Abstract
Objective: Ultrasound (US) is more sensitive and reliable than a clinical examination, and is better correlated with the disease activity in rheumatoid arthritis (RA). We conducted the present study to assess the value of US as a screening tool to predict therapeutic responses in RA patients treated with anti-tumor necrosis factor (TNF) drugs.
Methods: We retrospectively analyzed the cases of 86 consecutive RA patients who were classified by their DAS28-CRP scores at the 54th week. We assessed two US findings (i.e., the synovial hypertrophy index [SHI] and synovial vascularization) by grey-scale imaging and the Doppler synovitis index (DSI).
Results: When we applied cut-off points determined by a ROC curve analysis, patients with a lower total SHI (≤34) or DSI (≤7) at baseline were significantly more likely to reach remission (44 patients, 51.2%) as shown by the DAS28-CRP at 54 weeks. On the basis of these cut-off values, we dichotomized all variables and performed a logistic regression analysis using the 54-weeks data; the only predictive factors of remission with anti-TNF therapy were the patients' baseline DAS28-CRP ≤2.7 as low disease activity/remission, and the SHI.
Conclusion: An ultrasound assessment would be a highly useful predictor of the achievement of clinical remission.
1. Introduction
Biological disease-modifying antirheumatic drugs (bDMARDs) are changing the treatment landscape for patients with rheumatoid arthritis (RA), resulting in considerable and rapid clinical improvement in patients with active disease [Citation1]. Treatment monitoring is a challenge in daily rheumatological practice, and the development of new tools for assessing the activity of rheumatoid synovitis is a relevant topic of research in rheumatology [Citation1]. Currently, ultrasound (US) with the quantifications of joint B-mode synovitis (by grey scale [GS] imaging) and vascularization (by power Doppler [PD] imaging) is being considered as an extension of the clinical examination in RA, because it provides a direct visualization and assessment of synovitis [Citation2] and is useful for monitoring the response to RA therapy [Citation3]. Some studies have also shown the predictive value of US-detected subclinical synovitis — mainly the synovial PD signal — in relation to both radiographic damage progression and disease flare or relapse during RA therapy [Citation4–7].
However, a recent study investigating US predictors for clinical remission did not show US to be predictive for clinical remission [Citation8]. In the present study, to explore the usefulness of US in evaluating US-detected synovitis in RA patients before starting antitumor necrosis factor (TNF) treatment, we investigated the relationship between clinical remission and nonremission at 54 weeks by using the DAS28-CRP scores of a series of RA patients. We sought to determine the precise value of US as a screening tool to predict the therapeutic response in RA patients undergoing anti-TNF treatment.
2. Materials and methods
2.1. Patients
We retrospectively analyzed the cases of a total of 86 consecutive RA patients (65 women, 21 men) who fulfilled the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria [Citation9] from January 2013 to December 2017 when they began treatment with an anti-TNF drug (i.e., etanercept, adalimumab, infliximab, certolizumab pegol, or golimumab) at the Kindai University School of Medicine. We analyzed the patients' clinical and laboratory data, treatments (including dosages), the percentages of patients being treated with methotrexate (MTX) and prednisolone (PSL) along with the MTX and PSL dosages used, and US findings, at both baseline and at 54 weeks.
2.2. Compliance with ethical standards
This study was conducted according to the principles expressed in the Helsinki Declaration of 1983, and it was approved by the Research Ethics Committee of Kindai University of Medicine. For this retrospective cohort study, patient consent was not required.
2.3. Demographic characteristics and the assessment of clinical and laboratory data
The patients’ demographic characteristics recorded at baseline included age, sex, disease duration, and current therapy. The clinical data recorded included the 28-swollen joint count (SJC) and 28-tender joint count (TJC), and the score on a patient visual analogue scale (ptVAS) at baseline and 54 weeks. Each patient's score on the Health Assessment Questionnaire Disability Index (HAQDI) at baseline was also recorded. Each patient's CRP level was measured at baseline and at 54 weeks. The erythrocyte sedimentation rate (ESR), matrix metalloproteinase-3 (MMP-3), rheumatoid factor (RF), and anti-cyclic citrullinated peptide antibody (ACPA) values were measured at baseline.
