Abstract
To assess the efficacy and safety of switching to infliximab (IFX) from other biological disease-modifying anti-rheumatic drugs (bDMARDs) among Japanese patients with rheumatoid arthritis (RA) in daily practice. We examined 24 consecutive RA patients who had not achieved low disease activity (LDA) as the disease activity score-28 for rheumatoid arthritis with erythrocyte sedimentation rate (DAS28-ESR) despite previous bDMARD therapy in this cohort study. We attempted to determine predictive variables that are associated with achieving DAS28-ESR LDA at 22 weeks post-IFX introduction, by performing univariate analysis. The median DAS28-ESR at baseline was 5.41. Sixteen patients (66.7%) had been treated with a tumor necrosis factor inhibitor (TNF-i), and the other eight patients (33.3%) received a non-TNF-i (abatacept or tocilizumab). Nine patients (37.5%) achieved LDA or remission at 22 weeks. Univariate analyses showed that the variable to predict LDA achievement at 22 weeks was tender joints (>8 counts) at baseline (adjusted odds ratio, 0.10; 95% confidence interval, 0.01–0.63; p = .02). Nine adverse events were observed during the study period, and infection requiring hospitalization was observed in one patient. Switching to IFX is effective to achieve LDA or remission for RA patients refractory to bDMARDs.
1. Introduction
Infliximab (IFX) is the first biological disease-modifying anti-rheumatic drug (bDMARD) that became available for individuals with rheumatoid arthritis (RA) in Japan [Citation1,Citation2]. There is now abundant evidence showing a rapid response and a strong preventive effect on joint destruction in IFX treatment bio-naïve RA patients [Citation3–6].
According to the European League Against Rheumatism (EULAR) recommendations for the management of RA, both tumor necrosis factor-inhibitors (TNF-is) and bDMARDs with other modes of action are recommended equally for patients who failed to respond to their first treatment with a bDMARD [Citation7]. Although several studies showed the efficacy of switching from another TNF-i to IFX [Citation8–12], to the best of our knowledge, there no studies have evaluated the efficacy of switching from non-TNF agents to IFX in a comparison with TNF-i. Accordingly, the drug selection for RA patients who failed to respond to a first bDMARD treatment remains controversial and has been left to the discretion of the individual physicians.
In the present study, we assessed the efficacy and safety of switching to IFX from other bDMARDs among Japanese RA patients in daily clinical practice.
2. Materials and methods
2.1. Patients
In this cohort study, we examined the cases of 24 consecutive RA patients who had not achieved low disease activity (LDA) status according to DAS28-ESR (Disease Activity Score incorporating 28 joints and erythrocyte sedimentation rate)—LDA is defined as a score of ≥3.2 [Citation13]—despite undergoing bDMARD therapy with etanercept (ETN), adalimumab (ADA), golimumab (GLM), tocilizumab (TCZ) or abatacept (ABT). The patients were recruited from Nagasaki University Hospital, Sasebo Chuo Hospital, The Japanese Red Cross Nagasaki Genbaku Hospital, Japan Community Healthcare Organization, Isahaya General Hospital and Nagasaki Medical Hospital of Rheumatology from February 2006 through August 2015. The patients fulfilled the 1987 American College of Rheumatology (ACR) criteria [Citation14] or the 2010 ACR/EULAR rheumatoid arthritis classification criteria [Citation15]. They received IFX (3 mg/kg) at 0, 2, 6, 14 and 22 weeks. The dose escalation of IFX to 6 mg/kg was allowed after 14 weeks if DAS28-ESR remission had not been achieved according to medicinal application of the national insurance program in Japan. All of the patients were observed for 22 weeks after switching to IFX or until the last visit of IFX administration if the IFX was discontinued before 22 weeks.
All of the patients gave their informed consent to participate in the study. This study was approved by the Institutional Review Board of Nagasaki University Hospital (No. 140825272), and the Research Ethics Committees of Isahaya General Hospital (approved 11 November 2015), Sasebo Chuo Hospital (2014-37), Nagasaki Medical Hospital of Rheumatology (CR2013001) and Japanese Red Cross Nagasaki Genbaku Hospital (No. 375).
2.2. Clinical and laboratory assessments
We collected the patients' demographic information at the administration of IFX including age, sex, disease duration, Steinbrocker radiographic stage and functional class [Citation16], concomitant use of methotrexate (MTX), prednisolone (PSL) or other conventional synthetic disease modified anti-rheumatic drug (csDMARD), the 28-joint swollen joint count (SJC28), the 28-joint tender joint count (TJC28), the Modified Health Assessment Questionnaire (MHAQ) [Citation17] and blood tests including rheumatoid factor (RF; cut-off value 14 IU/ml), anti-cyclic citrullinated peptide antibodies (ACPA; cut-off value 4.5 U/ml), C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR).
