Abstract
This prospective, Phase 3, open-label, study (EudraCT: 2016-001631-12) evaluated pharmacokinetic (PK) characteristics of 3-/4-weekly Privigen® (IgPro10, CSL Behring, King of Prussia, PA, USA) in Japanese patients with PID. PK parameters including serum trough immunoglobulin (IgG) level before next infusion during the wash-in/wash-out phase (Ctrough), area under the concentration-time curve from time point zero to the last time point with quantifiable concentration (AUC0–last), dose-adjusted AUC0–last (dAUC), lowest and highest observed IgG levels (Cmin, Cmax), time to reach Cmax (Tmax), and total clearance (CL) were analyzed for both regimens of Privigen® (dose: 138–554 mg/kg body weight). Ten patients were included in this analysis (3-/4-weekly: n = 2/n = 8). Ctrough levels achieved ranged 7.96–10.05 g/L. Cmax was observed approximately 1 h after the start of the infusion in both regimens. Mean (SD [not applicable for 3-weekly data]) PK parameters: Cmax, 16.60 and 14.20 (5.53) g/L; Cmin, 10.60 and 8.53 (3.89) g/L; AUC0–last, 5971 and 6591 (2633) g*h/L; dAUC, 0.41 and 0.46 (0.19) g*h/L/mg; CL, 2.53 and 2.53 (1.00) mL/h and median Tmax was 1.19 and 1.14 h, for 3-/4-weekly dosing regimens, respectively. Privigen® PK characteristics in Japanese patients were similar between dosing regimens and to previously-reported results in non-Japanese patients with PID.
1. Introduction
Primary immunodeficiency (PID) is a heterogenous group of diseases characterized by intrinsic or genetic defects in one or more components of the immune system [Citation1–3]. More than 350 genetic disorders are categorized as a PID to date, with many new disorders frequently being recognized [Citation4–6]. Prevalence of the specific PIDs varies substantially across different geographical regions [Citation7]. PID results in increased susceptibility to various infections and many other organ-specific (e.g., respiratory and gastrointestinal) complications [Citation3,Citation8]. Antibody deficiencies represent the most common type of PID and are characterized by an inability to produce an effective antibody response to a number of pathogens [Citation3,Citation4,Citation8].
Immunoglobulin G (IgG) replacement therapy remains the mainstay of treatment for patients with PID, specifically for antibody deficiencies [Citation9–11]. Adequate IgG replacement therapy results in reduced infection rates and improved quality of life in patients with PID [Citation12,Citation13]. Intravenous IgG (IVIG) is one of the most common methods of IgG administration, usually delivered at an interval of 3–4 weeks with an infusion duration of 1–4 h, depending on individual dose and flow rate [Citation10,Citation11,Citation14]. IVIG infusions are usually conducted in either a hospital or an infusion center under the observation of qualified medical personnel, which makes IVIG suitable for patients who prefer not to self-infuse [Citation9–11]. IVIG results in a rapid and high increase in serum IgG concentration followed by a biphasic decline, starting with a relatively rapid decline over 2 days and a subsequent slow decline over the next few days, with an overall half-life of approximately 30–40 days [Citation9,Citation11].
Different IVIG formulations are available for the treatment of PID [Citation4]. These products differ in their concentration, osmolality, sugar, sodium, amino acid, and IgA content [Citation14]. Privigen® (IgPro10, CSL Behring, King of Prussia, PA, USA) is a ready-to-use, sterile, 10% liquid IVIG therapy, indicated for the treatment of PID, chronic immune thrombocytopenia (ITP) and chronic inflammatory demyelinating polyneuropathy (CIDP) in the United States [Citation15]. Privigen® is approved for the treatment of Guillain-Barré syndrome, Kawasaki disease and CIDP in the European Union [Citation16]. In Japan, Privigen® is approved for the treatment of CIDP in 2019 [Citation17]. Privigen® is the first and only IVIG designed with proline stabilization [Citation18], which limits the formation of idiotype/anti-idiotype dimers and protein aggregates and thereby improves stability and clinical tolerability [Citation19].