2.4. Definitions of disease activity
The established definitions in the evaluation of disease activity were used in this study. With respect to the DAS28-CRP disease activity categories [Citation10], we divided the patients into those at remission (DAS28-CRP <2.3) and nonremission (low, 2.3 < DAS28-CRP <2.7; moderate, DAS28-CRP 2.7–4.1; high, DAS28-CRP >4.1).
2.5. US assessment
The ultrasound equipment used was a GE Healthcare Venue 40 ultrasound system with a linear probe at 8–13 MHz and a Doppler frequency of 5–6.7 MHz. Each US examination for synovial hypertrophy (SH) and vascularization by grey-scale imaging and PD assessed 48 sites in 28 joints () as described by the Outcome Measures in Rheumatoid Arthritis Clinical Trial with a semiquantitative scale from 0 to 3 [Citation11]. At each joint region, the SH and the synovial PD were each scored semi-quantitatively on a scale of 0–3 (0, absent; 1, mild; 2, moderate; 3, marked). These scores corresponded to the maximum score for SH and the PD signal, respectively, obtained from any one of the synovial sites (i.e., a recess or joint) evaluated at each joint/joint region.
Table 1. Bilateral synovial intra-articular recesses and periarticular sites evaluated by ultrasound in bilateral joints.
Each US examination at baseline and at 54 weeks was performed by two rheumatologists in interpreting US findings, respectively (Y.N. and A.I.) who were blinded to all study findings. For each patient, we calculated a global index for SH (i.e., the SHI) and an index for Doppler synovitis (the DSI). The sum of the SHI and synovial PD signal scores obtained for each evaluated joint region, at 48 sites in 28 joints resulted in possible scores of 0–144 for the SHI and 0–144 for the DSI.
We used the kappa coefficient to evaluate the interobserver and intraobserver agreement of the US scores provided by the two rheumatologists based on their reading of captured images. The kappa coefficients of inter-observer reliability between the two observers were 0.78 for the SHI values and 0.91 for the PD signal scores. The intra-observer reliability assessment was good: kappa values 0.90 and 0.88 for the SHI and 0.93 and 0.88 for the PD signal scores by Y.N. and A.I., respectively.
2.6. Statistical analyses
The statistical analyses were performed using JMP ver. 13.0 software. For the continuous variables, summary statistics of mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate are presented. Categorical variables are presented as percentages. Comparisons between independent means were analyzed using the Mann-Whitney U-test. Relationships between categorical variables were evaluated by the chi-square test. Spearman’s correlation coefficients were computed to evaluate univariable associations between the clinical finding (i.e., the DAS28-CRP score) and the US indexes (SHI and DSI) based on their distribution.
To assess the value of the single clinical score (the DAS28-CRP score) and the two US indexes as predictors of treatment response during the subsequent 54 weeks of treatment, we performed receiver operating characteristic (ROC) analyses, and we then used the best cut-off obtained in the ROC analysis to dichotomize variables to be included in a multivariable logistic regression analysis performed to determine the independent contribution of each variable to the prediction of remission. p values <.05 were considered significant.
3. Results
3.1. Patient population
The cases of a total of 86 patients with RA were analyzed. The patients’ baseline clinical and laboratory data and the details of their treatment are provided in . The mean age was 54.8 years, and the mean disease duration was 6.9 years. The mean DAS28-CRP score was 4.3, and the mean HAQDI was 1.2. All patients were treated with an anti-TNF drug (etanercept, 28.6%; adalimumab, 24.8%; infliximab, 20.9%; certolizumab pegol, 18.7%; and golimumab, 7%); 46.5% of the patients were treated with a median PSL dose of 3.7 mg day−1, and 80% of the patients were treated with a median MTX dose of 5.7 mg week−1 ().
Table 2. The 86 RA patients’ baseline clinical and laboratory data, and treatment information.
3.2. Treatment responses
illustrates the clinical and US findings of treatment responses among the 81 RA patients treated with an anti-TNF drug. In the clinical assessment, the SJC, TJC, and ptVAS values at 54 weeks were all significantly decreased compared to those at baseline, as shown by the following median [25th–75th centiles] or mean (SD) data. SJC: 5.4 [1.5–9] versus 1.6 [0.9–2.2], p < .005. TJC: 6.4 [2–8] versus 3.0 [4.4–1.6], p < .0001. ptVAS: 55.3 (25.9) versus 25.9 (25.5), p < .0001.
