Citation2021), plasmonic metals (such as gold, silver, platinum) (Sarfraz et al., Citation2021; Zhang et al., Citation2018). These systems have demonstrated high efficacy in oxidizing MB through redox reactions. Nevertheless, strong differences in absolute rate constant values were observed due to: different reactor profiles, different lamps used with different emission spectra (Alkaykh, Mbarek, & Ali-Shattle, Citation2020). There is no standard condition to perform the photodegradation test. Moreover, the rate of the reaction can vary with : (i) MB concentration (Salama, Mohamed, Aboamera, Osman, & Khattab, Citation2018), (ii) the pH (Alkaim et al., Citation2014) and (iii) the surface charge of the catalyst (Singh et al., Citation2020). In fact, MB is a cationic dye, mainly adsorbs onto highly negatively charged photocatalyst (Azeez et al., Citation2018). Hence, the pH zero charge is unfortunately an important key parameter, H+ ions competes with cationic MB dye at the surface of the photocatalyst at pH lower than pH of zero charge (pHzc). Therefore, the test must be performed at pH slightly higher than pHzc. On the other hand, preparing an efficient, wide bandgap photocatalyst is still challenging. Therefore, an efficient strategy to decrease the rate of recombination of photogenerated electron-hole of the applied photocatalyst is of high interest. Different approaches have been investigated to boost the TiO2 response to photoexcitation: like photosensitization of TiO2 by dye (Li, Liang, Zhou, & Pan, Citation2020), metal ion doping (Liu, Jiang, Song, Duan, & Zhu, Citation2020), or creating heterojunction with metal oxides (Mohammed Redha, Abdulla Yusuf, Amin, & Bououdina, Citation2020). Another approach, by doping with metal oxides like transition and noble metals which showed a great influence on the photocatalyst efficiency and yields to the creation of electronic trap-centres (like oxygen vacancies) which quell the electron- hole recombination (Chakhtouna, Benzeid, Zari, & Bouhfid, Citation2021). However, all these synthesis methods led to limited success in photocatalytic activity enhancement. One way to improve the efficiency of photocatalytic materials is to develop “assisted” photocatalysis processes. In this respect, charge separation can be compelled by induced electric field coming from the energy discontinuity at the semiconductor/electrolyte interface. Tuning the electronic band structure in the interfacial region therefore appears as a very effective approach to enhance charge separation efficiency. In this respect, ferroelectric polarization is a promising strategy to modify band structures and charge transport performance in heterojunction-based semiconductors. The built-in electric polarization can lead to tremendous redistribution of charges in adjacent semiconductors. Generating locally such spontaneous polarization by addition of a ferroelectric material to the photocatalyst would provide a strong opportunity (to the best of our knowledge, not used up to now) to monitor the charge separation regardless of the synthesis limitations.
Few attempts have been done in the literature to build devices combining ferroelectrics and semiconductor materials by growing the strontium titanate (SrTiO3) phase onto TiO2 systems. Different methodologies were envisaged like TiO2 anodization (Janczarek & Kowalska, Citation2021) or rutile TiO2 nanowires obtained by controlled alkaline hydrothermal treatment (Zhang, Jing, Tan, Yu, & Ma, Citation2018) followed in all cases by in situ substitution by Sr or Ba under hydrothermal conditions. However, these methods suffer from a low scale production of anodized TiO2 materials or use of inappropriate low active rutile phase. TiO2 microspheres were also used but their very large size limits the proportion of TiO2 semiconductor phase influenced by ferroelectric domains (Hamisu, Gaya, & Gaya, Citation2020). Generally, improved photocatalytic performances were observed but without distinguishing the p-n heterojunction effects or induced ferroelectric polarization effects (if even considered). For example, the conduction band (CB) of SrTiO3 is lower than TiO2 (CB) which limits to some extend the recombination rate; but the role played by ferroelectricity was rarely considered in this case because SrTiO3 is generally considered paraelectric. This simplistic statement was however recently denied since under optical excitation, SrTiO3 was found to become ferroelectric (Liu, Lv, Fan, Xing, & Jia, Citation2019).
The objective here is to demonstrate the interest of assisted photocatalysis process. The photocatalytic activity will be enhanced by an adequate combination of ferroelectric strontium with TiO2 semiconductor of optimized 1 D morphology. To the best of our knowledge, Sr-doped TiO2 nanotubes for “assisted” photocatalysis has not yet been investigated. In this respect, charge separation can be boosted by the electric field created by strontium. In the first part, different wt% of strontium loadings were doped onto 1 D TiO2 nanotubes. The second part is devoted to characterization of Sr-NTs nanomaterials. Finally, kinetics models were applied for the photodegradation of model molecules (MB and Formic acid (FA)) and correlated to the structural, textural, morphological and electrical properties of Sr-NTs nanomaterials.
2. Experimental
2.1. Elaboration of Sr-doped TiO2 nanotubes
Firstly, 1 D TiO2 nanotubes material (NT) is obtained using a hydrothermal procedure as described in (Meksi et al., Citation2016). Secondly, NT was subsequently doped with different amounts of Strontium (0.2, 0.4, 0.6, 0.8 and 1.0 wt %) using dry impregnation method. The desired amount of strontium precursor, Sr(NO3)2 was first suspended in a volume of water corresponding to twice the porous volume (Vp) of the TiO2 nanotubes before being contacted with an adequate quantity of NT. The obtained paste was then dried in vacuum-oven at 40 °C for 24 h. The resulting nanomaterials are named x Sr- NT (with x the weight loading of strontium). Further post-thermal treatment was then conducted for the Sr-NT samples once again at 400 °C under air using a heating ramp of 2 °C/min. Samples were then named as x wt%Sr-NT.
2.2. Materials characterization
2.2.1. Catalyst characterization
XRD data were collected on an automatic diffractometer (XRD-7000, Shimadzu, Japan) using a Ni-filtered Cu Kα radiation source (λ = 1.54184 Å). The samples were measured at 2 theta range between 10 and 80° using a scan speed of 1°/min. x wt%Sr-NT nanomaterials structural environment was studied using Raman spectroscopy at excitation wavelength of 632 nm (iHR320, HORIBA, Japan). The textural properties of x wt%Sr-NT nanomaterials and their Sr free counterpart were measured using QUANT ACHROME Nova 1200e, USA. Prior to analysis, the samples were outgassed at 200 °C for 2 h. The UV–Vis DRS measurements were recorded on UVD-3200, Labomed Inc, USA. Bandgap energy values were evaluated using the Kubelka–Munk method. F(R)hν1/2 versus hν plots were built with F(R) = (1 − R)/2R, assuming an indirect bandgap transition. Transmission Electron Microscopy (TEM) was performed on a JEOL 2010 (200 kV) microscope to reveal the morphology of the different samples. The TEM analysis specimens were first dispersed in ethanol before dropwise addition and drying onto a holey carbon film supported on a Cu grid (300 mesh). The SEM images and SEM mapping of 0.2 wt% and 0.8 wt% Sr loaded TiO2 were studied using Scanning electron microscope at 20 kV (FEI, Inspect S50, USA). Photoluminescence (PL) of Sr-NTs are measured directly from the intensity and spectrum content of the photoluminescence (Shimadzu spectrofluorometer, RF5301 PC) with excitation wavelength of 290 nm. X-ray photoelectron spectroscopy (XPS) studies were carried out using a Thermo Scientific ESCALAB 250Xi equipped with a dual Al/Mg anode and a hemi-spherical analyzer operating at fixed pass energy of 50 eV. A 150 W monochromatic source (Al Ka = 1486.6 eV) was used to excite the samples. Impedance measurements were performed using PalmSens4 instrument. Samples (0.2 wt%Sr-NT and 0.8 wt%Sr-NT powder) in the form of a 13 mm diameter and a 2 mm thickness pellet were used. The material is placed between two glass coated with a conductive FTO electrodes. The complex impedance measurements were carried out at room temperature by scanning a frequency range from 0.1 Hz to 1 MHz with a voltage of 0.5 V.