The pharmacokinetic (PK) properties of Privigen® are well-established in non-Japanese patients with PID [Citation20–22]. However, to date, studies reporting PK characteristics of Privigen® in Japanese patients with PID have not been performed. This is, therefore, the first study that evaluated the PK characteristics of Privigen® specifically in Japanese patients with PID. Here, the disposition of Privigen® was evaluated and PK calculated following 3- or 4-weekly infusions of Privigen® and compared between dosing intervals.
2. Materials and methods
2.1. Study design
This PK analysis was part of a prospective Phase 3, open-label, single-arm study (EudraCT number: 2016-001631-12) designed to evaluate the PK parameters of 3- or 4-weekly Privigen® infusions in Japanese patients with PID. The study (Protocol No: 2016-0018 IgPro10_3004) was approved by the Institutional Review Boards of Tokyo Medical and Dental University, Kyushu University Hospital, Dokkyo Medical University Hospital, Kanagawa Children’s Medical Center, Haradoi Hospital, Hiroshima Citizen Hospital, Fukuoka University Hospital, and Gifu University Hospital. All patients provided informed consent.
2.2. Patient population
The study included male or female Japanese patients with a diagnosis of PID, aged ≥6 years with a bodyweight of ≥19 kg at the time of recruitment, who were on stable doses (within ±10% of an average dose in mg/kg in the last 6 months) of any IVIG product currently approved in Japan administered at regular 3- or 4-weekly intervals for ≥6 months prior to study entry, and who reported ≥1 historic IgG trough level of ≥5 g/L during the 6 months prior to study entry.
Key exclusion criteria included patients with a newly-diagnosed PID, who were not receiving IgG replacement therapy, with ongoing active serious infection at the time of screening, with ongoing or history of concomitant malignancies of lymphoid cells, who had allergic reactions to IgG in the past 3 months prior to study entry or who were receiving concomitant treatment with steroids at the time of screening.
Patients received either 3-weekly or 4-weekly infusions of IgPro10 based on their pre-study treatment schedule as prescribed by their physician and this treatment schedule was not modified during the duration of the study.
2.3. Dosing and sampling
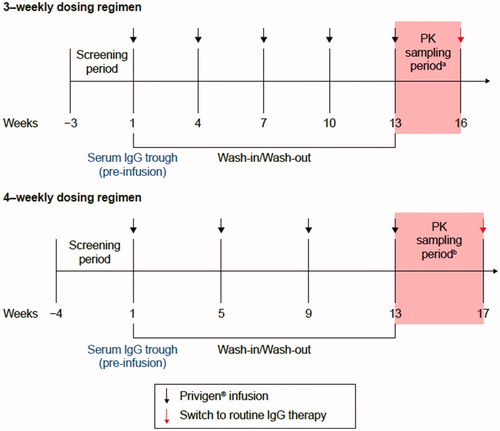
The Privigen® dose was selected as per the patient’s last steady-state dose recorded for the previous IVIG product. The dose range for Privigen® was anticipated to be approximately 200–600 mg/kg per dosing cycle. Individual stable doses outside this range were not considered as a protocol deviation. Based on the IVIG dosing cycle of patients, the duration of the wash-in/wash-out for each of the enrolled patients was estimated to be up to 4 months. Serum samples for PK analysis were collected during the last dosing cycle of Privigen®, starting at Week 13. PK sampling for 3-weekly and 4-weekly intravenous infusions is presented in .
Figure 1. Study overview. aSampling period for 3-weekly dosing regimen: sampling points included −60 minutes to −1 minute prior to infusion, 3–20 minutes post infusion, 24 ± 2 h, 3 ± 1 days, 7 ± 1 days, 10 ± 1 days, 14 ± 1 days, 21 ± 1 days post infusion; bsampling period for 4-weekly dosing regimen: sampling points were the same as for 3-weekly regimena plus 28 ± 2 days post infusion. IgG: immunoglobulin G; PK: pharmacokinetic.
