Abstract
Chronic active Epstein–Barr virus infection (CAEBV) is one of the Epstein–Barr virus (EBV)-positive T- or NK-cell lymphoproliferative diseases. It is characterized by clonal proliferation of EBV-infected T or NK cells and their infiltration into systemic organs, leading to their failure. Inflammatory symptoms, fever, lymphadenopathy and liver dysfunction are main clinical findings of CAEBV. EBV itself contributes to the survival of the host cells via induction of CD40 and CD137 expression and constitutive activation of NF-κB. Accumulation of gene mutations in the infected cells may lead to the development of highly malignant lymphoma or leukemia. Furthermore, constitutive activation of STAT3 is detected in the infected cells, which not only promotes cell survival but also enhances production of inflammatory cytokines. Currently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only effective treatment strategy for eradication of EBV-infected T or NK cells. However, active disease at the time of allo-HSCT (defined as presence of fever, liver dysfunction, progressive skin lesions, vasculitis or uveitis) is a negative prognostic factor. Establishment of chemotherapy regimens for effective resolution of disease activity in patients with CAEBV is a key imperative. Based on the recently unraveled molecular mechanisms CAEBV development, pathways mediated by NF-κB or JAK/STAT are potential novel therapeutic targets.
1. Introduction
Epstein–Barr virus (EBV) is a ubiquitous double-stranded DNA virus categorized as a member of the human herpes virus family. Once infected with EBV, human beings remain infected for life due to persistence of latent infection of B cells. EBV renders the infected B cells immortal. In immunocompromised conditions, proliferation of EBV-infected B cells can cause B-cell neoplasms. A vast majority of immunodeficiency-associated lymphoproliferative disorders is attributable to this phenomenon.
Several T- and NK-lymphoid neoplasms test positive for the EBV genome. Chronic active EBV infection (CAEBV) is one of the EBV-positive T- or NK-cell lymphoproliferative diseases (EBV-T/NK-LPDs). The condition was originally reported in Western countries, but has been reported and studied mainly in Japan and neighboring countries. In the revised WHO classification of tumors of hematopoietic and lymphoid tissues (2016), CAEBV was classified in the category of EBV-positive T- or NK-cell neoplasms [Citation1]. Subsequently, CAEBV has drawn international attention, with several case reports having been reported worldwide. Although CAEBV is a fatal condition, some patients recently achieved long-term survival with allogeneic hematopoietic stem cell transplantation (allo-HSCT). Furthermore, the mechanisms by which certain patients with EBV-infected T or NK cells develop CAEBV is currently an active area of research. This review describes the current state of CAEBV under the new WHO classification. The key focus is on the mechanism of development of CAEBV, the pertinent diagnostic and therapeutic strategies, and future research directions.
2. History of CAEBV
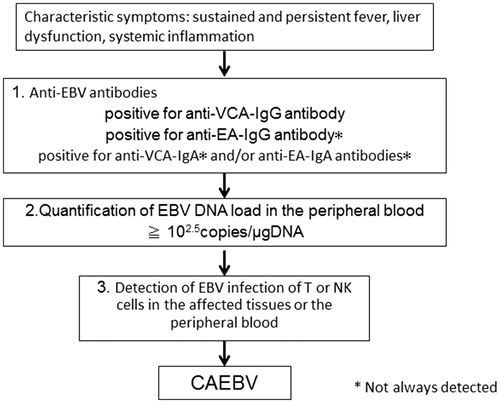
Chronic active EBV infection is characterized by persistent or recurrent systemic inflammation accompanied by EBV infection of T or NK cells. CAEBV was originally reported as a condition marked by sustained inflammatory symptoms such as fever, lymphadenopathy and liver dysfunction (the so-called infectious mononucleosis [IM]-like symptoms) associated with high titers of anti-EBV antibodies in the peripheral blood (PB). The first report pertaining to suspected CAEBV was a case-series report of 53 patients in the US in 1948 [Citation2]. The patients who had fever and splenomegaly lasting ‘from