2.2.2. Photocatalytic experiments
The photocatalytic activity of Sr doped TiO2 nanomaterials were applied in the photodegradation of methylene blue (MB) under visible light and formic acid (FA) under UV-A. The photodegradation of MB was performed using visible lamp (HQI-E 400 W/n) having a maximum emission at 520 nm and coated with UV filter. Typically, 30 mg of xwt% Sr-NT catalyst was added to 30 mL of 20 ppm aqueous solution of MB. The solution was stirred under dark for an hour to reach adsorption equilibrium and then the lamp was turned on. The methylene blue samples were withdrawn at different time intervals (Ct) and analyzed by UV-visible spectrophotometer. In order to elucidate the photodegradation kinetic model, the concentrations of MB were varied between 5 and 50 mg.L−1 (0.014 to 0.14 mmol.L−1). The photodegradation of FA was performed in a photoreactor (pyrex, 100 ml) with 12.5 cm2 of optical window area. Sr-NTs concentration was maintained at 1.0 g.L−1. The solution pH was in the range of 3.0 ± 0.2. A PL-L (18 W) lamp with emission at 365 nm was used for UV irradiation. An optical Corning filter 0.52 was employed to cut-off wavelength below 340 nm. A radiant flux of 5 mW.min−1 was fixed for all experiments. Before the study, the suspensions were facilitated to reach equilibrium condition by stirring well in darkness for 30 min. The formic acid concentration of the solution after equilibration was then measured and used as the initial concentration (C0). Formic acid samples were taken for analysis at different intervals (C) from the photoreactor while the photocatalyst was separated from the liquid phase by filtration. Withdrawn samples were then analyzed with a HPLC Shimadzu Nexera i-series equipped with Hypersil Gold column (5 mm, 150 × 4.6 mm); using acetonitrile/water (30/70 v/v) as mobile phase and UV-visible detector (λ = 210 nm) at a flow rate of 0.8 mL.min−1.
3. Results and discussion
3.1 X-Ray diffraction (XRD)
illustrates the X-ray diffraction patterns of xwt% Sr- NT; x = (0.2%, 0.4%, 0.6%, 0.8%, 1.0%) and their free strontium material TiO2 (NT) taken as reference. The NT displayed the presence of crystalline TiO2 nanostructure typical to that of anatase phase and agrees with JCPDS No. 21-1272. Moreover, the addition of strontium does not lead the appearance of new peaks corresponding to SrO (32.85°, 38.74°, 46.95° and 58.40°). Nevertheless, Sr doping induced slightly shift in (101) plane of anatase phase (), which indicates distortion in lattice of TiO2 (Keerthana et al., Citation2022) by creation of defects (Ti3+ and/or oxygen vacancies). Hence, PL and XPS measurements were used in the later section to highlight this hypothesis. In our case, the presence of strontium oxide or strontium titanates (SrTiO3) is highly probable even if we do not detect their corresponding rays in the XRD patterns of doped samples. This could be explained by (i) the low content of strontium which is lower than XRD sensitivity or (ii) Sr might be highly dispersed onto TiO2. Similarly, doping TiO2 with large ionic radius like rare elements lanthanides (e.g. La, Ce) anatase crystallite size and generates surface defects like oxygen vacancies (Xue et al., Citation2011). Following this finding, calculations were established, in the case of our materials, and determined from the broadening of (101) plane of anatase phase by using Scherrer formula (Secundino-Sánchez et al., Citation2022):
(1)
(1)
Where L is the crystallite size; K is taken 1; λ is the wavelength of the X-Ray radiation (CuKα = 0.15406 nm) and βi is the line width at half-maximum height considering the correction (βi = {(βmeasured)2 – (βinstrumental)2}0.5). After taking into account all parameters, crystallites size results of samples are summarized in .
Figure 1. XRD patterns of: (A) xwt% Sr-NT, x = {0, 0.2, 0.4, 0.6, 0.8 and 1.0} and (B) zoom of (101) peak.
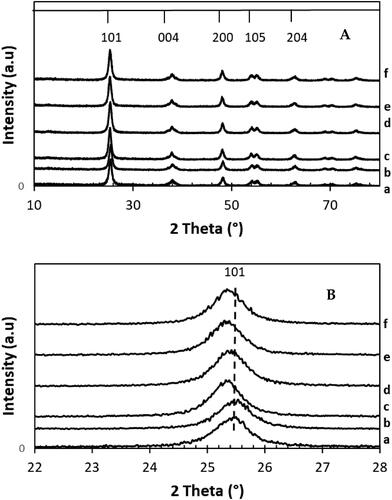
Table 1. Textural and structural properties of x wt% Sr -NT catalysts, x = {0, 0.2, 0.4, 0.6, 0.8 and 1}.
From , Anatase crystallite sizes are similar for all the xwt% Sr- NT nanomaterials with values around 11.12 ± 0.06 nm showing the absence of crystallite size variation compared to the Sr-free 0NT reference (12 nm). This result might be explained by better stabilizing of the structural properties in presence of Strontium.
