Abstract
Here we present a 45-year-old male patient who suffered from psoriatic arthritis (PsA), for which adalimumab (80 mg every other week) was effective. Dose escalation of a TNF-α antagonist should be considered for patients showing an inadequate response for disease stabilization in PsA.
1. Introduction
Psoriatic arthritis (PsA) is a progressive and chronic inflammatory arthritis [Citation1]. This leads to physical limitations and reduces the quality of life. Here, we report a 45-year-old male patient who suffered from PsA. Because his PsA was a severe, he had become depressive. The patient’s arthritic signs preceded skin signs by 20 years. Although adalimumab (ADA) (40 mg every other week) was started, polyarthritis and psoriasis had not resolved. After the ADA dose was increased to 80 mg (every other week), both polyarthritis and psoriasis were notably resolved. Thus, we believe that this case provides clinical insight into treatment for PsA.
2. Case
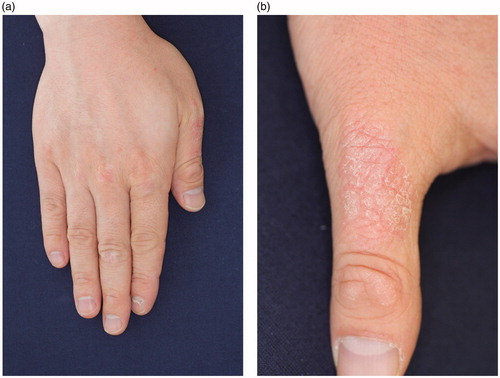
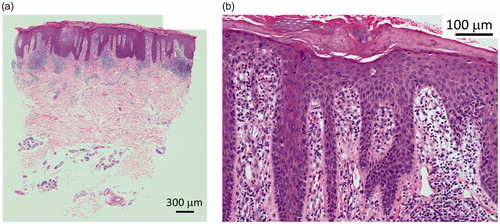
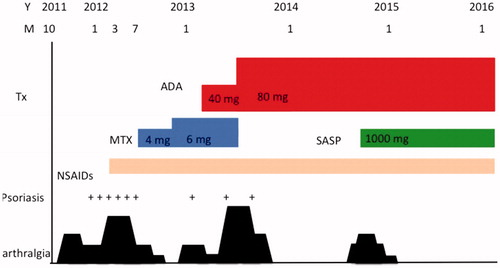
A 45-year-old man was seen to our outpatient clinic because of back pain and polyarthritis in March 2012. He had been suffering from back pain occasionally since his 20’s. Before visiting our outpatient clinic, he had experienced pain in the PIP joints, neck, and back since January 2011. On admission, he suffered from arthritis in both knees and the left hip, arthritis in PIP, and MCP in the right hand. There were some eruptions on his hands () and left ankle. Laboratory data were as follows: ESR: 10 mm/h, WBC: 6700/µL (neutrophils: 59.9%, lymphocytes: 34.2%, monocytes: 2.4%), Hb: 12.6 g/dL, PLT: 20.2 × 104/µL, and C-reactive protein (CRP): 0.03 mg/dL. Urinalysis did not demonstrate any protein on a dipstick test. Creatinine was 1.11 mg/dl. Liver functions were within normal limits. Immunological tests demonstrated that immunoglobulin (Ig) G, IgA, and IgM were 1117, 136, and 63 mg/dL, respectively. RF was <3. Anti-nuclear antibody and anti-CCP antibody were negative. Serologic HLA analysis showed A24, A33, B40, and B58. Radiography findings of the chest, hands, feet, and knees were within normal limits. Sacroiliac joints were normal on both radiography and computed tomography. In June 2012, skin biopsy from his right thumb was performed (). Keratinocyte hyperplasia was seen by pathology report and his eruption was compatible for psoriasis. Eruption in his right MCP joint was consistent with psoriasis. Finally, he was diagnosed with PsA, and NSAIDs was started, although it was not effective, then methotrexate (MTX) (4 mg/week) was started. In November 2012, the dosage of MTX was increased to 6 mg/week; then, polyarthritis was resolved. In February 2013, the patient suffered from polyarthritis again, the dose of MTX was not able to increased because he felt nausea, thus ADA (40 mg every other week) was started. Because his arthritis was not resolved, in July 2013, the dose of ADA was increased to 80 mg (every other week) and MTX was stopped. Finally, his arthritis and psoriasis were resolved. In October 2014, as his back pain worsened and CRP was elevated to 0.38, Salazosulfapyridine (SASP) (1000 mg/day) was added. He is currently in a favorable condition, taking SASP and ADA (80 mg every other week). Other clinical manifestations of PsA are well controlled ().