The primary endpoint in this study was the rate of achievement of LDA or remission (DAS28-ESR <3.2) at week 22 after the introduction of IFX. We attempted to determine the predictive variables that are associated with the achievement of DAS28-ESR LDA or remission at 22 weeks by performing multiple logistic regression analyses. We also analyzed the DAS28-CRP, the Simplified Disease Activity Index (SDAI) [Citation18], the Clinical Disease Activity Index (CDAI) [Citation19] and the proportion of patients who achieved a EULAR 'good' or 'moderate' response based on DAS28-ESR [Citation20] at weeks 0, 2, 6, 14 and 22. A good EULAR response is defined as a decrease in the DAS28-ESR of >1.2 points, resulting in a score of ≤3.2. A moderate EULAR response is defined as a decrease of >0.6 and resulting in a score of <5.1 points [Citation20]. To assess the safety of IFX, we also analyzed the adverse events during the observation period.
2.3. Statistical analysis
Continuous variables are shown as median ± quartile. Fisher’s exact test was used to compare categorical variables. To determine the predictive variables associated with the achievement of DAS28-ESR LDA or remission at 22 weeks, we performed univariate analyses. The cut-off values in univariate analyses were determined by the following methods (determined by the ROC curve: age, disease duration, MTX dosage, tender and swollen joint, patient's and evaluator's global VAS, CRP and ESR; determined by the clinical significance: DAS-CRP, DAS-ESR, SDAI, CDAI and MHAQ). The last observation carried forward (LOCF) method was applied when patients discontinued the IFX. JMP® 11 software (SAS Institute, Cary, NC) was used for all statistical analyses. p-values <.05 were considered significant.
3. Results
3.1. Patient characteristics
The patients' demographic and clinical characteristics are summarized in . The median age was 58 years, and the median disease duration was 11.5 years. The median MTX dosage was 8 mg/week, and the median DAS28-ESR at baseline was 5.41. Seventeen patients (70.8%) were RF-positive, 18 (75%) were ACPA-positive, 16 (66.7%) had been treated with a TNF-i (ETN or ADA or CZP or GLM) as the previous bDMARD and the other eight patients (33.3%) had been treated with a non-TNF-i (ABT or TCZ). Three patients (12.5%) had been treated with a csDMARD (salazosulfapyridine or tacrolimus) in addition to MTX. There were no patients who were added to csDMARDs or corticosteroid during the study period.
Table 1. Demographic characteristics at baseline (n = 24).
3.2. Clinical efficacy of IFX at 22 weeks
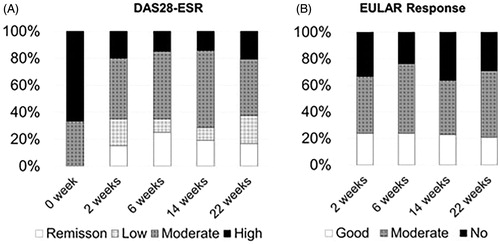
The median DAS28-ESR was 3.75 at 22 weeks. Nine patients (37.5%) achieved the LDA at 22 weeks (). The EULAR response (good or moderate) rate was 70.8%, and 20.8% of the patients achieved a good response ().
3.3. Prediction of LDA at 22 weeks
To determine the predictive variables associated with the achievement of DAS28-ESR LDA or remission at 22 weeks after the introduction of IFX, we evaluated the 22 variables shown in (by a univariate analysis). The univariate analyses identified that tender joints (>8 counts) was a prognostic factor to predict LDA achievement at 22 weeks (adjusted odds ratio, 0.10; 95% confidence interval, 0.01–0.63; p = .02), whereas the other baseline clinical variables including MTX dosage, disease duration and the previous usage of TNF-i were not associated with LDA achievement ().
Table 2. Predictors of low disease activity (LDA) or remission at 22 weeks by univariate analysis.
3.4. Safety data
The adverse events observed during the study period are shown in . Nine adverse events were observed during the study period, and an infection requiring hospitalization was observed in one patient.
Table 3. Safety (n = 24).
The persistence rate of IFX at 22 weeks was 75%. Reasons for discontinuation were infection: two patients, infusion reaction: one patient, headache: one patient, skin eruption: one patient, and insufficient effect: one patient. Of the six patients who discontinued IFX during the observation period, only one was considered as LDA achieved by LOCF method.
4. Discussion
The results of our present analyses demonstrated the efficacy of switching to IFX from other bDMARDs among Japanese RA patients in daily clinical practice. 37.5% of the patients achieved LDA or remission and 70.8% of patients achieved a good or moderate EULAR response, even though they failed to respond to previous bDMARD treatment. These findings are consistent with the results of a retrospective clinical study that analyzed the efficacy of IFX among bio-naïve RA patients in a Japanese population (i.e., 38.8% of the patients achieved LDA or remission, and 84.5% of the patients achieved a good or moderate overall response at 22 weeks) [Citation21]. That study also revealed that male sex, RF negativity, low CRP, lower swollen joint count and a low prednisolone dose were significantly related to the clinical response [Citation21].
In the present study, the univariate analyses revealed that tender joints (>8 counts) was a predictor for LDA achievement at 22 weeks. In addition to the swollen joint count (SJC), tender joint counts (TJC) have traditionally been used to assess for disease activity. Since TJC has better interobserver reliability compared to the SJC, it has a higher weighting than the SJC in the DAS calculation [Citation22]. Moreover, it has been also reported that TJC is more sensitive to change [Citation23]. Accordingly, assessing the TJC before switching bDMARDs to IFX could be useful to predict clinical response in daily practice.