three months to longer than four years after the initial attack’ were diagnosed as having chronic IM. Three of them developed ‘lymphoblastoma’. Subsequently, similar cases were reported by another researcher [Citation3]. The condition was considered as a form of sustained IM and named CAEBV. However, the cases were not consistent with sustained IM. In 1988, Jones and colleagues detected clonal proliferation of EBV-infected T-cells in CAEBV [Citation4]. Thereafter, similar reports were published from Japan and East Asia, which claimed the detection of EBV-positive NK cells in CAEBV. After the 1980s, it was confirmed that CAEBV is a progressive disease and that EBV-infected cells infiltrate multiple organs leading to their dysfunction. Furthermore, conditions with characteristic skin lesions such as hypersensitivity to mosquito bite (HMB) or hydroa vacciniforme (HV) that show EBV-infected T or NK cells exhibit a similar disease course as that of CAEBV. In 2005, the Japanese investigators Okano et al. [Citation5] proposed the first diagnostic guidelines for CAEBV as follows: persistent and recurrent IM-like symptoms, unusual pattern of anti-EBV antibodies with raised anti-VCA and anti-EA and/or detection of increased EBV genomes in the affected tissues, including the PB, and chronic illness which cannot be explained by other known disease processes at the time of diagnosis. The guidelines mentioned that some CAEBV patients showed characteristic skin lesions such as HMB or HV. It also mentioned that hemophagocytic lymphohistiocytosis (HLH) or lymphoproliferative disorder (LPD)/lymphoma originating from T- or NK-cell lineage often developed during the course. This was the first suggested diagnostic criteria for CAEBV that sought to clarify the outline of the disease. Subsequently, the WHO classification of tumors of the hematopoietic and lymphoid tissues revised in 2008 first described CAEBV as a systemic EBV-T-LPD of the childhood [Citation6]. In 2012, Kimura et al. [Citation7] reported a prospective analysis of 108 patients with EBV-T/NK-LPDs. They suggested the following subtypes of EBV-T/NK-LPDs: CAEBV; severe mosquito bite allergy (sMBA); HV-like lymphoproliferative disorder; and EBV-HLH. In the report, CAEBV was described as presence of systemic inflammation while sMBA and HV-LPD were defined as diseases in which the lesions were limited to the skin. Based on these reports, the new WHO classification revised in 2016 defined CAEBV as an EBV-T/NK-LPD [Citation1]. In Japan, the Research group of Measures against Intractable Diseases under the aegis of the Ministry of Health, Labor and Welfare, suggested the addition of EBV infection of T or NK cells to the Okano’s guidelines as the diagnostic criteria for CAEBV (). The criteria matched the new WHO classification. shows a schematic illustration of the diagnostic schema for CAEBV.
Table 1. Diagnostic criteria for CAEBV by the Research group of Measures against Intractable Diseases by Ministry of Health, Labour and Welfare of Japan.
3. Epidemiology of CAEBV
Although the accurate incidence of CAEBV is not clear, a Japanese study group estimated an annual incidence of 23.8 cases/year in Japan. CAEBV has been considered as a disease of the childhood. However, the report mentioned an increase in the number of adult patients. There are some differences between the childhood-onset and adult-onset disease with respect to the clinical features and prognosis [Citation8,Citation9]. Indeed, these may well be different disorders. Most CAEBV cases have been reported from Japan and East Asia. Some investigators suggested a linkage between genetic factors and development of CAEBV [Citation10]. However, there has been no scientific evidence to support the hypothesis.