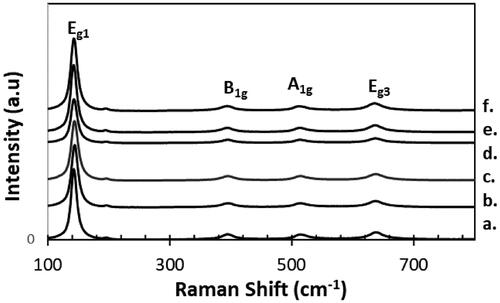
3.2. Raman spectroscopy
Raman spectra of pure NT nanotubes and x wt%-Sr-NT nanomaterials are shown in . The appearance of peaks at 143, 394, 514 and 638 cm−1 are assigned to active modes E1g, B1g, A1g and Eg of anatase phase. The Raman signature clearly shows the presence of anatase phase, in good agreement with XRD results. However, a red shift was observed corresponding to the E1g mode of the anatase phase. NT has a mode of vibration at 149 cm−1 against 152 cm−1 for 0.6 wt% Sr-NT and 153 cm−1 for 0.8 wt% Sr-NT ( supplementary information). Such shift was ascribed to the creation of surface defects like oxygen vacancies (Scepanovic, Grujic-Brojcin, Dohcevic-Mitrovic, & Popovic, Citation2007). Moreover, spectra of low doped materials (0.2 and 0.4 wt%Sr) show a small blue shift and extending of the principal peak (144 cm−1). This effect was explained in literature by the distortion of anatase crystalline lattice or also the non-uniform distribution of the particle size and to the co-existence of several phases (Zhang, Bang, Tang, & Kamat, Citation2010). However, according to XRD patterns, distortion of the anatase crystalline lattice is eliminated but possibility of co-existence of other phases like SrTiO3 or SrO are not excluded.
3.3. UV-vis diffuse reflection
UV-vis DRS spectra were also acquired for NT and different wt% Sr doped NT catalysts (). The spectra showed a strong absorption band at about 360 nm corresponding to TiO2, Sr-doped TiO2 showed a wavelength shift to lower values (340 nm for 0.2 wt% Sr-NT against 240 nm for 1 wt% Sr-NT) (). Furthermore, the band gap energies were determined for doped and undoped strontium samples () using Kubelka-Munk method (Machado & da Hora Machado, Citation2020) by plotting F(R) hυ1/2 against Energy (E) ( supplementary information).
Figure 3. UV-visible spectra (reflectance) of xwt% Sr-NT: a. 0 wt% Sr, b.0.2 wt% Sr, c. 0.4 wt% Sr, d. 0.6 wt% Sr, e. 0.8 wt% Sr and f. 1.0 wt% Sr.
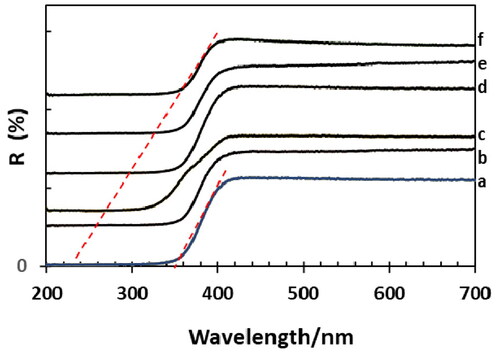
From , the undoped TiO2 NT material shows a band gap energy about 3.20 eV, value near to anatase one Eg (∼3.17 eV) which agrees with XRD and Raman spectroscopies results. For doped samples, Eg increases slightly to reach 3.43 ± 0.02 eV. It should be noted that this absence of Eg shift can be expected if SrTiO3 is formed at the surface of TiO2 nanotubes since SrTiO3 and TiO2 are known to present similar bandgap values (Ohtani, Citation2010).
3.4. Textural properties
The textural changes of NT with different wt% Sr loadings (0.2–1.0) was analyzed using N2 adsorption-desorption measurement. The results are summarized in and in supplementary information. NT shows a type IV isotherm pattern with H3 hysteresis loop at p/p0 range of 0.45, while with different wt% loading, the hysteresis loop tends to extend at higher p/p0 ∼ 0.7. Such isotherm with narrow and broad hysteresis indicates presence of micropore and mesopores and are characteristic of intergranular porosity resulting from particles aggregation to form slot like pores (Lei et al., Citation2001). The textural properties of NT and xwt% Sr-NT materials () show a slight decrease of the surface area from 128 m2 g−1 NT to 95 m2 g−1 after doping with 0.4 wt%Sr. Compared to NT, the surface area varies in the range of 8.5%–17% for respectively 0.6 wt%Sr and 0.8 wt%Sr-NT materials. This suggests that some porosity vanished probably by partial blockage in the presence of strontium oxide species. The pore diameter obtained from BJH method varies slightly (22 ± 0.65 nm). Based on previous Xray results the particle size (or coherent domain) is constant ∼11 nm. Therefore, this homogeneous pore diameter distribution is most probably related to intergranular porosity resulting from physical agglomeration of xwt% Sr-NT materials by Van der Waals interaction.
3.5. Morphological properties
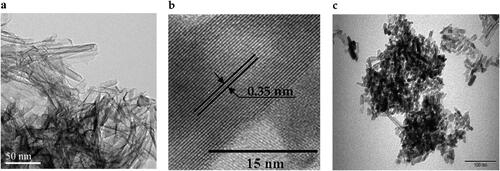
The morphological properties of representative nanomaterials NT and 0.8 wt% Sr-NT was examined with TEM and HRTEM ().
From , NT possess a tubular morphology with an outer diameter of ca. 15 nm (). HR-TEM image of NT shows an interlayer space of 0.34 nm corresponding to the (101) plane of the anatase phase (), being in good agreement with X-ray diffraction and Raman observations. By contrast, doping NT with Sr ions followed by post-thermal treatment (calcination at 400 °C) yields to mixture of nanotubes and nanorods ().
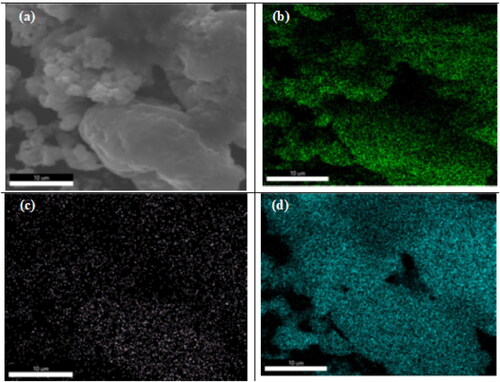
The FE-SEM-EDS mapping of 0.8 wt%Sr-NT () and Ti, Sr and O elements are presented in . The mapping shows the presence of Sr coexisting with NT nanoparticles. The presence of elements Sr, O and Ti are homogeneously distributed.
From EDAX results, the atomic molar ratio Sr/Ti are 0.0067 and 0.020 for respectively 0.2 wt%Sr and 0.8 wt%Sr-NT (, Supplementary information). This result clearly indicates that dry impregnation method yields to excellent Sr ions distribution onto the surface of NT material.
3.6. Photoluminescence (PL) emission spectroscopy measurements
Photoluminescence (PL) spectra were also acquired for the same series of xSr-NT samples with x = 0, 0.2 and 0.8 (). Results were recorded using an excitation source centred at 330 nm while spectra are herein presented in the 330–700 nm wavelength range.
Figure 6. PL signal of a. Sr-free; b. 0.2 wt% Sr and c. 0.8 wt%Sr NT nanomaterials calcined at 400 °C.