The question regarding what type of bDMARD is the best when previous bDMARDs have failed to control the disease activity of RA remains unanswered. A randomized clinical trial showed that a non-TNF-i was more effective for achieving good or moderate EULAR responses compared to a second TNF-i among RA patients who showed an insufficient response to the first TNF-i [Citation24]. In addition, an observational clinical study showed that a TNF-i is more effective than ABT for RA patients with insufficient responses to TCZ as evaluated by the CDAI [Citation25]. However, in the subgroup analysis of that study focusing on the patients who had a history of TNF-i failure [Citation25], the proportion of patients achieving LDA or remission was comparable between the current TNF-i and ABT.
In the present study, 66.7% of the previous bDMARDs before the switch to IFX were TNF-i, and 33.3% were non-TNF-i. The type of previous bDMARD (i.e., TNF-i vs. non-TNF-i) did not affect the achievement of LDA or remission at 22 weeks. Our observations indicate that IFX improved the conditions of the RA patients who had an insufficient response to any type of bDMARD. Consistent with our data, the EULAR recommendation states that ‘if a first TNF inhibitor therapy has failed, patients may receive another TNF inhibitor or a biological agent with another mode of action’ [Citation7].
A 26-week open-label, assessor-blinded study of the switch to IFX in RA patients with an inadequate response to ADA or ETN demonstrated similar clinical efficacy at week 26 between the patients received 3 mg/kg throughout and the patients who required dose escalation to 5 mg/kg or 7 mg/kg [Citation11]. These results are consistent with our present finding that the achievement of LDA or remission at 22 weeks was similar with or without a dose escalation of IFX. It was reported that the production of anti-drug antibodies (ADAbs) to IFX lowers the serum concentration of IFX as well as its efficacy [Citation11,Citation26,Citation27], and thus, the measurement of the serum concentrations of IFX and/or ADAbs to IFX may be useful to predict the efficacy of IFX as a switched bDMARD.
Our study has several limitations to acknowledge. This was a retrospective study with a small number of patients. We were not able to perform multivariate analyses because the sample size was not sufficient. Therefore, it has not been evaluated whether TJC is an independent factor predicting LDA achievement. In addition, a statistical power may be insufficient to conclude that other clinical variables at baseline were not predictors of achievement of LDA. Furthermore, the observation period was only 22 weeks, which could be too short to evaluate the true efficacy of IFX, because the structural damage in RA progresses over a period of years. Taken together, a prospective study in a larger cohort and/or with long-term observation is required to confirm our results.
In conclusion, our findings indicate that switching to IFX is effective to achieve LDA or remission for RA patients who are refractory to previous bDMARD treatment, especially among patients with low number of tender joints.
Disclosure statement
AK has received grant support, research support or speaker bureau fees from AbbVie, Asahikasei, Astellas, BMS, Chugai, Daiichi-Sankyo, Eli Lilly and Company, Kyowa-Kirin, Mitsubishi-Tanabe, MSD, Nakatani foundation, Novartis, Ono, Pfizer, Takeda and Taishotoyama.
References
- Abe T, Takeuchi T, Miyasaka N, et al. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol. 2006;33:37–44.
- Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:189–194.
- Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602.
- St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–3443.
- van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009;68:914–921.
- Takeuchi T, Miyasaka N, Inoue K, et al. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol. 2009;19:478–487.
- Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509.
- Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893–899.
- Hansen KE, Hildebrand JP, Genovese MC, et al. The efficacy of switching from etanercept to infliximab in patients with rheumatoid arthritis. J Rheumatol. 2004;31:1098–1102.
- van Vollenhoven R, Harju A, Brannemark S, et al. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis. 2003;62:1195–1198.
- Fleischmann R, Goldman JA, Leirisalo-Repo M, et al. Infliximab efficacy in rheumatoid arthritis after an inadequate response to etanercept or adalimumab: results of a target-driven active switch study. Curr Med Res Opin. 2014;30:2139–2149.
- Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890–896.
- Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588.
- Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc. 1949;140:659–662.
- Pincus T, Summey JA, Soraci SA Jr., et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353.
- Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 2003;42:244–257.
- Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–R806.
- van Gestel AM, Prevoo ML, van 't Hof MA, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39:34–40.
- Yamanaka H, Tanaka Y, Sekiguchi N, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM). Mod Rheumatol. 2007;17:28–32.
- van der Heijde DM, van 't Hof M, van Riel PL, et al. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579–581.
- Anderson JJ, Felson DT, Meenan RF, et al. Which traditional measures should be used in rheumatoid arthritis clinical trials? Arthritis Care Res. 1989;32:1093–1099.
- Gottenberg JE, Brocq O, Perdriger A, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug. A randomized clinical trial. JAMA. 2016;316:1172–1180.
- Akiyama M, Kaneko Y, Kondo H, et al. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829–2834.
- Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165–178.
- Bendtzen K, Geborek P, Svenson M, et al. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006;54:3782–3789.