2.4. PK parameters
PK parameters were calculated by non-compartmental analysis (NCA) using Model 202 for constant infusion in Phoenix™ WinNonlin® Version 6.3 (Pharsight Corp., St. Louis, MO, USA). Actual sampling times calculated relative to the start of dose infusion, as well as the actual doses, were used for the analysis. PK parameters calculated in the present analysis included: area under the concentration-time curve (AUC) from time point zero to the last time point with quantifiable concentration (AUC0–last), calculated using log-linear trapezoidal method; dose-adjusted AUC0–last (dAUC,) calculated as AUC0–last/total dose administered (actual dose*body weight); trough IgG level prior to the next infusion during the wash-in/wash-out phase (Ctrough); the lowest (Cmin) and highest (Cmax) observed IgG level within a dosing interval; the time to reach Cmax (Tmax) and total clearance (CL), calculated as dose/AUC0–last where actual doses were used for the calculation of CL. Calculated PK parameters were compared between 3-weekly and 4-weekly intravenous infusions of Privigen®.
2.5. PK statistical analysis
The PK analysis was based on the “Pharmacokinetic Analysis Set” (PKAS). The PKAS included all patients who received ≥1 dose or a partial dose of Privigen® and for whom ≥1 measurable IgG concentration was available following Privigen® infusion without any major deviations related to Privigen® administration. Individual concentration-time profiles and mean (standard deviation [SD]) values by treatment and patient were plotted. For all PK parameters, except Tmax, summary statistics were calculated. Mean, SD, median, minimum, and maximum were calculated in addition to the coefficient of variation (CV), geometric mean, geometric percent CV (CV%), and 95% confidence interval (CI) around the geometric mean. The geometric parameters were derived by log-transforming the concentrations and calculating mean, SD and 95% CI of the log-transformed data. The parameters were back-transformed to the original scale by exponentiation. For Tmax, n, median, minimum and maximum were calculated.
3. Results
3.1. Patient demographics and disposition
Overall, the study enrolled 11 patients; 2 patients on 3-weekly infusions, and 9 patients on 4-weekly infusions. One patient in the 4-weekly infusion group left the study before PK sampling and was thus excluded from the PKAS. This patient had a history of psoriasis at the time of screening and was withdrawn from the study to receive a prohibited immunosuppressive concomitant medication. Worsening of psoriasis was not considered to be related to Privigen® based on the patient’s medical history. The remaining 10 patients (3-weekly infusions, n = 2 and 4-weekly infusions, n = 8) had no major protocol deviations and were included in the PK analysis.
The study included Japanese patients with a female to male ratio of 1:4. The median (range) age of patients was 30 (9–67) years, and the majority (8 patients, 80%) were 18–64 years of age; the remaining patients were aged 2–11 years (n = 1) and 65–84 years (n = 1) (). Overall, the median (range) body weight was 62.1 (27.5–78.9) kg with a median (range) body mass index of 21.4 (16.3–28.3) and a high proportion of patients (6 patients, 60%) were diagnosed with X-linked agammaglobulinemia, resulting in the inclusion of a large proportion of male patients. (). Serum IgG concentration at the first diagnosis of PID and serum IgG trough levels at baseline are shown in .
Table 1. Baseline demographic characteristics of patients (PKAS).
3.2. Privigen® infusion parameters
Privigen® doses were determined based on the individual pre-study treatment schedule of each patient and ranged from 138 to 556 mg/kg per dosing cycle. The mean (SD) doses were similar between 3- and 4-weekly groups, at 272.7 (30.82) mg/kg and 283.4 (132.15) mg/kg, respectively. Administration and doses of Privigen® were well adhered to, with 90% of the patients able to tolerate infusions at a flow rate of 8 mg/kg/min (5.6 mL/kg/h), and about 50% for flow rates of up to 12 mg/kg/min (7.2 mL/kg/h).