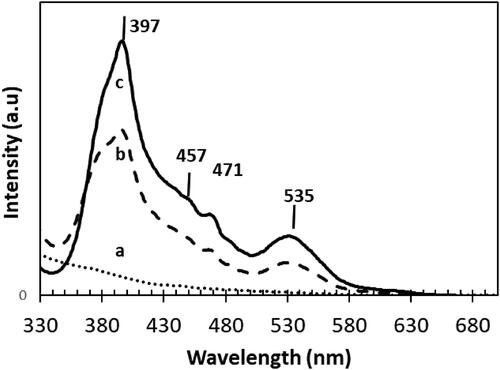
In case of Sr doped NT, four emission peaks were observed in the PL spectra. At the excitation of 397 nm, the emission signalling self-electron trapping tendency by holes inside bulk titania lattice structure (Lei et al., Citation2001). Two emission peaks at about 457 nm and 535 nm were observed indicating a generated photoelectrons recombination with titania oxygen surface defect sites (Liu, Li, Sedhain, Lin, & Jiang, Citation2008). The emission peak 471 nm indicates the excited charge transfer transition from titania Ti3+ species to octahedral unit (TiO62-) (Li, Li, Hou, Cheah, & Choy, Citation2005). A much higher PL intensity is found for Sr-NT than for NT. In addition, a high intense emission peak at about 535 nm indicates the generation of surface defects with Sr doping due to distortion of TiO6 octahedra (Choudhury, Borah, & Choudhury, Citation2013). This phenomenon is particularly attributed to the ion size mismatch between Sr2+ (1.18 Å) and Ti4+ (0.68 Å) species, which eventually helps to generate oxygen vacancies and restore neutral charge. Increasing Sr loading from 0.2 to 0.8 wt% leads to the intensification of the PL intensity especially for 0.8 wt% Sr-NT with a dominant peaks at 397 nm, 471 nm and 535 nm. Such enhancement in peak intensity shows that increasing Sr loading influences the surface defect sites generation. Though an increase in surface oxygen vacancies occurs, the electrons transport from conduction to valence band (radiative recombination centres) becomes less, while act as luminescence sinkers and decreases the intensity of emission (Choudhury, Borah, & Choudhury, Citation2013). Overall, the PL characterization study shows that strontium doping on NT creates surface oxygen vacancies, assist trapping photogenerated electrons leaving holes for photocatalytic process. In the next section XPS analysis was used to provide more insights about the modification of surface structure in presence of strontium.
3.7. XPS measurements
XPS was employed to determine the elemental composition and surface chemical states of NT, 0.2 wt%Sr-NT and 0.8 wt% Sr-NT nanomaterials. Results are reported in and . As shown in , Ti 2p, Sr 3d, O 1 s and Ti 2 s can be readily observed in the fully scanned spectra, indicating the presence of Ti, O and Sr at the surface of xwt% Sr-NT nanomaterials in good agreement with SEM mapping (b–d). As displayed in , the core O1s peak at 529.14 eV attributed to bulk oxygen (O2-) bound to Ti4+ is observed for all nanomaterials (Wang et al., Citation2021). The introduction of Sr yields to the formation of: (i) Ti-OH bonds and/or oxygenated Ti3+ species (530.08 eV) and (ii) oxygen vacancy (Ov) at 530.72 eV for respectively 0.2 wt%Sr-NT and 0.8 wt%Sr-NT. These results confirm PL measurements, which showed the emission peaks at 457 and 535 nm for surface oxygen defect sites. Ti2p3/2 core-level spectra () present a bimodal signal at 458.18 eV and 456.8 eV suggesting the presence of Ti4+ (Liu, Yang, & Wang, Citation2009) and Ti3+ species (Di Valentin, Pacchioni, & Selloni, Citation2009). Herein, the predominance of Ti3+(defects) increases vs strontium loading in following order 10 and 14% for respectively 0.2 wt%Sr-NT and 0.8 wt%Sr-NT. From , the ratio of O2-/Ti4+ increases versus strontium loading and confirms the formation of surface defects (Ti3+); being consistent with the results of PL measurements and ascribed to lattice distortion as described by X-ray diffraction. XPS peaks of Sr 3d found at 134.2 eV in is attributed to electron binding energy of Sr 3d5/2, confirming the presence of Sr2+ (Wang et al., Citation2021) as identified by SEM mapping. Moreover, it must be pointed out that experimental ratio between the intensities of XPS peaks of Sr 3d5/2 for 0.8 wt% Sr and 0.2 wt% Sr () is equal to 3.45, which is lower than the theoretical one of 4. This result can be explained by possible Sr ions migration inside the bulk.
Figure 7. XPS spectra of Sr-free and 0.2 wt% Sr and 0.8 wt%Sr-doped NT nanomaterials calcined at 400 °C: (a) full scan survey of all elements and high-revolution spectra of (b) O 1 s (c) Ti 2p3/2, (d) Sr 3d5/2.
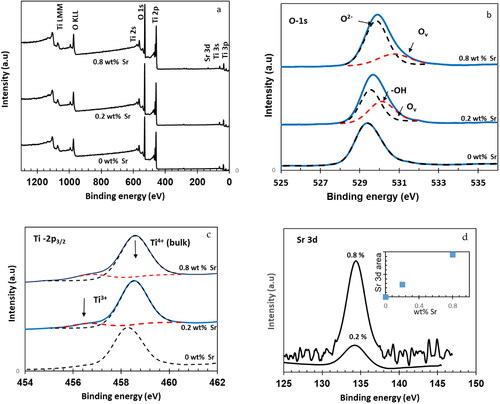
Table 2. XPS results of different xwt% Sr-NT nanomaterials: surface state, binding energy, predominance, molar ratio O2-/Ti4+.
3.8. Photocatalytic properties
3.8.1. MB photodegradation kinetics
The photodegradation of MB onto xwt% Sr-NT is investigated under visible light radiation.
The photodegradation rate of MB can be expressed as
(3)
(3)
In order to investigate the reaction kinetics, we apply to the experimental data to two kinetic models: (i) pseudo-first order and (ii) pseudo- second order. The results are summarized in .
Table 3. Rate constant values (k1 and k2) obtained using pseudo-first order and pseudo-second order models and MB photodegradation.
illustrate the kinetics parameters obtained from two models. The correlation coefficient R2 values of pseudo-first-order model are tad-higher with respect to those of pseudo-second order model. Hence, we can assume that the kinetic parameters of MB photodegradation over xwt%Sr-NT catalysts are best interpreted by pseudo-first order kinetic model. From , it seems that k1 (min−1) increases versus Sr loading. The direct photolysis of MB (2%) is quite low, the MB photodegradation increases in the following order: 0.2 wt%Sr-NT< 0.4 wt%Sr-NT <0.6 wt%Sr-NT <1wt%Sr-NT <0.8 wt%Sr-NT. An optimum MB degradation (56%) is observed for 0.8 wt% Sr-NT. Therefore, the photocatalytic activity is boosted mainly due to low recombination rate prompted by possible formation of heterojunction between Sr and TiO2-MB. The photogenerated (electron-hole) pairs are most probably separated on the interface between ferroelectric strontium and TiO2. Herein, the introduction of Sr ions induced in the creation of oxygen vacancies (Ov) as demonstrated earlier by PL and XPS measurements. The oxygen vacancies act as traps for photogenerated electrons which results in increase of holes lifetime. Nevertheless, we cannot discard the hypothesis that strontium might create local electric field which limits the recombination of photogenerated (e-hole) pairs and results in enhanced MB photodegradation.