4. A case presentation
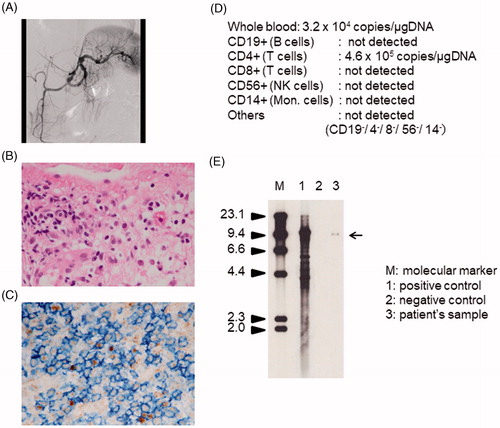
I will present a typical adult case of CAEBV. A 41-year-old man started with blurred vision caused by uveitis 2 years before admission. Subsequently, he developed persistent fever, liver dysfunction, and body weight loss about 6 months prior to admission. He had a history of breathlessness since 3 months prior to the admission and was diagnosed as a case of cardiac failure. He was referred to our hospital. Angiography showed severe systemic vasculitis (). His cardiac ejection fraction was about 20%. Biopsy of the cardiac muscle revealed infiltration of lymphocytes (). He was diagnosed with undetermined vasculitis and myositis and referred to the Department of Rheumatology at our hospital. No signs of infectious disease or immunological disorders were detected. However, the titers of anti-EBV antibodies were markedly high as follows: anti-VCA-IgG antibody, ×5120; anti-EA-DR IgG antibody, ×640; anti-EBNA antibody, ×10. Anti-VCA-IgM was undetectable. The EBV-DNA load in PB was extremely high as follows: PB mononuclear cells (PBMCs), 1.2 × 105 copy/μg DNA; whole blood, 3.2 × 104 copies/μg DNA. Owing to suspected CAEBV, we examined the EBV-infected cells using PB. PBMCs obtained from patient were sorted into CD19-, CD4-, CD8- and CD56-positive fractions using antibody-conjugated magnetic beads. EBV-DNA levels in each fraction were evaluated by real-time polymerase chain reaction (PCR). The fraction which had the highest load was determined as an EBV-infected cell fraction. The results are shown in . We confirmed that the patient’s EBV-infected cells were CD4-positive. We performed immunological staining and EBV-encoded small RNA in situ hybridization. As shown in , the infiltrating cells were CD4- and EBV-positive. Then, a diagnosis of CAEBV was made. Clonal proliferation of EBV-infected cells was determined by Southern blotting (). He received all-HSCT after reduced-intensity conditioning (RIC). The patient’s inflammatory symptoms resolved after transplantation. EBV-infected cells disappeared and EBV-DNA turned undetectable in PB. He has been well for 3 years after allo-HSCT.
Figure 2. Clinical findings of a male patient with CAEBV. (A) Angiogram of the splenic artery showing multiple aneurysms. (B,C) Histopathological specimen of cardiac muscle tissue. Hematoxylin–eosin staining (B) and double staining for anti-CD4 antibody (blue) and EBV-encoded small RNA in situ hybridization (brown) (C). Infiltrating cells were positive for CD4 and EBV. (D) Results of EBV-infected cell analysis using PBMCs. Cells positive for CD4 expression were determined as EBV-infected. (E) Southern blotting analysis for the EBV-terminal repeat in PBMCs. The clonal band was detected in the lane 3 (the patient’s sample).
5. Clinical features of CAEBV
CAEBV has two main elements, i.e., systemic inflammation and neoplastic disease. In CAEBV, clonal proliferation of EBV-infected T or NK cells and their infiltration into systemic organs leads to organ failure. This evidence defines CAEBV as a lymphoid neoplasm. However, CAEBV is rarely associated with solid tumors. The main clinical findings of CAEBV are those associated with inflammation. In a Japanese large cohort study of 108 patients by Kimura et al. [Citation7], the main symptoms of CAEBV were fever (91% patients), liver dysfunction (77%), thrombocytopenia (59%), and anemia (44%). In addition, EBV-infected T or NK cells had infiltrated not only lymphoid tissues but also other organs such as the skin, lungs, heart, intestine, central and peripheral nervous systems, and eyes; indeed, every organ is a potential target. Furthermore, CAEBV causes vasculitis due to direct invasion by the infected cells as well as to the immune reaction resulting from activation of cells. Vasculitis may lead to vascular aneurysms, ischemic organ damage and uveitis.