3.3. Serum IgG concentrations and PK parameters
All PK parameters analyzed in this study were similar between the two dosing regimens with no significant differences (). However, results should be interpreted with caution due to the small sample size in the 3-weekly infusion group (n = 2). Because of this, SD, CV%, and 95% CI data were not available for the PK parameters of the 3-weekly infusion group parameters, and thus are reported only for the 4-weekly infusion group.
Table 2. Serum IgG PK parameters by dosing regimen (PKAS).
Mean (SD) Ctrough levels were similar between 3- and 4-weekly infusion groups, and ranged from 7.96 to 10.05 g/L. Geometric mean (95% CI) of Ctrough levels was also similar between 3-weekly and 4-weekly infusion groups (). The change in Ctrough levels from ‘prior to initial infusion’ to ‘prior to last infusion’ was negligible in both infusion groups with a difference (mean [SD]) of −0.86 (0.50) g/L in the 3-weekly and −0.30 (0.44) g/L in the 4-weekly infusion group.
The individual patient serum IgG concentration-time profile curves showed variability between patients, and one patient, who received 4-weekly infusions, had particularly high levels of IgG. Mean (SD) serum IgG concentrations are presented in , where Cmax was observed approximately 1 h after the start of the infusion in both groups, with a gradual decline in serum IgG concentration from 24 h post-infusion to 21 days (3-weekly) or 28 days (4-weekly) post-infusion. Mean (SD) Cmax was similar between the infusion groups at 16.6 and 14.2 (5.53) g/L for 3- and 4-weekly infusions, respectively. Geometric mean (95% CI) of Cmax was also similar between 3- and 4-weekly infusion groups (). Similarly, mean (SD) Cmin and corresponding geometric mean (95% CI) were similar between the infusion groups (). Tmax was slightly higher in the 3-weekly infusion group (median [range]: 1.19 [0.92–1.47] h) than the 4-weekly infusion group (1.14 [0.62–23.37] h).
Figure 2. Mean (SD) serum IgG concentration-time data by dosing regimen (PKAS). The first two time points (60–1 minutes prior to infusion and 3–20 minutes post infusion) are presented on a different scale. Profiles are shifted for ease of comparison. IgG: immunoglobulin G; PKAS: pharmacokinetic analysis set; SD: standard deviation.
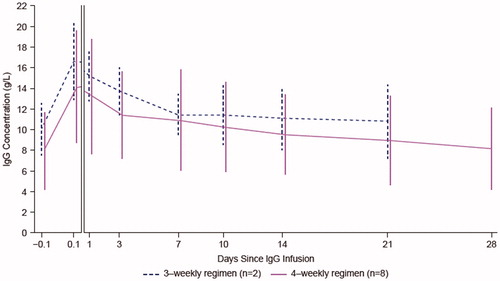
Although there were generally no notable differences, mean (SD) and geometric mean (95% CI) AUC0–last were found to be slightly higher for the 4-weekly infusion group than the 3-weekly infusion group (mean [SD] 5971 and 6591 [2633] g*h/L; geometric mean [95% CI] 5891 and 6239 [4713–8258] g*h/L for 3- and 4-weekly infusion groups, respectively).
The mean (SD) and geometric mean (95% CI) of dAUC were similar between 3-weekly and 4-weekly infusion groups, as was the mean (SD) and geometric mean (95% CI) of CL ().
4. Discussion
This was the first clinical study to evaluate the PK properties of Privigen® in Japanese patients with PID. In this study, PK assessments were performed after a standard 12 weeks wash-in/wash-out period to eliminate any significant carryover of the prestudy IgG therapies and allow for the evaluation of steady-state dosing of Privigen®. The PK properties of Privigen® were found to be similar following 3-weekly and 4-weekly intravenous administration, with no notable differences in any of the evaluated PK parameters. Serum IgG concentrations (Cmax, Cmin) and AUC(0–last) were similar between dosing regimens, considering the limited number of patients in the 3-weekly infusion group. This indicates the possibility that equivalent therapeutic IgG levels can be achieved with a 4-weekly regimen as with a 3-weekly regimen, which offers flexibility in switching from 4-weekly to 3-weekly dosing, for example, in cases where a 4-weekly regimen does not ensure the desired efficacy, in terms of low Ctrough levels, high IgG clearance or recurrent infections.