3.8.2. MB photodegradation isotherms-Langmuir-Hinshelwood (LH) kinetic model
The photodegradation of MB is followed at different concentrations. Herein, the initial rate (r0) is plotted versus the concentration of MB at the equilibrium (Ce) ().
Figure 8. Kinetic study of MB photodegradation: rate vs equilibrium concentration for a. 0 wt% Sr, b.0.2 wt% Sr, c. 0.4 wt% Sr, d. 0.6 wt% Sr, e. 0.8 wt% Sr and f. 1.0 wt% Sr.
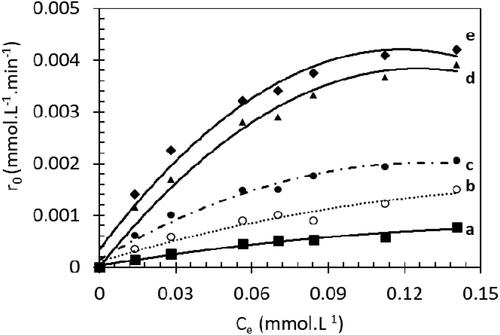
reveals that the degradation rate (r0) increases versus MB concentration and reaches a plateau. We can highlight mainly two domains: at low MB concentration (30 mg.L−1 ∼ 0.08 mmol.L−1), r0 increases linearly with MB concentration which fits with simplified LH model (1 ≫ k KLH Ce) EquationEq. (2)(2)
(2) . Then a first order kinetic is observed.
For high MB concentrations, ( 1 ≪ k KLH Ce), the initial rate is zero order to MB (15) and a plateau is reached.
The linearization of EquationEq. (2)(2)
(2) ( in supplementary information) indicates that our results fit well with Langmuir-Hinshelwood model (L-H). In addition, LH model was found to be successful for the degradation of several organic compounds (Zhang, Yang, Xie, Zhang, & Li, Citation2009).
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
(6)
(6)
with r0 is the initial rate of MB disappearance (mmol.L−1.min−1), k is the rate constant (mmol.L−1.min−1), KLH is the adsorption constant under visible conditions (L.mmol−1), and Ce is MB concentration at the adsorption equilibrium (mmol.L−1) and θ is the surface coverage . The kinetic parameters (kLH, KLH and θ) are determined from the linearization form of LH model (2) ( and in supplementary information).
Table 4. Rate constant (kLH), Adsorption constant (KLH) values and surface coverage θ determined using a Langmuir–Hinshelwood model for the Methylene Blue photodegradation.
From , the photocatalytic activities are boosted by increasing strontium loading onto TiO2 nanotubes (NT) nanomaterials. 0.8 Sr-NT catalyst shows kLH value 28 times higher compared to photolysis reaction; the MB degradation reached 56%. The photocatalytic activities vary in the order: 0.2 wt% Sr-NT <0.4 wt% Sr-NT < 0.6 wt% Sr-NT < 1Sr wt%-NT< 0.8 wt% Sr NT. Above 0.8 wt%Sr, the activity slightly decreases by possible charges accumulation.
Our results indicate the beneficial effect of Strontium doped TiO2 NTs. In fact, at the same surface coverage (θ = 0.999), the rate of MB degradation is reflected by enhancement of kLH values vs strontium loading ( in supplementary information).
The degradation kinetics of MB under visible light can be described by the apparent first-order kinetics model. Wu et al. Citation2017 has demonstrated that pseudo-first order of MB photodegradation onto g-C3N4 -RGO-TiO2 is about 4.7 and 3.2 times higher than those of the pure g-C3N4 (0.0029 min−1) and direct Z-scheme g-C3N4-TiO2 (0.0043 min−1), respectively. The photocatalytic activity of Bi2MoO6/TiO2 nanofiber heterojunction film is enhanced by increasing Bi2MoO6 loading (Li et al., Citation2017). Hence, 0.08Bi2MoO6/TiO2 nanofiber (0.035 min−1) is about 25 and 2.8 times more active than pure TiO2 nanofiber (0.0043 min−1) and 0.02 Bi2MoO6/TiO2 (0.0123 min−1) respectively. Recently, Waimbo et al. Citation2020 has demonstrated that the photodegradation of MB onto CuWO4 nanoparticles under visible light shows a pseudo-rate constant of 0.00235 min−1 using H2O2 as oxidant. In this study, the generated OH radicals act as the dominant reactive oxygen species (ROS) and is responsible for the degradation of MB until complete mineralization into carbon dioxide (CO2) and water (Zong et al. Citation2021, Okla et al. Citation2022). 0.8 wt%-NT catalyst shows good activity in absence of hydrogen peroxide, thus Sr-NT materials will be promising candidate for water treatment in the industrial effluents at low cost.
Furthermore, to avoid any competitive adsorption between MB molecule and its by-products onto the surface of xwt%Sr-TiNT materials; the formic acid (HCOOH) was selected as target pollutant. Because of its simple chemical formula (C1), the degradation leads to the formation of carbon dioxide (CO2) and water. In addition to that, Formic Acid is one of the final intermediate products of most of the organic pollutant degraded, so it could be interesting to learn about the kinetic photodegradation of this by-product. The properties of Sr-NT nanomaterials under UV-A light, subsequently, formic acid photodegradation was investigated onto NT, 0.2 wt%Sr-NT and 0.8 wt%Sr-NT materials (). The predominance of Ti species (Ti4+, Ti3+) and oxygen vacancies obtained from XPS are summarized in .
Figure 9. (a) Variation of C/C0 in the photodegradation of formic acid (FA, 100 mg.L−1) under UV-A light, (b) predominance of Ti species (Ti4+, Ti3+) and oxygen vacancies (Ov).
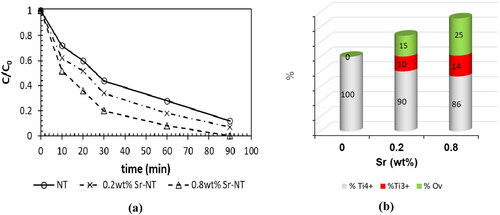
From , the initial photodegradation rate is obviously enhanced in presence of Sr. In fact, the initial rate increases in the following order: NT< 0.2 wt%Sr-NT < 0.8 wt%Sr-NT; here NT material is taken as reference catalyst. The catalyst 0.8 wt%Sr-NT shows a degradation rate of 98 mmol.L−1.min−1 against 51 mmol.L−1.min−1 for 0.2 wt%Sr-NT. Therefore, the photocatalytic activity was enhanced mainly by factor of two due to the high efficiency of charge separation induced by the synergistic effect between Sr and TiO2. , shows that the percentages of defects (Ti3+) and oxygen vacancies (from XPS) increase vs strontium loading. The highest amount of oxygen vacancies is obtained for 0.8 wt% Sr-NT catalyst. Therefore, the photocatalytic properties are indirectly affected by strontium ions. Hence, the rate of recombination of photogenerated electron-hole decreases due to the creation of oxygen vacancies onto Sr-NT nanomaterials and results in electron trapping. Most notably, the holes are used to produce hydroxyl radicals (OH°) which are highly reactive electrophilic oxidants. So, they decompose directly formic acid to CO2 and H2O.