Two characteristics skin symptoms are referred to as CAEBV-related findings. sMBA is characterized by local skin inflammation followed by high fever, lymphadenopathy and liver dysfunction following the bite of Aedes (Stegomyia) albopictus, also known as the Asian tiger mosquito. The puncture sites ulcerate and often leave scars on healing. sMBA occurs due to the hyper-reactive response of the patients’ lymphocytes to the mosquito’s saliva [Citation11]. In 1997, Ishihara et al. [Citation12] detected monoclonal proliferation of EBV-positive T and NK cells in the PB of sMBA patients. Subsequent reports indicated that sMBA may lead to fatal disorders such as T- or NK-cell lymphoma or HLH. Therefore, sMBA is now classified as a T- or NK-cell neoplasm in the WHO classification of EBV-T/NK-LPDs [Citation13]. HV is characterized by sunlight-induced vesicle and can be accompanied by systemic inflammation with detection of EBV-infected clonally proliferating T or NK cells [Citation11]. The condition is defined as an HV-like lymphoproliferative disorder (HV-LPD) in the WHO 2016 classification [Citation1]. The diagnoses of sMBA and HV-LPD are made if the lesions are limited to the skin. Some CAEBV patients have sMBA or HV-LPD. The difference between these skin-limited diseases and CAEBV is not well characterized. Analysis of a large number of patients using a standardized diagnostic criteria may help characterize the differences.
CAEBV is a progressive disease. The two aspects of the disease, i.e., systemic inflammation and neoplasm, develop during the disease course and may eventually end up as two lethal conditions, i.e., HLH and chemotherapy-resistant lymphoma, respectively. The duration from the disease onset to the development of these conditions may vary from several months to several decades. Development of means to predict and prevent the development of these conditions is a key imperative.
6. Current problems of CAEBV
Several issues pertaining to CAEBV require further clarification or improvement; these relate to the diagnosis, treatment and mechanism of development of CAEBV.
Why is it difficult to make a diagnosis of CAEBV? One of the reasons is the diversity of its clinical symptoms. shows the list of departments that CAEBV patients initially visited at our hospital. Patients may visit various departments and hematologists do not always check CAEBV patients at the first visit. Every doctor, including surgeons, should be aware of this disease and consider it as a differential diagnosis in case of sustained inflammation. Second, it is difficult to determine EBV-infected cells. Since most cases of CAEBV do not show solid tumors, pathological examination is typically not useful. Instead, EBV-infected T or NK cells are detected in PB. Unfixed PB was used for detection of the phenotypes of the infected cells in a vast majority of patients using flow cytometry or antibody-conjugated magnetic beads sorting. The procedure to sort PBMCs into various fractions is costly and requires special skills. Although the quantification of the EBV-DNA load by PCR was approved for coverage under the Japanese health insurance in 2018, it is necessary to develop versatile methods to detect EBV-infected cells in PB.
Table 2. Departments where patients with CAEBV first visited at our institution.
Allo-HSCT with RIC is currently the only curative treatment strategy for CAEBV. In other words, there is no effective chemotherapy that can eradicate EBV-infected neoplastic cells in patients with CAEBV. However, allo-HSCT is not feasible for all patients. CAEBV patients often experience disease-induced damage of vital organs such as the liver, heart and lungs. Furthermore, the number of elderly patients with CAEBV is increasing, although it was originally reported as a childhood disorder. These patients are not suitable candidates for allo-HSCT. In addition, presence of disease activity at the initiation of conditioning therapy is a negative prognostic factor for outcomes of allo-HSCT.