3. Discussion
This patient suffered from PsA. After the start of ADA (40 mg every other week), polyarthritis and psoriasis were only partially resolved. After the dose increase of ADA to 80 mg (every other week), both his arthritis and psoriasis were notably resolved. Thus, in this case, 80 mg of ADA was needed to control the arthritis and psoriasis. We believe that this case provides clinical insight into treatment for PsA.
PsA shares genetic and clinical features with other forms of spondyloarthritis and is grouped with these disorders. Compared with skin leasion, arthritis is rare in Psoriasis, but recent studies based on the Classification Criteria for Psoriatic Arthritis (CASPA criteria) indicate that it occurs in up to 30% of patients with psoriasis. It has been reported that in PsA patients, the majority (84%) experience skin symptoms prior to the onset of joint symptoms. Simultaneous onsets of arthritis and psoriatic symptoms occurred in 13% of patients. Only 3% of patients experienced joint involvement preceding skin involvement [Citation1]. Lenert P et al reported a HLA-27 positive polyarthritis preceding the onset of psoriasis [Citation2]. The case developed acute polyarthritis affecting both peripheral joints and the left sacroiliac joint, preceding the appearance of typical skin manifestations for almost two months [Citation2]. The present patient had suffered from back pain for more than 20 years and polyarthritis for a year prior to the onset of skin eruption. After biopsy of the skin, the patient was finally diagnosed with PsA.
The pathophysiology of PsA is still unclear. PsA has varied clinical features, such as enthesitis, dactylitis, nail dystrophy, uveitis, and osteitis. However, it has become clear that tumor necrosis factor (TNF)-α, interleukin (IL)-17, and IL-23 play a crucial role [Citation3]. TNF-α, transforming growth factor-β, IL-17A, and adhesion molecules have been detected in synovia of PsA patients [Citation4]. Immature dendritic cells in PsA show the increased expression of Toll-like receptor2, and this induces a T-helper-1 (Th)-1 cell response with upregulated production of TNF-α, IL-12, and interferon-γ. Activation of the TNF and IL-23-Th17 pathway and evidence for the role of pathogenic CD8-positive memory T cells has a crucial role of PsA. The target of these cells or pathway has led to successful therapy [Citation5].
Bone involvement in PsA is heterogeneous. It has been reported that in the psoriatic synovium, the receptor activator of NF-κB ligand (RANKL) has been upregulated. And moreover, a study has shown that osteoclast precursors derived from circulating CD14+ monocytes are markedly elevated in the peripheral blood of patients with PsA. Treatment with anti-TNF agents significantly reduced the level of circulating precursors [Citation6].
PsA is a highly heritable polygenic disease, and joint involvement occurs in genetically predisposed subjects. HLA alleles and studies of heritability and genome-wide association studies have provided many evidence that PsA has a genetic component. This is stranger than and distinct from that of psoriasis [Citation7]. It has been reported that HLA-Cw 0602 is associated with psoriasis. HLA-B 08, 38, 39, and 27 are associated with PsA. The specific HLA susceptibility genes may define different subphenotypes of PsA. HLA-B27 was reported to be associated with enthesitis, symmetric sacroiliitis, and dactylitis, and HLA-B8 was associated with joint fusion, deformities, asymmetric sacroiliitis, and dactylitis [Citation8].
