Abstract
Older adults are mostly affected by chronic lymphocytic leukemia (CLL). The present study aimed to evaluate oxidative stress in CLL and to assess its impact on IL-9, Th9 cells levels and prognosis of cases. Seventy Egyptian CLL patients and 15 healthy controls were included. Th9 cell and immunophenotyping of abnormal B cells were assessed by flow cytometry, IL-9 level using ELISA, IL-9 mRNA by qRT-PCR, cytogenetics using FISH, and oxidative stress parameters were determined spectrophotometrically and with native gel electrophoresis. Oxidative stress was elevated in CLL that correlated with abnormal immunophenotyping, cytogenetic changes, bad prognosis, Th9 cells, and overexpression of IL-9. Levels of IL-9 and Th9 cells were strongly correlated with oxidative stress and bad prognostic markers in CLL, indicating that these cells may contribute to CLL by novel mechanisms that could include oxidant injury.
Graphical Abstract
1. Introduction
Chronic lymphocytic leukemia (CLL) patients in early stages can live for many years without treatment but rather with a ‘watch and wait’ strategy. If symptoms got worse or patients were in advanced stage, treatment can be administered [Citation1]. There are some prognostic factors for CLL; lymphocyte doubling time (LDT), a measure of time required for circulating lymphocytes to be duplicated, less than 12 months marks increased risk [Citation2]; mutation in certain genes, such as 17p or 11q deletions, indicates poor prognosis, and median overall survival time of 3 and 7 years, respectively, while CLL patients who carry trisomy 12 or with normal cytogenetic status achieve a longer overall survival time of 9 years; whereas 13q deletion represent the best prognosis with a median survival time of 11 years [Citation3]; CLL cells expressing CD38, more than 30%, indicates worse prognosis [Citation4]; CLL cells expressing more than 20% of Zeta-associated protein-70 (ZAP-70), a cytoplasmic protein not expressed by B cells but rather expressed by T cells, indicates bad prognosis and lower survival rates [Citation5].
T helper (Th) cell subsets differentiate from naïve CD4+ T cells after exposure to a specific cytokine environment. Th9 cells, unlike Th cells subsets, are a recent addition and characterized by secretion of interleukin-9 (IL-9) [Citation6] and it is only recently researchers have understood the development and function of Th9 cells. Transforming growth factor-β (TGF-β) and IL-4 can drive Th9 cells development from naïve CD4 cells. IL-9 can induce tumor advancement in many cancers including CLL [Citation7].
Oxidative stress has been defined as an increase in the production of free radicals more than antioxidant system in the body [Citation8]. The imbalance between them leads to potential cellular damage. Most cells have adequate antioxidant and repair systems that enable them to recognize and remove oxidized biomolecules at mild degree of oxidative stress [Citation8]. Oxidative stress is involved in recruitment of many diseases including autoimmunity, allergies, chronic inflammation, metastatic cancer, and angiogenesis [Citation9]. Damage to organic molecules in the cell such as DNA, lipids, proteins, and vasculature is a result of such alterations. Oxidative damage to genes causes the development of ageing, mutation, and cancer [Citation10]. Aforementioned data indicated that IL-9 and oxidative stress have a role in cancer. So in this study, the aim was to study the oxidative stress in CLL patients by measuring malondialdehyde (MDA), as the indices of lipid peroxidation, protein carbonyl (PC) as a product of oxidized proteins, and antioxidants including superoxide dismutase (SOD) and vitamin C in blood samples taken from CLL patients and correlate them with the abnormal immunophenotyping, cytogenetic changes and clinical features in CLL. Moreover, SOD and catalase activities were validated by using native gel electrophoresis. This study correlated between these oxidative stress parameters and circulating Th9 cells and its signature cytokine, IL-9, in CLL patients.
2. Material and methods
2.1. Subjects
The subjects were 70 Egyptian chronic lymphocytic leukemia (CLL) patients from the Oncology Center of Mansoura University, Egypt and healthy volunteers (n = 15) as a control group. All individuals gave informed consent to participate as a subject in this study. The CLL patients included 36 males and 34 females, with an age range of 34–87 years. Healthy controls were 8 males and 7 females, with an age range of 40–72 years. Detailed subject characteristics were summarized (Supplementary data, Tables S1 and S2)
2.2. Laboratory investigations
The subjects were tested for routine clinical and laboratory investigations including complete blood count (CBC), hepatitis testing, liver and kidney function tests.
2.3. Flow cytometric immunophenotypic analysis
Detailed procedures of flow cytometric immunophenotyping analysis were performed as described in Sabry et al. [Citation11,Citation12].