Two reports indicated poor outcomes of allo-HSCT in CAEBV patients with active disease [Citation7,Citation14]. Active disease is defined as condition accompanied by any of following: fever, liver dysfunction, progressive skin lesions, vasculitis or uveitis, accompanied by a significant increase in EBV-DNA copies. Patients who lack these clinical findings are categorized as having inactive disease. These reports suggest that establishment of chemotherapy regimens that effectively resolve the disease activity is indispensable for treatment of active CAEBV.
7. Mechanisms of development of CAEBV
Epstein–Barr virus is a ubiquitous virus; almost all adults across the world are infected by the virus. It is not clear as to why EBV infects T or NK cells leading to the development of CAEBV in limited patients. In recent years, the mechanisms are being clarified gradually but certainly.
Most reports of CAEBV have originated from Japan and East Asia. This indicates a potential genetic determinant of the disease. Currently, members of a Japanese study group are using next-generation sequencing to investigate the genetic factors that contribute to the development of CAEBV.
How does EBV infect T and NK cells? EBV infects its target B cells via the receptor CD21 on the cell surface. T-cells were shown to exhibit weak expression of CD21 [Citation15]. In addition, an in vitro study demonstrated that activated NK cells conjugated to CD21-positive EBV-infected B cells transiently acquire a weak CD21 expression by synaptic transfer of a few receptor molecules onto their surface [Citation16]. A similar mechanism exists in T-cells [Citation17]. Furthermore, another in vitro infection assay using a high amount of EBV load demonstrated successful establishment of EBV infection of T or NK cells [Citation18,Citation19]. Additionally, in vivo EBV infection of T or NK cells were detected in patients with active IM. These findings indicate that EBV can infect T or NK cells under conditions of high viral load [Citation20]. However, EBV infection of T or NK cells is considered to be a transient phenomenon in vivo. In IM, EBV-positive T-cells disappeared 1 year after the disease onset [Citation21]. Therefore, the mechanism of sustained EBV infection of T or NK cells in CAEBV is not clear. There are two plausible mechanisms that can explain this phenomenon, i.e., suppressed immune reaction to the infected cells or characteristics of the virus itself. A decrease in the number of cytotoxic T-cells or their impaired function has been reported in CAEBV [Citation22]. A patient with perforin mutation [Citation23] who developed a CAEBV-like condition has been reported. Other undetermined immune suppressive disorders may also contribute to this phenomenon. Factors related to the virus itself may also play a major role. Results of ongoing genome-wide analyses are expected to clarify some of these issues.
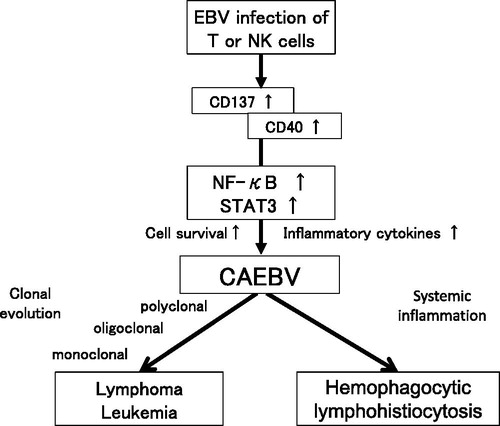
Epstein–Barr virus infection of B cells renders them immortal. The next question is how EBV-infected T or NK cells transform into neoplastic cells. Several studies have shown that EBV infection induces activation of survival-promoting molecules or pathways. Imadome et al. [Citation24] reported positive expression of CD40 on EBV-infected T or NK cells obtained from patients with CAEBV. They performed in vitro infection of T-cells with EBV and observed induction of surface expression of CD40 [Citation18]. CD40 is originally expressed on activated B cells. Since the ligand of CD40, CD40L, is originally expressed on the surface of activated T-cells, it was hypothesized that the induced surface expression of CD40 on T-cells associated with CD40L activates intracellular signaling molecules, such as NF-κB, that mediate cell survival. They also confirmed that CD40 on EBV-infected T-cells activates CD40L, which mediates survival-promoting intracellular signaling in an autocrine or paracrine manner and suppresses their apoptosis. Another co-stimulatory molecule, CD137, may help to promote the survival of EBV-infected T-cells. Yoshimori et al. [Citation25] found CD137 expression on EBV-infected T or NK cells in CAEBV, and its expression could be induced by in vitro EBV infection of T-cells. Stimulation of CD137 by CD137 ligand suppressed etoposide-induced cell survival. Furthermore, Takada et al. [Citation26] found constitutive activation of NF-κB, a transcription factor that mediates cell survival signals, in EBV-infected T or NK cells in CAEBV. They also reported that in vitro EBV infection of T-cells induced constitutive activation of NF-κB and suppressed serum-depletion and etoposide-induced apoptosis of the infected cells. NF-κB exists downstream of CD40 and CD137. These findings suggest that EBV infection directly induces cell survival of T or NK cells via survival-promoting pathways such as that of NF-κB.