The efficacy and safety of ADA, a fully human TNF-α antagonist indicated for the treatment of patients with psoriasis, were demonstrated in randomized, placebo-controlled, phase 2/3 trials in Japan [Citation9–11].
ADA has been approved in European Union, the USA and Japan for the treatment of moderate-to-severe plaque psoriasis and PsA at an initial dose of 80 mg followed by 40 mg every other week, with a provision in Japan for dose escalation to 80 mg every other week following an inadequate response to 40 mg every other week [Citation12].
Spontaneous remission of PsA is extremely rare. In an observational trial involving patients treated with TNF agents, the rate of partial remission was 23% [Citation13]. In the present patient, after the dose of ADA was increased to 80 mg (every other week), both his arthritis and psoriasis were notably resolved. ADA at 40 mg every other week maybe the optimal starting dose, as patients with a suboptimal response to 40 mg every other week have the option to change to 80 mg every other week.
Use of the anti-P40 antibody ustekinumab, directed against the shared subunit of IL-12 and IL-23, is effective for the treatment of psoriasis and PsA, although results for the skin are more impressive than those for the joints [Citation14]. In this case, joint symptoms were revealed more than 20 years prior to the onset of skin eruption, which was not severe.
In summary, we present a 45-year-old male patient who suffered from PsA, for which ADA (80 mg every other week) was effective. In the case of severe forms of arthritis, dose escalation may be an effective treatment option. Again, dose escalation of a TNF-α antagonist should be considered for patients showing an inadequate response for disease stabilization in PsA.
Author contributions
Y Nanke prepared for this manuscript. TK. NS, HY and SK involved for treatment of this patient.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gottlieb AB, Mease PJ, Jackson M, et al. Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists’s offices. J Derma Treat. 2006;17:279–287.
- Lenert P, Jovanovic M, Mitic I, et al. Psoriatic arthritis: case report of acute HLA-B27 positive polyarthritis preceding the onset of typical skin changes. Med Pregl. 1997;50:387–390.
- Yeremenko N, Paramarta JE, Baeten D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol. 2014;26:361–371.
- Ritchlin C, Haas-Smith SA, Hicks D, et al. Pattern of cytokine production in psoriatic synovium. J Rheumatol. 1998;25:1544–1552.
- Douglas JV, Ursula F. The pathogenesis of psoriatic arthritis. Lancet. 2018;391:2273–2284.
- Ritchlin CT, Haas-Smith SA, Li P, et al. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–831.
- Stuart PE, Nair RP, Tsoi LG, et al. Genome-wide association anaylysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet. 2015;97:816–836.
- Haroon M, Winchester R, Giles JT, et al. Certain class of HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann Rheum Dis. 2016;75:155–162.
- Asahina A, Ohtsuki M, Etoh T, et al. Adalimumab treatment optimization for psoriasis: results of a long-term phase 2/3 Japanese study. J Derma Treat. 2015;42:1–11.
- Asahina A, Torii H, Ohtsuki M, et al. Safety and efficacy of adalimumab treatment in Japanese patients with psoriasis: results of SALSA study. J Dermatol. 2016;43:1257–1266.
- Asahina A, Nakagawa H, Etoh T, et al. Adalimumab M04-688 Study Group. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis (2013 version). Dermatological Association. J Dermatol. 2013;40:683–695.
- Leonardi C, Sobell JM, Crowley JJ, et al. Efficacy, safety and medication cost implications of adalimumab 40 mg weekly dosing in patients with psoriasis with suboptimal response to 40 mg every other week dosing: results form an open –label study. Br J Dermatol. 2012;167:658–667.
- Lubrabo E, Massimo Perrotta F, Manara M, et al. Predictors of loss of remission and disease flares in patients with axial spondyloarthritis receiving antitumor necrosis factore treatment: a retrospective study. J Rheumatol. 2016;43:1541–1546.
- Weitz JE, Ritchlin CT. Ustekinumab: targeting the IL-17 pathway to improve outcomes in psoriatic arthritis. Expert Opin Biol Ther. 2014;14:515–526.