2.4. Cytogenetics
FISH analysis was performed on peripheral blood samples from patients with CLL in Cytogenetics Laboratory in Oncology Center, Mansoura University using probes mentioned in a preceding paper [Citation11].
2.5. Estimation of serum ascorbic acid
A trichloroacetic acid was added to serum samples to precipitate proteins. Trichloroacetic acid filtrate of samples is shaken with charcoal for 5 min and centrifuged. The acetic acid was adsorbed on charcoal and ascorbic acid is oxidized to dehydroascorbic acid. The supernatant was treated with 2,4-dinitrophenylhydrazine (2,4-DNPH) and thiourea to form the 2,4-dinitrophenylosazone. A reddish complex was produced after adding strong sulfuric acid. The color was measured at 515 nm [Citation13].
2.6. Estimation of serum MDA
The level of MDA level was estimated by a method described previously by Ohkawa et al. [Citation14]. The method depended on the reaction between thiobarbituric acid (TBA) and MDA in acidic medium at 94 °C for 30 min to yield thiobarbituric acid reactive product which is pink and measured at 532 nm.
2.7. Estimation of serum PC
PC was detected using a modified method of the traditional one proposed by Levine et al. [Citation15]. This method depended on 2,4-DNPH reaction with carbonyl groups. The hydrazone derivatives of carbonyl groups produced from the reaction of PC groups with DNPH in alkaline medium was measured at 450 nm [Citation16].
2.8. Estimation of SOD activity by colorimetric assay
This assay relied on the ability of SOD to inhibit the phenazine methosulphate-mediated reduction of nitro blue tetrazolium (NBT) dye according to the method described by Nishikimie et al. [Citation17]. The increase in absorbance was recorded for 5 min at 560 nm at 25 °C.
2.9. Detection of SOD and catalase activity by using native gel electrophoresis
The SOD activity gel assay relied on SOD ability to suppress the reduction of NBT and was previously described by Ornstein [Citation18]. The assay principle was based on the ability of SOD to remove O2˙− and the areas where it is active will be clear (achromatic bands) [Citation19].
Catalase activity assay utilized a procedure measuring peroxide removal [Citation20]. The removal of peroxide by catalase will not allow for the potassium ferricyanide (a yellow substance) to be reduced to potassium ferrocyanide that will react with ferric chloride to form a Prussian blue precipitate [Citation21]. 12% or 8% gel was used for SOD or catalase, respectively. The experiments were repeated three times in order to confirm the finding and correlate it with the results of colorimetric kinetic assay.
2.10. Analysis of the changes in circulating Th9 cells by flow cytometry
Th9 cell level were defined as the number of IL-9+ cells among T cell population that were CD5+ CD19− and after further characterization of cellular subtype by staining with antibodies to CD4 as mentioned in Sabry et al. [Citation11].
2.11. Quantification of IL-9 mRNA by qRT-PCR
The quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis for quantification of IL-9 mRNA was done as defined previously by Lv et al. [Citation22] and mentioned by Sabry et al. [Citation11].
2.12. Quantification of serum level of IL-9
Blood samples were taken from patients and control groups and the serum was separated and stored at −20 °C till analysis. The kit Human IL-9 DuoSet ELISA (R&D Systems) was used for estimating the level of IL-9 and performed according to instructions of the manufacturer and as mentioned in Sabry et al. [Citation11].
2.13. Statistical analysis
Statistical analysis of data was carried out by using statistical computer programs SPSS (statistical package for social science) program (SPSS Inc., version 20, Chicago, IL), and the GraphPad Prism 3.0 software (GraphPad Software Inc, San Diego, CA, USA). Comparison between the mean value of two groups was analyzed using t-test or Man Whitney (for non-parametric). Multiple comparisons were done by using one-way analysis of variance (ANOVA) test. Areas under receiver operating characteristic (ROC) curves were conducted for determination of cut-off values and differentiation between control and CLL subjects. Survival analysis was done by Kaplan–Meier test and Log-Rank test was used to determine the statistical significance of differences among curves. Cox regression analysis was used for prediction of time to start first treatment (TST). Pairwise parametric correlations were detected by computing the Pearson’s r statistic, whereas Spearman’s correlation coefficient was used for non-parametric correlations. A p value less than .05 was considered statistically significant value.