STAT3 is a transactivation factor that mediates proliferation and anti-apoptotic signaling. It is activated in various cancer cells and contributes to neoplastic transformation of cells [Citation27]. STAT3 also mediates the intracellular signaling downstream of the cytokine receptors and regulates inflammation [Citation28]. We observed constitutive activation of STAT3 in EBV-positive T or NK-cell lines established from EBV-T/NK-lymphoid neoplasms as well as in neoplastic EBV-positive T or NK cells obtained from CAEBV patients [Citation29]. Of note, we observed no mutation in the SH2 domain of the STAT3 gene, which is an essential site for activation of molecules that are hot spots of activating mutation in other T or NK-cell tumors; therefore, it seems likely that the upstream molecules may contribute to the activation. Several studies have suggested that STAT3 is activated downstream of LMP1 through the activation of NF-κB [Citation30,Citation31]. Interestingly, Onozawa et al. [Citation29] found that inhibition of the tyrosine kinase JAK1/2, which phosphorylates STAT3, by its inhibitor ruxolitinib, inhibited STAT3 activation in EBV-positive T or NK-cell lines. Ruxolitinib was shown to suppress proliferation and to induce apoptosis of these cells. Moreover, ruxolitinib suppressed the mRNA expressions of interferon gamma, tumor necrosis factor-alpha and interleukin-6 in EBV-positive T or NK cells. These results indicated that the JAK1/2–STAT3 pathway contributes to the development of both inflammatory and neoplastic aspects of CAEBV.
Collectively, these findings suggest that EBV-related factors play a major role in the development of CAEBV. The suggested mechanisms that underlie the development of CAEBV are summarized in .
8. Current treatment strategy for CAEBV
The treatment objective for CAEBV is to control the neoplastic and inflammatory elements of the disease. Once HLH or lymphoma develops, CAEBV can be fatal. Therefore, initiation of treatment prior to their development is recommended. According to Kimura’s report, the survival rates of patients with EBV-T/NK-LPDs was 44% and the median follow-up period of patients who died was 46 months [Citation7]. Unfortunately, chemotherapy regimens for CAEBV that can eradicate EBV-infected T or NK cells are yet to be established. Currently, the only effective curative treatment strategy is allo-HSCT. In the study, the 15-year OS rate of patients treated with allo-HSCT was 60.6% compared to 25.7% for patients who were not treated with allo-HSCT [Citation7]. The OS with allo-HSCT was significantly higher than that without allo-HSCT. Furthermore, in a retrospective study of patients treated with allo-HSCT at their hospital, Kawa et al. [Citation32] reported that the 3-year OS rate of patients who received RIC (85.0%) was significantly better than that of patients who received myeloablative conditioning (54.5%). These findings indicate that the therapeutic effects of allo-HSCT were attributable to the replacement and reconstruction of the hematopoietic and immune systems by allogeneic grafts rather than to the anti-tumor effects of chemo- and radiotherapies. In other words, immunological dysfunction plays a pivotal role in the development of CAEBV.