For better understanding, the electrical properties of Sr-NT materials was thoroughly discussed in the next paragraph.
3.9. Electrical properties
To attempt to explain of the changes in catalytic properties between 0.2 wt%-Sr NT and 0.8 wt%Sr-NT catalysts, additional impedance measurements were performed. The complex impedance measurements were performed at room temperature by scanning from low to high frequencies (from 0.1 Hz to 1 MHz) using a voltage of 0.5 V. The experimental and theoretical curves were summarized in .
Figure 10. Experimental and theoretical impedance diagrams of the 0.2 wt% Sr-NT and 0.8 wt% Sr-NT (Inset the corresponding equivalent circuit).
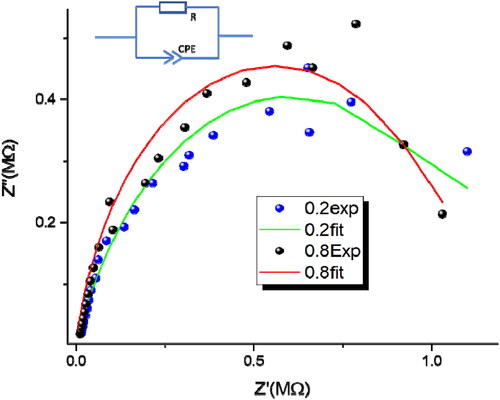
Figure10 shows the electrical response of Sr-NT materials by the Nyquist diagram, the points representative of the complex impedance Z are arranged in an approximately circular arc. The equivalent electrical circuit of the Sr-NT catalysts (shown in the inset of ). The impedance model cannot be often represented by combining resistance (R) and capacitance (C) circuit. However, Constant Phase Element CPE are usually regarded as circuit-fitting parameters (Liu, Citation1985). The application of R-CPE circuit (instead of R-C circuit) allowed researchers to overcome the distortion of the impedance semicircles due to the inhomogeneities of defects distribution and to grain boundaries (Komornicki, Radecka, & Rekas, Citation2001)
The total impedance of the circuit is given by EquationEq. (7)(7)
(7)
(7)
(7)
where the impedance of the CPE is defined via EquationEq. (8)
(8)
(8) (Jorcin, Orazem, Pébère, & Tribollet, Citation2006):
(8)
(8)
where (
) and
is the angular frequency (
),
is a constant independent of frequency, and
is a dimensionless parameter determining the degree of deviation from an exact semicircle (Sdiri, Elhouichet, Azeza, & Mokhtar, Citation2013). When n = 1, EquationEq. (7)
(7)
(7) yields the impedance of a capacitor, where
The resistance
is the intercept of the impedance curve with
axis. The experimental semicircles have been fitted by the ORIGINLAB software based on the following relationships.
(9)
(9)
(10)
(10)
From , the maximum Imaginary impedance spectra Z″ slightly shifted to high frequency by increasing the percentage of Sr. The modeling of the impedance results gives us the parameters of the equivalent circuit ().
Table 5. Impedance results obtained by fitting the experimental data to the equivalent electrical circuit shown in .
From , a slight decrease in resistance (8%) with simultaneously marked increase in capacitor are observed when going from 0.2 wt%Sr-NT to 0.8 wt% Sr-NT. While the electron lifetime τ increases from 269 μs to 5450 μs. Herein, τ parameter could be considered as the time required to discharge the capacitor. This means that the τ parameter also reflects the time that photogenerated charges remain available at the semiconductor interface for generating reactive oxygen species (ROS) able to degrade organic pollutants such as: formic acid and MB. This observation helps us to understand the enhancement of photocatalytic activity as observed in the photodegradation of MB under visible light and FA under UV-A light. For MB photodegradation, kLH=0.0054 mmol.L−1.min−1 is obtained for 0.8 wt%Sr-NT against 0.0013 mmol.L−1.min−1 for 0.2 wt%Sr-NT. A high charges accumulation is obtained with 0.8 wt%Sr-NT (high capacitor) due to highest number of oxygen vacancies (identified by PL and XPS). In addition, the conductivity increases slightly from 0.2 Sr to 0.8 Sr (the reverse of resistance value).
4. Conclusion
In the present study, the addition of low amounts of strontium to TiO2 nanotubes was herein studied in order to determine its potential role in enhancing the photooxidation ability of 1 D titanium oxide semiconductor. Attention was therefore paid here to the influence of strontium on the structural, textural, optical, morphological and electrical properties of the resulting materials named as xSr-NT, with x between 0.2 and 1.0 wt% Sr. Results emphasize interesting textural and structural properties of xSr-NT nanomaterials. X-ray diffraction patterns shows that Sr doping induces slight shift of the main (101) diffraction peak of the anatase phase, indicating a possible distortion in the TiO2 lattice in good agreement with Raman results. UV-visible DRS highlights the absence of any Eg shift (3.43 ± 0.02 eV) as expected if SrTiO3 is formed at the surface of TiO2. While the photoluminescence and XPS analysis show that the role of strontium is mainly to generate surface oxygen vacancies able to trap photogenerated electrons and leaving photogenerated holes available for oxidation reactions. Finally, photodegradation of MB yields to mineralization and the electric properties of xSr-NT clearly show that photogenerated charges remain available for longer time at the semiconductor interface for generating reactive oxygen species.
Credit authorship contribution statement
Dr. Nuhad Alomair contributed in conceptualization, methodology, investigation and writing original draft and approving final version.
Supplemental Material
Download MS Word (719.6 KB)Acknowledgments
The author would like to acknowledge the funding (IF-2020-019-BASRC), Imam Abdulrahman Bin Faisal University, Basic and Applied Scientific Research Centre obtained from Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahmad, A., Mohd-Setapar, S. H., Chuong, C. S., Khatoon, A., Wani, W. A., Kumar, R., & Rafatullah, M. (2015). Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Advances, 5(39), 30801–30818. doi:10.1039/C4RA16959J
- Alencar, L., Passos, L. M. S., Soares, C. M. F., Lima, A. S., & Souza, R. L. (2020). Efficiency method for methylene blue recovery using aqueous two-phase systems based on cholinium-ionic liquids. Journal of Fashion Technology & Textile Engineering, 6, 13–20. doi:10.19080/CTFTTE.2020.06.555678
- Alkaim, A. F., Aljeboree, A. M., Alrazaq, N. A., Baqir, S. J., Hussein, F. H., & Lilo, A. J. (2014). Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian Journal of Chemistry, 26(24), 8445–8448. doi:10.14233/ajchem.2014.17908
- Alkaykh, S., Mbarek, A., & Ali-Shattle, E. E. (2020). Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon, 6(4), e03663. 10.1016/j.heliyon.2020.e03663.