3. Results
3.1. Oxidative stress parameters in CLL
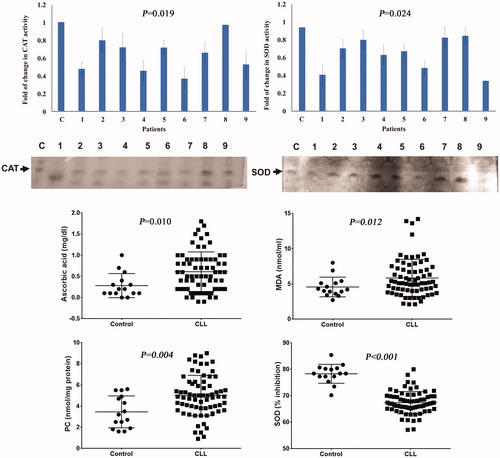
Serum levels of ascorbic acid, MDA, PC, and SOD were detected, using spectrophotometric assay, in CLL patients as well as healthy controls to investigate the possible involvement of oxidative stress in CLL. The data showed that ascorbic acid, MDA, and PC levels were significantly elevated in CLL patients than healthy controls (p = .010, p = .012, p = .004, respectively). SOD was significantly decreased in CLL group than healthy controls (p < .001) ().
Figure 1. Serum levels of oxidative stress parameters, using spectrophotometric and native gel electrophoresis, in CLL patients. Ascorbic acid, MDA, and PC levels were significantly elevated in CLL patients more than their levels in healthy controls. SOD was significantly decreased in CLL group than healthy controls. The results of native gel electrophoresis were obtained from three independent experiments. There were significant decreases in SOD and catalase levels in CLL patients when compared with their corresponding levels in healthy controls.
3.2. Determination of SOD and catalase activities using native gel electrophoresis in CLL patients
CLL patients in addition to healthy controls were examined for serum levels of SOD and catalase using native gel electrophoresis in order to confirm the spectrophotometric analysis. There was significant decrease in SOD (p = .024) and catalase (p = .019) levels in CLL patients when compared with their corresponding levels in healthy controls (). A significant positive correlation was found between serum levels of SOD and catalase in selected CLL patients (r = 0.733, p = .025).
3.3. Relation of ascorbic acid, MDA, PC, and SOD with the Del(17p) and clinical parameters of CLL patients
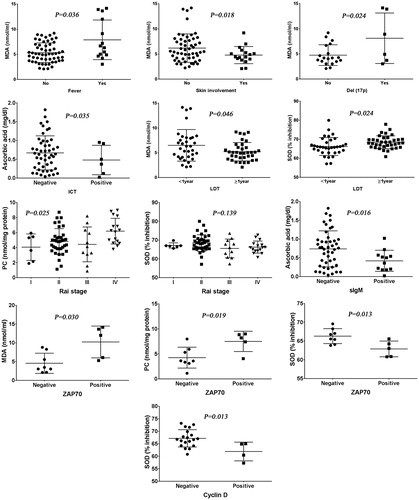
The possibility of interaction between oxidative stress and clinical parameters as well as other available prognostic factors of CLL patients was examined. MDA level was significantly higher in CLL patients with fever, absence of skin involvement, and Del(17p) (p = .036, p = .018, p = .024, respectively). Patients with LDT less than one year had significant high levels of MDA and low levels of SOD (p = .046, p = .024, respectively). Ascorbic acid level increased in patients negative to indirect Coombs test (ICT) (p = .035). PC was statistically different among different Rai stages of CLL patients using one-way analysis of variance (ANOVA) test. In post-hoc comparisons, PC level was higher in Rai stage IV than Rai stages I, II, and III (p = .017, p = .014, p = .016, respectively). Moreover, SOD level was observed to be higher in Rai stage II than Rai stage III (p = .048) ().
Figure 2. Relation of ascorbic acid, MDA, PC, and SOD with the different clinical and prognostic parameters of CLL as well as immunophenotyping of abnormal B cells. MDA level was increased in CLL patients with fever, absence of skin involvement, and Del(17p). Patients with LDT less than one year had significant high levels of MDA and low levels of SOD. Ascorbic acid level was higher in cases negative for ICT. PC level was higher in Rai stage IV than Rai stages I, II, and III (p = .017, p = .014, p = .016, respectively). Moreover, SOD level was observed to be higher in Rai stage II than Rai stage III (p = .048). Ascorbic acid was decreased in CLL cases positive for sIgM. Positive cases for ZAP-70 were strongly associated with higher levels of MDA and PC, and lower level of SOD. CLL patients who were positive for cyclin D had lower levels of SOD.
3.4. Relation of serum levels of ascorbic acid, MDA, PC, and SOD with immunophenotyping of abnormal B cells in CLL patients
In CLL patients, there were no significant differences regarding serum levels of ascorbic acid, MDA, PC, or SOD associated with positive and negative cases for CD79b, Flinder Medical Centre (FMC)7, CD38, B cell lymphoma (BCL)2, and BCL6. Serum level of ascorbic acid was significantly lower in CLL patients positive for surface immunoglobulin M (sIgM) (p = .016). Positive cases for ZAP-70 were highly linked with increased levels of MDA and PC (p = .030, p = .019, respectively), and lower level of SOD (p = .013), but without significant difference regarding serum ascorbic acid level. CLL patients who were positive for cell cycle cyclin D had lower levels of SOD ().