What is the most effective chemotherapy to reduce the disease activity? Sawada and his colleagues at the Osaka Medical Center and Research Institute for Maternal and Child Health suggested a sequential treatment strategy consisting of prednisolone, cyclosporine A and etoposide (the so-called cooling therapy) as the first step, and combination chemotherapies, such as CHOP and Capizzi [Citation33]. The last recommended step was RIC followed by allo-HSCT. In order to determine the effects of chemotherapy, we performed a nationwide survey. Regrettably, the rates of resolution of disease activity in patients with CAEBV were very low (approximately 10%; manuscript in preparation). In order to improve the outcomes of CAEBV patients, it is indispensable to establish more effective chemotherapy for CAEBV.
Some reagents are candidates as new treatments for CAEBV based on the molecular mechanisms of the development of CAEBV. As mentioned in the previous section, NF-κB is constitutively activated in EBV-positive T or NK cells and may help suppress apoptosis of infected cells [Citation26]. It was reported that bortezomib suppressed proliferation and induced apoptosis of EBV-infected cell lines including T or NK cells [Citation34]. The JAK/STAT pathway is another potential therapeutic target. This pathway is commonly used by multiple cytokine receptors and is involved in the establishment of inflammation. As mentioned above, a JAK inhibitor, ruxolitinib, suppressed proliferation and cytokine production of EBV-positive T or NK cells. JAK inhibitors are clinically used and effective for cytokine-associated inflammatory diseases such as rheumatoid arthritis, ulcerative colitis and graft-versus-host disease [Citation35–37]. Since CAEBV shows sustained inflammation accompanied by hypercytokinemia, similarly to these diseases, the effects of JAK inhibitors on the inflammatory symptoms and disease activity are expected.
9. Summary
Chronic active Epstein–Barr virus infection has been recognized as an endemic disease of East Asia. However, the number of reported patients is increasing due to the description in WHO 2016 and development of diagnostic criteria. CAEBV is still associated with a poor prognosis. It is necessary to clarify the molecular mechanisms of disease development to establish effective treatment strategies.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390.
- Isaacs R. Chronic infectious mononucleosis. Blood. 1948;3:858–861.
- Straus SE. The chronic mononucleosis syndrome. J Infect Dis. 1988;157:405–412.
- Jones JF, Shurin S, Abramowsky C, et al. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med. 1988;318:733–741.
- Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80:64–69.
- Quintanilla-Martinez L, Kimura H, Jaffe ES. EBV-positive T-cell lymphoproliferative disorders of childhood. World Health Organization Classification of Tumors Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008.
- Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673–686.
- Arai A, Imadome K, Watanabe Y, et al. Clinical features of adult-onset chronic active Epstein-Barr virus infection: a retrospective analysis. Int J Hematol. 2011;93:602–609.
- Kawamoto K, Miyoshi H, Suzuki T, et al. A distinct subtype of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorder: adult patients with chronic active Epstein-Barr virus infection-like features. Haematologica. 2018;103:1018–1028.
- Fujiwara S, Kimura H, Imadome K, et al. Current research on chronic active Epstein-Barr virus infection in Japan. Pediatr Int. 2014;56:159–166.
- Asada H, Saito-Katsuragi M, Niizeki H, et al. Mosquito salivary gland extracts induce EBV-infected NK cell oncogenesis via CD4 T cells in patients with hypersensitivity to mosquito bites. J Invest Dermatol. 2005;125:956–961.
- Ishihara S, Okada S, Wakiguchi H, et al. Clonal lymphoproliferation following chronic active Epstein-Barr virus infection and hypersensitivity to mosquito bites. Am J Hematol. 1997;54:276–281.
- Quintanilla-Martinez L, Kimura H, Jaffe ES. EBV-positive T-cell lymphoproliferative disorders of childhood. In: Jaffe E, Harris N, Stein H, editors. World Health Organization classification of tumors pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. p. 527.
- Arai A, Sakashita C, Hirose C, et al. Hematopoietic stem cell transplantation for adults with EBV-positive T- or NK-cell lymphoproliferative disorders: efficacy and predictive markers. Bone Marrow Transplant. 2016;51:879–882.