Figure 1. Comparisons of clinical disease activity and US indexes between baseline and 54 weeks in RA patients treated with an anti-TNF agent. At 54 weeks, the patients' swollen joint count (SJC), tender joint count (TJC), patient visual analogue scale (ptVAS), and the mean DAS28-CRP as clinical parameters were significantly decreased compared to their baseline values. As US parameters, the synovial hypertrophy index (SHI) and Doppler synovitis index (DSI) at 54 weeks were significantly decreased compared to baseline. Values are median [25th–75th centiles] or mean (SD), unless otherwise indicated. *p < .05, **p < .005, ***p < .0005, ****p < .0001 for the numerical values at baseline versus 54 weeks by Mann-Whitney U-test.
![Figure 1. Comparisons of clinical disease activity and US indexes between baseline and 54 weeks in RA patients treated with an anti-TNF agent. At 54 weeks, the patients' swollen joint count (SJC), tender joint count (TJC), patient visual analogue scale (ptVAS), and the mean DAS28-CRP as clinical parameters were significantly decreased compared to their baseline values. As US parameters, the synovial hypertrophy index (SHI) and Doppler synovitis index (DSI) at 54 weeks were significantly decreased compared to baseline. Values are median [25th–75th centiles] or mean (SD), unless otherwise indicated. *p < .05, **p < .005, ***p < .0005, ****p < .0001 for the numerical values at baseline versus 54 weeks by Mann-Whitney U-test.](/cms/asset/6cf43a43-7434-4c75-a5c5-dc89efaf1329/timm_a_1531192_f0001_b.jpg)
At 54 weeks, the mean DAS28-CRP score as a clinical measure of disease activity was significantly decreased compared to that at baseline: mean 4.2 (SD 1.3) versus 2.6 (1.5), p < 0.0001. Regarding the two US parameters, there were significant decreases in both the SHI and DSI at 54 weeks compared to baseline, as follows. SHI: 36.5 median [45.4–20.0 25th–75th centiles] versus 18.0 [24.0–13.0]. DSI: 6.5 [13.3–1.0] versus 0.0 [1.3–0.0], p < .005.
3.3. Relationship between the clinical parameter (DAS28-CRP score) and the US indexes (SHI and DSI) of disease activity
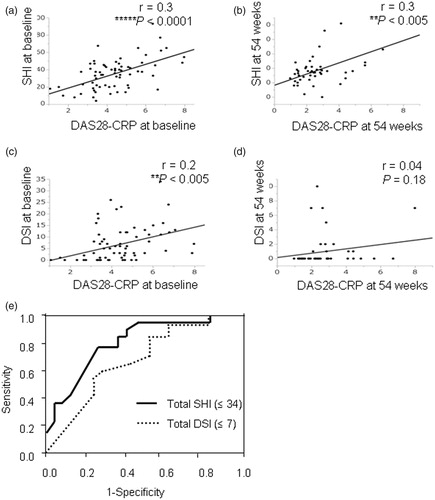
shows the patients’ DAS28-CRP and US data at baseline and 54 weeks. The SHI showed weak but significant correlations with the baseline and 54-week DAS28-CRP scores (r = 0.3, p < .0001 and p < .005; ). A weak correlation of the DSI with the DAS28-CRP score at baseline was also observed (r = 0.2, p < .005; ), but there was no correlation between the DSI and the DAS28-CRP score at 54 weeks (r = 0.04, p = .18; ).
Figure 2. Correlations between clinical disease activity and US indexes, and the ROC curves of US findings for predicting remission. The relationship of DAS28-CRP and US findings at baseline and 54 weeks. (A,B) The SHI showed a significant correlation with the DAS28-CRP score at baseline and 54 weeks. (C) A weak correlation of the DSI was found with the DAS28-CRP score at baseline. (D) There was no correlation of the DSI with the DAS28-CRP score at 54 weeks. (E) The SHI (i.e., ≤34) and DSI (i.e., ≤7) cut-off point at baseline provided the highest sensitivity and specificity for remission at 54 weeks (SHI; 86.3% and 71.5%, DSI; 77.2% and 48.8%, respectively). **p < .005, ****p < .0001 for the SHI and DSI versus DAS28-CRP by Spearman’s correlation.