- Azeez, F., Al-Hetlani, E., Arafa, M., Abdelmonem, Y., Nazeer, A. A., Amin, M. O., & Madkour, M. (2018). The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Scientific Reports, 8(1), 7104. 10.1038/s41598-018-25673-5.
- Barnes, R. J., Molina, R., Xu, J., Dobson, P. J., & Thompson, I. P. (2013). Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria. Journal of Nanoparticle Research, 15(2), 1432. doi:10.1007/s11051-013-1432-9
- Bilińska, L., & Gmurek, M. (2021). Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actionsdye by-products removal by catalytic and synergistic actions. Water Resources and Industry, 26, 100160. doi:10.1016/j.wri.2021.100160
- Chakhtouna, H., Benzeid, H., Zari, N., Qaiss, A. E. K., & Bouhfid, R. (2021). Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environmental Science and Pollution Research International, 28(33), 44638–44666. doi:10.1007/s11356-021-14996-y
- Choudhury, B., Borah, B., & Choudhury, A. (2013). Ce–Nd codoping effect on the structural and optical properties of TiO2 nanoparticles. Materials Science and Engineering: B, 178(4), 239–247. doi:10.1016/j.mseb.2012.11.017
- Derakhshan, Z., Baghapour, M. A., Ranjbar, M., & Faramarzian, M. (2013). Adsorption of methylene blue dye from aqueous solutions by modified pumice stone: kinetics and equilibrium studies. Health Scope, 2(3), 136–144. doi:10.17795/jhealthscope-12492
- Di Valentin, C., Pacchioni, G., & Selloni, A. (2009). Reduced and n type doped TiO2: Nature of Ti3+ species. The Journal of Physical Chemistry C, 113(48), 20543–20552. doi:10.1021/jp9061797
- Hamisu, A., Gaya, U., & Gaya, A. (2020). Effect of alkali strength on the hydrothermal growth of photoactive TiO2 nanowires. Journal of Nanostructures, 10(3), 639–651. doi:10.22052/JNS.2020.03.017
- Janczarek, M., & Kowalska, E. (2021). Defective dopant-free TiO2 as an efficient visible light-active photocatalyst. Catalysts, 11(8), 978. doi:10.3390/catal11080978
- Jorcin, J. B., Orazem, M. E., Pébère, N., & Tribollet, B. (2006). CPE analysis by local electrochemical impedance spectroscopy. Electrochimica Acta, 51(8–9), 1473–1479. doi:10.1016/j.electacta.2005.02.128
- Keerthana, S. P., Yuvakkumar, R., Ravi, G., Hong, S. I., Al-Sehemi, A. G., & Velauthapillai, D. (2022). Fabrication of Ce doped TiO2 for efficient organic pollutants removal from wastewater. Chemosphere, 293, 133540. doi:10.1016/j.chemosphere.2022.133540
- Komornicki, S., Radecka, M., & Rekas, M. (2001). Frequency-dependent electrical properties in the system SnO2-TiO2. Journal of Materials Science: Materials in Electronics, 12(1), 11–16. doi:10.1023/A:1011208310147
- Lei, Y., Zhang, L. D., Meng, G. W., Li, G. H., Zhang, X. Y., Liang, C. H., … Wang, S. X. (2001). Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Applied Physics Letters, 78(8), 1125–1127. doi:10.1063/1.1350959
- Li, W., Liang, R., Zhou, N. Y., & Pan, Z. (2020). Carbon black-doped anatase TiO2 nanorods for solar light-induced photocatalytic degradation of methylene blue. ACS Omega, 5(17), 10042–10051. doi:10.1021/acsomega.0c00504
- Li, F. B., Li, X. Z., Hou, M. F., Cheah, K. W., & Choy, W. C. H. (2005). Enhanced photocatalytic activity of Ce3+-TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control. Applied Catalysis A: General, 285(1-2), 181–189. doi:10.1016/j.apcata.2005.02.025
- Liu, S. H. (1985). Fractal model for the ac response of a rough interface. Physical Review Letters, 55(5), 529–532. doi:10.1103/PhysRevLett.55.529
- Liu, L., Jiang, W., Song, X., Duan, Q., & Zhu, E. (2020). A novel strategy of lock-in effect between conjugated polymer and TiO2 towards dramatic enhancement of photocatalytic activity under visible light. Scientific Reports, 10(1), 6513. doi:10.1038/s41598-020-63623-2
- Liu, J., Li, J., Sedhain, A., Lin, J., & Jiang, H. (2008). Structure and photoluminescence study of TiO2 nanoneedle texture along vertically aligned carbon nanofiber arrays. The Journal of Physical Chemistry C, 112(44), 17127–17132. doi:10.1021/jp8060653
- Liu, X., Lv, S., Fan, B., Xing, A., & Jia, B. (2019). Ferroelectric polarization-enhanced photocatalysis in BaTiO3-TiO2 core-shell heterostructures. Nanomaterials, 9(8), 1116. doi:10.3390/nano9081116
- Liu, G., Yang, H. G., Wang, X., Cheng, L., Lu, H., Wang, L., … Cheng, H.-M. (2009). Enhanced photoactivity of oxygen-deficient anatase TiO2 sheets with dominant {001} facets. The Journal of Physical Chemistry C, 113(52), 21784–21788. doi:10.1021/jp907749r
- Li, H., Zhang, T., Pan, C., Pu, C., Hu, Y., Hu, X., … Fan, J. (2017). Self-assembled Bi2MoO6/TiO2 nanofiber heterojunction film with enhanced photocatalytic activities. Applied Surface Science, 391, 303–310. doi:10.1016/j.apsusc.2016.06.167
- Lu, D., Wang, H., Zhao, X., Kondamareddy, K. K., Ding, J., Li, C., & Fang, P. (2017). Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen. ACS Sustainable Chemistry & Engineering, 5(2), 1436–1445. doi:10.1021/acssuschemeng.6b02010
- Machado, W. A., & da Hora Machado, A. E. (2020). Characterization and evaluation of the photocatalytic activity of oxides based on TiO2 synthesized by hydrolysis controlled by the use of water/acetone mixtures. PeerJ Materials Science, 2, e11. doi:10.7717/peerj-matsci.11
- Meksi, M., Turki, A., Kochkar, H., Bousselmi, L., Guillard, C., & Berhault, G. (2016). The role of lanthanum in the enhancement of photocatalytic properties of TiO2 nanomaterials obtained by calcination of hydrogenotitanate nanotubes. Applied Catalysis B: Environmental, 181, 651–660. doi:10.1016/j.apcatb.2015.08.037