Negligible changes in Ctrough levels from ‘prior to initial infusion’ to ‘prior to last infusion’ indicate that stable serum IgG trough levels were maintained both during the study and following a switch from the prestudy IVIG products. Ctrough levels at ≥5 g/L are associated with therapeutic protection against infections for many patients with PID, and levels ≥9 g/L offer further enhanced protection in patients with PID [Citation20,Citation23]. However, there is no absolute protective serum IgG level applicable to all patients with PID, and experts in the field agree that IgG dosing often has to be adjusted individually to achieve optimal therapeutic effect [Citation10–12]. Ctrough levels observed with 3-weekly and 4-weekly infusions in the present study (7.95–10.90 g/L) were within a range generally accepted to be sufficient to confer protection from infection in the majority of patients with PID.
The PK parameters calculated in Japanese patients in this study were similar to those previously reported in non-Japanese patients. Ctrough levels achieved with Privigen® infusions in the present study are similar to those observed in a previous Privigen® PK study conducted in non-Japanese patients with PID, which reported a relatively constant Ctrough levels (approximately 9–10 g/L) throughout the 12-month study period [Citation20]. Similar to the maintenance of Ctrough levels reported in the present study, individual Ctrough levels in this previous study were stable over time and remained similar to pre-study levels. There was no statistically significant difference in Ctrough levels between 3-weekly and 4-weekly regimens in this previous study [Citation20], which validates the similar Ctrough levels observed between the regimens in the current study.
In another study of non-Japanese patients with PID conducted at multiple sites in the United States and Germany, 3- or 4-weekly Privigen® was administered from 4 to 26 months (data unpublished) [Citation22]. In this previous study, median (range) Cmin was 10.2 (5.8–14.7) g/L, Cmax was 23.4 (10.4–34.6) g/L, AUC0–last was 366 (197–443) day*g/L, Tmax was 2.3 (1.3–26.3) h and CL was 1.33 (0.87–2.09) mL/day/kg [Citation22]. The higher Cmax and AUC0–last reported in this previous study of non-Japanese patients may be due to the higher median dose of 444.4 mg/kg of Privigen® administered, compared with the present study, where the median dose was 280.9 mg/kg [Citation22]. Despite this difference, the serum IgG concentration-time profile observed in the study of Privigen® PK study in non-Japanese patients [Citation22] is similar to the profile observed in the present study.
In the present PK analysis, the wash-in/wash-out period and sampling points for PK assessments were similar to previous Privigen® studies conducted in non-Japanese patients, enabling the comparison of PK profile between Japanese and non-Japanese patients with PID. However, limitations include the small number of patients, particularly in the 3-weekly infusion group, which restricts the interpretation of results. It can also not be excluded that variability in the dose range, sample size, and duration of treatment in the present study compared with previous studies may affect the comparison of PK parameters between Japanese and non-Japanese patients. In addition, there was no comparator product used in this study.
In conclusion, there were no substantial differences in PK parameters between groups receiving 3-weekly or 4-weekly infusions of Privigen®, which can improve the flexibility of IVIG therapy with Privigen® in patients with PID. Given the small number of patients in the 3-weekly regimen group in this study, further investigation with larger patient samples is warranted to evaluate any differences in PK parameters between the two regimens. In addition, this study suggests for the first time that the PK characteristics of Privigen® are similar between Japanese and non-Japanese patients with PID.
Acknowledgments
Editorial and graphical support was provided by Heather Shawcross, Ph.D., of Fishawack Communications GmbH, a member of the Fishawack Group of Companies, supported by CSL Behring.
Disclosure statement
The data summarized in this study are from CSL Behring-sponsored clinical trials. GB, MAT, JH, and MAR are employees of CSL Behring. GB, MAT, JH, and MAR own CSL Behring shares. TM has received grants from CSL Behring and serves as an associate editor-in-chief on the editorial board of Immunological medicine.