3.5. Correlations between serum levels of ascorbic acid, MDA, PC, SOD, and their relation with Th9 cells and IL-9 levels as well as clinical and laboratory measurements in patients with CLL
The associations between some oxidative stress parameters and their relation with levels of Th9 cells and serum IL-9 in CLL patients were investigated. MDA was positively correlated with PC (p = .013), Th9 cells (p < .001), IL-9 serum level (p = .001) and gene expression of IL-9 (p = .021), and negatively correlated with SOD (p = .020). Serum level of PC was positively correlated with Th9 cells (p = .030), IL-9 serum level (p = .044) and gene expression of IL-9 (p = .015), and negatively correlated with SOD (p = .002). SOD level was negatively correlated with both Th9 cells (p = .007), and IL-9 serum level (p = .008). No correlation was observed between ascorbic acid level and levels of MDA, PC, or SOD ().
Figure 3. Correlations between serum levels of ascorbic acid, MDA, PC, SOD, and their relation with Th9 cells and IL-9 levels as well as clinical and laboratory measurements in patients with CLL. Malondialdehyde was positively correlated with PC, Th9 cells, IL-9 serum level and expression, and negatively correlated with SOD. Serum level of PC was positively correlated with Th9 cells, IL-9 serum level and expression, and negatively correlated with SOD. SOD level was negatively correlated with both Th9 cells, and IL-9 serum level. There was significant negative correlation between serum levels of creatinine and ascorbic acid, RBCs count and PC level, and serum levels of total bilirubin and MDA; as well as ssignificant positive correlations between Hb concentration and serum level of SOD, and platelets count and MDA level.
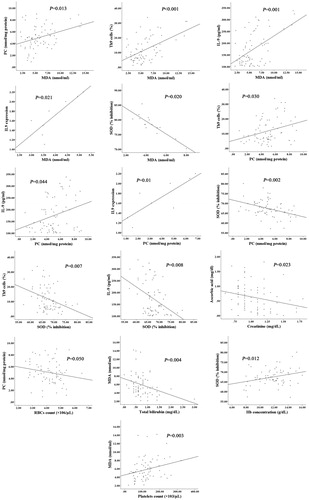
The correlation between serum levels of ascorbic acid, MDA, PC and SOD, and clinical parameters was studied. There were significant negative correlations between serum levels of creatinine and ascorbic acid (r = −0.271, p = .023), RBCs count and PC level (r = −0.235, p = .050), and serum levels of total bilirubin and MDA (r = −0.344, p = .004). Significant positive correlations were observed between Hb concentration and serum level of SOD (r = 0.297, p = .012), and platelets count and MDA level (r = 0.355, p = .003) ().
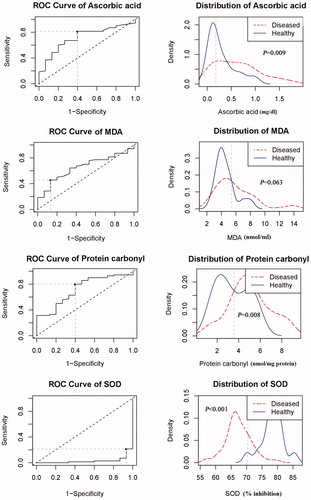
3.6. Area under ROC curves (AUC) of serum levels of ascorbic acid, MDA, PC and SOD
AUC of serum levels of ascorbic acid, MDA, PC and SOD were conducted for determination of cut-off values and differentiation between patients and control groups (). Ascorbic acid, PC, and SOD showed significant AUCs (p = .009, p = .008, p < .001, respectively) that could differentiate between patients and controls. The cut–off level of serum ascorbic acid 0.18 mg/dl (AUC = 0.716, 95% CI = 0.587–0.848, p = .009, sensitivity = 81.4%, specificity = 60%), PC 5.4 nmol/mg protein (AUC = 0.718, 95% CI = 0.579–0.856, p = .008, sensitivity = 80%, specificity = 60%), and SOD 70.4% inhibition (AUC = 0. 0.031, 95% CI = 0.000–0.066, p < .001, sensitivity = 21.4%, specificity = 6.7%) was decided by using ROC Curve analysis. On the other hand, MDA did not show AUC significance (p = .063) for discrimination between control and CLL subjects. The cut–off level of MDA 5.4 nmol/ml (AUC = 0.653, 95% CI = 0.520–0.787, p = .063) showed sensitivity of 45.7% and specificity of 86.7%.