3.4. Baseline factors predictive of RA remission
Remission was achieved at 54 weeks by 44 of the 86 patients (51.2%) (). No significant differences were revealed between the no-remission (n = 42) and remission (n = 44) groups at 54 weeks regarding age, gender, disease duration, the titers of RF or ACPA, the CRP level, the ESR, MMP-3, TJC, SJC, Pt-VAS, DAS28-CRP score or the dosages of PSL and MTX at baseline. However, the HAQDI scores at baseline showed a significant difference between the no-remission and remission groups: 1.3 (0.2) versus 0.8 (0.2), respectively; p < .005 (). In both the no-remission and remission groups, both the SHI and DSI recorded at baseline differed significantly from the corresponding index values obtained at 54 weeks as follows. SHI: No-remission, 41 [22–49.5] versus Remission, 32 [15.5–35.3], p < .005. DSI: No-remission, 6 [1–15] versus Remission, 1 [0–7], p < .05.
Table 3. Predictors of remission at baseline.
When we used the cut-off points determined by the ROC curve analyses (), the patients with a lower total SHI (≤34) or a lower DSI (≤7) at baseline were significantly more likely to reach remission as represented by the DAS28-CRP scores at 54 weeks compared to the patients with a higher total SHI or DSI at baseline. The SHI cut-off point (i.e., ≤34) provided 86.3% sensitivity and 71.5% specificity for remission at 54 weeks. The DSI cut-off point (i.e., ≤7) provided 77.2% sensitivity and 48.8% specificity for remission at 54 weeks.
Using these SHI and DSI cut-off points, we dichotomized all variables and performed a logistic regression. The results revealed that there were only two predictive factors of remission at 54 weeks: a baseline DAS28 ≤ 2.7 as low disease activity/remission, and an SHI ≤34 (). Female gender, RF positivity, and ACPA positivity were not significantly associated with remission at 54 weeks. The odds ratio (OR) [95% confidence interval] were 3.1 [1.5–11.2] for DAS28 ≤ 2.7 and 6.7 [1.9–27.4] for SHI ≤34.
Table 4. Association between remission at 54 weeks and US findings at baseline.
4. Discussion
Several studies have indicated that ultrasonography with grey-scale and power Doppler techniques has contributed to the detection and monitoring of synovitis in RA target joints [Citation2,Citation12,Citation13]. Other investigations revealed that changes in US findings are associated with the clinical and laboratory responses of RA patients to anti-TNF drugs [1Citation4–21]. Naredo et al. reported the predictive value of cumulative joint counts for the synovial PD signal in relation to radiologic progression in patients with early RA who started treatment with a DMARD [Citation22]. Cumulative counts of joints with the PD signal along with RF levels and laboratory markers of inflammation were described as predictors of radiologic progression [Citation14]. and were reported to reflect pathogenic destructive roles of persistent synovial angiogenesis and hypervascularization in rheumatoid joints [Citation23].
In contrast, Ten Cate et al. reported that ultrasound routinely performed at baseline provided no additional prognostic information when added to clinical, laboratory, and radiographic parameters for predicting failure to achieve DAS28 remission at 1 year in early RA patients treated to target. The absence of remission was associated only with a high DAS28 value at the time of diagnosis and RF positivity [Citation8]. In the present study, we observed significant improvements in the ultrasound parameters of SHI and DSI as part of the clinical and laboratory assessment of disease activity throughout the follow-up period. We were also able to revaluate the usefulness of ultrasound in evaluating the ultrasound-detected SHI as a predictor of the therapeutic response, by using the DAS28-CRP score at 54 weeks after the start of anti-TNF treatment. We speculate that the ultrasound findings described herein could also be analyzed by evaluating a greater number of joints and using the SHI and DSI in comparisons with previous reports [Citation8].
Unexpectedly, the baseline DSI was not identified as a significant predictor of therapeutic response in our present investigation. It remains unclear whether the DSI only reflects the present joint inflammatory activity or is truly a predictor of therapeutic response, since our study’s small sample size was insufficient for assessing significance.