References
- Bousfiha A, Jeddane L, Al-Herz W, et al. The 2015 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2015;35(8):727–738.
- Picard C, Al-Herz W, Bousfiha A, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35(8):696–726.
- Wood P, Stanworth S, Burton J, et al. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol. 2007;149(3):410–423.
- McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol. 2018;14(S2):61.
- Bousfiha A, Jeddane L, Picard C, et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2018;38(1):129–143.
- Ortega-López MC, Garay J, Pinilla ML. Efficacy, safety and quality of life in patients receiving subcutaneous IgG treatment: experience in Bogotá, Colombia. Immunotherapy. 2018;10(10):861–869.
- Modell V, Knaus M, Modell F, et al. Global overview of primary immunodeficiencies: a report from Jeffrey Modell Centers worldwide focused on diagnosis, treatment, and discovery. Immunol Res. 2014;60(1):132–144.
- Ballow M. Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol. 2002;109(4):581–591.
- Espanol T, Prevot J, Drabwell J, et al. Improving current immunoglobulin therapy for patients with primary immunodeficiency: quality of life and views on treatment. Patient Prefer Adherence. 2014;8:621–629.
- Jolles S, Orange JS, Gardulf A, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179(2):146–160.
- Krivan G, Jolles S, Granados EL, et al. New insights in the use of immunoglobulins for the management of immune deficiency (PID) patients. Am J Clin Exp Immunol. 2017;6(5):76–83.
- Aghamohammadi A, Moin M, Farhoudi A, et al. Efficacy of intravenous immunoglobulin on the prevention of pneumonia in patients with agammaglobulinemia. FEMS Immunol Med Microbiol. 2004;40(2):113–118.
- Kearns S, Kristofek L, Bolgar W, et al. Clinical profile, dosing, and quality-of-life outcomes in primary immune deficiency patients treated at home with immunoglobulin G: data from the IDEaL Patient Registry. J Manag Care Spec Pharm. 2017;23:400–406.
- Albin S, Cunningham-Rundles C. An update on the use of immunoglobulin for the treatment of immunodeficiency disorders. Immunotherapy. 2014;6(10):1113–1126.
- Privigen®, Immune Globulin Intravenous (Human), 10% Liquid; CSL Behring AG, US PI, September. 2017 [cited 2019 Oct 28]. Available from: https://www.fda.gov/media/83304/download
- Privigen®100 mg/ml solution for infusion, EU, Summary of product characteristics, 2017 [cited 2019 Oct 28]. Available from: https://www.ema.europa.eu/en/documents/product-information/privigen-epar-product-information_en.pdf
- CSL Behring. Japan’s Ministry of Health, Labour and Welfare Approves CSL Behring Immunoglobulins to Treat Patients with Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP). [cited 2019 Sep 11]. Available from: https://www.cslbehring.com/newsroom/2019/20190326-japan-cidp.
- Sleasman JW, Duff CM, Dunaway T, et al. Tolerability of a new 10% liquid immunoglobulin for intravenous use, Privigen, at different infusion rates. J Clin Immunol. 2010;30(3):442–448.
- Bolli R, Woodtli K, Bartschi M, et al. L-Proline reduces IgG dimer content and enhances the stability of intravenous immunoglobulin (IVIG) solutions. Biologicals. 2010;38(1):150–157.
- Stein MR, Nelson RP, Church JA, et al. Safety and efficacy of Privigen, a novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29(1):137–144.
- Tortorici MA, Lawo JP, Weide R, et al. Privigen(R) has similar pharmacokinetic properties in primary and secondary immune deficiency. Int Immunopharmacol. 2019; 66:119–126.
- Wasserman RL, Church JA, Peter HH, et al. Pharmacokinetics of a new 10% intravenous immunoglobulin in patients receiving replacement therapy for primary immunodeficiency. Eur J Pharm Sci. 2009;37(3-4):272–278.
- Eijkhout HW, van Der Meer JW, Kallenberg CG, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135(3):165–174.