- Mohammed Redha, Z., Abdulla Yusuf, H., Amin, R., & Bououdina, M. (2020). The study of photocatalytic degradation of a commercial azo reactive dye in a simple design reusable miniaturized reactor with interchangeable TiO2 nanofilm. Arab Journal of Basic and Applied Sciences, 27(1), 287–298. doi:10.1080/25765299.2020.1800163
- Ohtani, B. (2010). Photocatalysis A to Z—What we know and what we do not know in a scientific sense. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 11(4), 157–178. doi:10.1016/j.jphotochemrev.2011.02.001
- Okla, M. K., Harini, G., Dawoud, T. M., Akshhayya, C., Mohebaldin, A., AL-ghamdi, A. A., … Khan, S. S. (2022). Fabrication of MnFe2O4 spheres modified CeO2 nano-flakes for sustainable photodegradation of MB dye and antimicrobial activity: A brief computational investigation on reactive sites and degradation pathway. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 641, 128566. doi:10.1016/j.colsurfa.2022.128566
- Pandey, S., Do, J. Y., Kim, J., & Kang, M. (2020). Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. International Journal of Biological Macromolecules, 143, 60–75. doi:10.1016/j.ijbiomac.2019.12.002
- Rao, H., Lu, Z., Liu, X., Ge, H., Zhang, Z., Zou, P., … Wang, Y. (2016). Visible light-driven photocatalytic degradation performance for methylene blue with different multi-morphological features of ZnS. RSC Advances, 6(52), 46299–46307. doi:10.1039/C6RA05212F
- Salama, A., Mohamed, A., Aboamera, N. M., Osman, T. A., & Khattab, A. (2018). Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Applied Nanoscience, 8(1-2), 155–161. doi.org/ doi:10.1007/s13204-018-0660-9
- Sarfraz, N., Khan, I., Sarfaraz, N., Khan, I., Sarfraz, N., & Khan, I. (2021). Plasmonic gold nanoparticles (AuNPs): properties, synthesis and their advanced energy, environmental and biomedical applications. Chemistry, an Asian Journal, 16(7), 720–742. doi:10.1002/asia.202001202.
- Scepanovic, M. J., Grujic-Brojcin, M., Dohcevic-Mitrovic, Z. D., & Popovic, Z. V. (2007). Temperature dependence of the lowest frequency Eg Raman mode in laser-synthesized anatase TiO2 nanopowder. Applied Physics A, 86, 365–371. doi:10.1007/s00339-006-3775-x
- Sdiri, N., Elhouichet, H., Azeza, B., & Mokhtar, F. (2013). Studies of (90-x) P2O5xB2O310Fe2O3 glasses by Mossbauer effect and impedance spectroscopy methods. Journal of Non-Crystalline Solids, 371–372, 22–27. doi:10.1016/j.jnoncrysol.2013.04.002
- Secundino-Sánchez, O., Díaz-Reyes, J., Sánchez-Ramírez, J. F., Arias-Cerón, J. S., Galván-Arellano, M., & Vázquez-Cuchillo, O. (2022). Controlled synthesis of electrospun TiO2 nanofibers and their photocatalytic application in the decolouration of Remazol Black B azo dye. Catalysis Today, 392–393, 13–22. doi:10.1016/j.cattod.2021.10.003
- Singh, R. K., Behera, S. S., Singh, K. R., Mishra, S., Panigrahi, B., Sahoo, T. R., … Mandal, D. (2020). Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. Journal of Photochemistry and Photobiology A: Chemistry, 400, 112704. doi:10.1016/j.jphotochem.2020.112704
- Waimbo, M., Anduwan, G., Renagi, O., Badhula, S., Michael, K., Park, J., … Kim, Y. S. (2020). Improved charge separation through H2O2 assisted copper tungstate for enhanced photocatalytic efficiency for the degradation of organic dyes under simulated sun light. Journal of Photochemistry and Photobiology. B, Biology, 204, 111781. doi:10.1016/j.jphotobiol.2020.111781
- Wang, Z., Gao, M., Li, X., Ning, J., Zhou, Z., & Li, G. (2020). Efficient adsorption of methylene blue from aqueous solution by graphene oxide modified persimmon tannins. Materials Science & Engineering. C, Materials for Biological Applications, 108, 110196. doi:10.1016/j.msec.2019.110196
- Wang, X., Hu, C., An, H., Zhu, D., Zhong, Y., Wang, D., … Zhou, H. (2021). Photocatalytic removal of MB and hydrogen evolution in water by (Sr0.6Bi0.305)2Bi2O7/TiO2 heterostructures under visible-light irradiation. Applied Surface Science, 544, 148920. doi:10.1016/j.apsusc.2020.148920
- Wu, F., Li, X., Liu, W., & Zhang, S. (2017). Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nano hetero junctions. Applied Surface Science, 405, 60–70. doi:10.1016/j.apsusc.2017.01.285
- Xue, W., Zhang, G., Xu, X., Yang, X., Liu, C., & Xu, Y. (2011). Preparation of titania nanotubes doped with cerium and their photocatalytic activity for glyphosate. Chemical Engineering Journal, 167(1), 397–402. doi:10.1016/j.cej.2011.01.007
- Zhang, J., Bang, J. H., Tang, C., & Kamat, P. V. (2010). Tailored TiO2-SrTiO3 heterostructure nanotube arrays for improved photoelectrochemical performance . ACS Nano, 4(1), 387–395. doi:10.1021/nn901087c
- Zhang, Z., Jing, W., Tan, X., Yu, T., & Ma, J. (2018). High-efficiency photocatalytic performance of Cr–SrTiO3-modified black TiO2 nanotube arrays. Journal of Materials Science, 53(8), 6170–6182. doi:10.1007/s10853-017-1977-6
- Zhang, Z., Li, X., Chen, H., Shao, G., Zhang, R., & Lu, H. (2018). Synthesis and properties of Ag/ZnO/g-C3N4 ternary micro/nanocomposites by microwave-assisted method. Materials Research Express, 5(1), 015021. doi:10.1088/2053-1591/aaa1dc
- Zhang, L., Yang, H., Xie, X., Zhang, F., & Li, L. (2009). Preparation and photocatalytic activity of hollow ZnSe microspheres via Ostwald ripening. Journal of Alloys and Compounds, 473(1-2), 65–70. doi:10.1016/j.jallcom.2008.06.018
- Zhu, L., Hong, M., & Ho, G. W. (2015). Fabrication of wheat grain textured TiO2/CuO composite nanofibers for enhanced solar H2 generation and degradation performance. Nano Energy, 11, 28–37. doi:10.1016/j.nanoen.2014.09.032
- Zong, M., Song, D., Zhang, X., Huang, X., Lu, X., & Rosso, K. M. (2021). Facet-dependent photodegradation of methylene blue by hematite nanoplates in visible light. Environmental Science & Technology, 55(1), 677–688. doi:10.1021/acs.est.0c05592