- Fischer E, Delibrias C, Kazatchkine MD. Expression of CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J Immunol. 1991;146:865–869.
- Tabiasco J, Vercellone A, Meggetto F, et al. Acquisition of viral receptor by NK cells through immunological synapse. J Immunol. 2003;170:5993–5998.
- Stinchcombe JC, Bossi G, Booth S, et al. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761.
- Imadome K, Shimizu N, Arai A, et al. Coexpression of CD40 and CD40 ligand in Epstein-Barr virus-infected T and NK cells and their role in cell survival. J Infect Dis. 2005;192:1340–1348.
- Isobe Y, Sugimoto K, Matsuura I, et al. Epstein-Barr virus renders the infected natural killer cell line, NKL resistant to doxorubicin-induced apoptosis. Br J Cancer. 2008;99:1816–1822.
- Anagnostopoulos I, Hummel M, Kreschel C, et al. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750.
- Arai A, Yamaguchi T, Komatsu H, et al. Infectious mononucleosis accompanied by clonal proliferation of EBV-infected cells and infection of CD8-positive cells. Int J Hematol. 2014;99:671–675.
- Shibayama H, Imadome KI, Onozawa E, et al. Virus-specific cytotoxic T cells in chronic active Epstein-Barr virus infection. Rinsho Ketsueki. 2017;58:583–588.
- Katano H, Ali MA, Patera AC, et al. Chronic active Epstein-Barr virus infection associated with mutations in perforin that impair its maturation. Blood. 2003;103:1244–1252.
- Imadome K, Shirakata M, Shimizu N, et al. CD40 ligand is a critical effector of Epstein-Barr virus in host cell survival and transformation. Proc Natl Acad Sci USA. 2003;100:7836–7840.
- Yoshimori M, Imadome KI, Komatsu H, et al. CD137 Expression is induced by Epstein-Barr virus infection through LMP1 in T or NK cells and mediates survival promoting signals. PLoS One. 2014;9:e112564.
- Takada H, Imadome KI, Shibayama H, et al. EBV induces persistent NF-κB activation and contributes to survival of EBV-positive neoplastic T- or NK-cells. PLoS One. 2017;12:e0174136.
- Scott LM, Gandhi MK. Deregulated JAK/STAT signalling in lymphomagenesis, and its implications for the development of new targeted therapies. Blood Rev. 2015;29:405–415.
- Arora L, Kumar AP, Arfuso F, et al. The role of signal transducer and activator of transcription 3 (STAT3) and its targeted inhibition in hematological malignancies. Cancers (Basel). 2018;10:piiE327.
- Onozawa E, Shibayama H, Takada H, et al. STAT3 is constitutively activated in chronic active Epstein-Barr virus infection and can be a therapeutic target. Oncotarget. 2018;9:31077–31089.
- Chen H, Hutt-Fletcher L, Cao L, et al. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J Virol. 2003;77:4139–4148.
- Nosbaum A, Prevel N, Truong HA, et al. Cutting edge: regulatory t cells facilitate cutaneous wound healing. J Immunol. 2016;196:2010–2014.
- Kawa K, Sawada A, Sato M, et al. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant. 2011;46:77–83.
- Sawada A, Inoue M, Kawa K. How we treat chronic active Epstein-Barr virus infection. Int J Hematol. 2017;105:406–418.
- Iwata S, Yano S, Ito Y, et al. Bortezomib induces apoptosis in T lymphoma cells and natural killer lymphoma cells independent of Epstein-Barr virus infection. Int J Cancer. 2011;129:2263–2273.
- Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10:117–127.
- Kasembeli MM, Bharadwaj U, Robinson P, et al. Contribution of STAT3 to inflammatory and fibrotic diseases and prospects for its targeting for treatment. Int J Mol Sci. 2018;19:pii:E2299.
- Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 2012;120:1367–1379.