The limitations in this study should be considered. The main limitations are the small sample size and short follow-up period of this retrospective cohort. The short length of the study period raises a question as to how long the ultrasound assessment conducted before the start of anti-TNF treatment would remain as a predictor of achieving clinical remission. Studies with larger numbers of patients and longer follow-up periods are needed to estimate the efficacy of bDMARDs and to establish the optimal ultrasound-based strategies.
Another limitation is related to the ultrasound examination itself. Ultrasonography is known to be observer-dependent and was performed in this study by two different observers. However, the observers performed several tests, and good reliability between them was confirmed, thus diminishing the risk of error in practice and interpretation. Before treatment strategies based on ultrasound assessments of the SHI and DSI before the start of anti-TNF treatment can be established, further studies are necessary.
In conclusion, our results suggest that a feasible therapeutic strategy is as follows: before beginning the administration of an anti-TNF drug in a patient with RA, an ultrasound assessment should be performed as a highly useful predictor of the achievement of clinical remission.
Acknowledgements
The authors thank K. Niki and M. Kawanishi for the expert technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheum. 2003;48:35–45.
- Naredo E, Bonilla G, Gamero F, et al. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis. 2005;64:375–381.
- Naredo E, Möller I, Cruz A, et al. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:2248–2256.
- Scire CA, Montecucco C, Codullo V, et al. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology. 2009;48:1092–1097.
- Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–2967.
- Foltz V, Gandjbakhch F, Etchepare F, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012;64:67–76.
- Saleem B, Brown AK, Quinn M, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis. 2012;71:1316–1321.
- Horton SC, Tan AL, Freeston JE, et al. Discordance between the predictors of clinical and imaging remission in patients with early rheumatoid arthritis in clinical practice: implications for the use of ultrasound within a treatment-to-target strategy. Rheumatology (Oxford). 2016;55:1177–1187.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581.
- Inoue E, Yamanaka H, Hara M, et al. Comparison of disease activity score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann Rheum Dis. 2007;66:407–409.
- Wakefield RJ, Balint PV, Szkudlarek M, et al; OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487.
- Wakefield RJ, Green MJ, Marzo-Ortega H, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63:382–385.
- Walther M, Harms H, Krenn V, et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–338.
- Taylor PC, Steuer A, Gruber J, et al. Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo-controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum. 2004;50:1107–1116.
- Hau M. High resolution ultrasound detects a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor alfa receptor (etanercept). Ann Rheum Dis. 2002;61:55–58.
- Ribbens C, André B, Marcelis S, et al. Rheumatoid hand joint synovitis: gray-scale and power Doppler US quantifications following anti-tumor necrosis factor-alpha treatment: pilot study. Radiology. 2003;229:562–569.
- Terslev L, Torp-Pedersen S, Qvistgaard E, et al. Effects of treatment with etanercept (Enbrel, TNRF:Fc) on rheumatoid arthritis evaluated by Doppler ultrasonography. Ann Rheum Dis. 2003;62:178–181.
- Fiocco U, Ferro F, Vezzù M, et al. Rheumatoid and psoriatic knee synovitis: clinical, grey scale, and power Doppler ultrasound assessment of the response to etanercept. Ann Rheum Dis. 2005;64:899–905.
- Filippucci E, Iagnocco A, Salaffi F, et al. Power Doppler sonography monitoring of synovial perfusion at the wrist joints in patients with rheumatoid arthritis treated with adalimumab. Ann Rheum Dis. 2006;65:1433–1437.
- Iagnocco A, Perella C, Naredo E, et al. Etanercept in the treatment of rheumatoid arthritis: clinical follow-up over one year by ultrasonography. Clin Rheumatol. 2008;27:491–496.
- Iagnocco A, Filippucci E, Perella C, et al. Clinical and ultrasonographic monitoring of response to adalimumab treatment in rheumatoid arthritis. J Rheumatol. 2008;35:35–40.
- Naredo E, Collado P, Cruz A, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: Predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57:116–124.
- Pap T, Distler O. Linking angiogenesis to bone destruction in arthritis. Arthritis Rheum. 2005;52:1346–1348.