Figure 4. The ROC curves and distribution graphs of ascorbic acid, MDA, protein carbonyl, and SOD levels for discrimination between CLL and control groups. Ascorbic acid, protein carbonyl, and SOD showed significant AUCs that could discriminate between cases and control groups, while MDA did not show AUC significance for discrimination between control and CLL subjects.
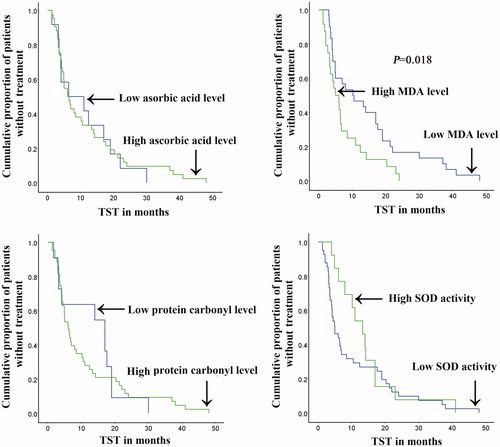
3.7. Comparison of time to start treatment (TST) according to ascorbic acid, MDA, PC, and SOD levels in CLL patients
The TST did not show significant difference in CLL patients with high and low levels of ascorbic acid, PC and SOD. However patients with low level of MDA had significantly longer TST (p = .018) than that associated with high MDA level ().
Figure 5. The TST according to ascorbic acid, MDA, protein carbonyl and SOD levels in studied CLL patients. A significantly longer TST was associated with low serum MDA level than that associated with high MDA level. There was no significant difference regarding TST between CLL patients with low and high levels of ascorbic acid, PC and SOD.
3.8. Th9 cells, IL9, and MDA are significant independent risk factors for shorter TST in CLL
Cox regression analysis was conducted for prediction of TST within studied CLL cases, using age, gender, LDT, absolute lymphocytic count (ALC) at diagnosis, ZAP 70 and CD38 positivity, Rai staging, circulating Th9 cell frequencies, serum levels of IL9, ascorbic acid, MDA, PC, and SOD. Advanced Rai stage, Th9 cells, IL9, and MDA levels were considered as significant independent risk factors for shorter TST in studied CLL cases in univariable (p = .014, p = .002, p = .004, p = .026, respectively) but not multivariable analyses (Supplementary data, Table S3).
4. Discussion
In recent decades, numerous prognostic factors are studied in CLL such as, CD38 and ZAP-70 expression which serve as independent prognostic factors and correlated with bad prognosis in CLL patients [Citation23]. This study aimed to evaluate the level of oxidative stress in patients with CLL followed by evaluation of its association with prognosis in CLL patients and to study cross-relation between these oxidative stress parameters and to assess their association with circulating Th9 cells and serum IL-9 in CLL patients.
In the present study, plasma levels of MDA and PC increased in CLL patients than controls. These results are in agreement with Zelen et al. [Citation24], Pujari and Jadkar [Citation25], and Abd El-Aziz et al. [Citation26]. Higher levels of MDA and PC in CLL patients indicate increased levels of oxidative stress in CLL. Results obtained from the current study are in accordance with a previous study by Bakan et al. [Citation27], who reported lower serum level of SOD activity as well as higher concentration of MDA in CLL patients when compared with healthy subjects. Decreased level of SOD causes accumulation of hydrogen peroxide (H2O2) and superoxide anion (O2•−) in cancer cells that is a cause for the increased plasma levels of MDA and PC in CLL patients when compared to controls [Citation28].
The possibility of interaction between oxidative stress parameters and clinical parameters as well as other available prognostic factors of CLL was examined. The level of MDA was elevated in CLL patients suffering from skin involvement, fever, and Del(17p). Patients with CLL who have short LDT less than one year had significant high levels of MDA and low levels of SOD. Similarly, Abd El-Aziz et al. [Citation26] showed significant higher concentrations of MDA in CLL patients with shorter lymphocytic doubling time and those who have advanced stage. Decreased level of ascorbic acid in patients positive to indirect Coombs test (ICT) was observed. The depletion of plasma ascorbic acid in CLL patients positive to ICT may contribute to abnormalities in polyunsaturated fatty acids of the cell membrane due to its attack by free radicals. In addition, increased level of PC and decreased level of SOD were observed in advanced Rai stages.
ZAP-70-positive CLL patients ZAP-70 had higher levels of MDA and PC, and lower level of SOD. On the contrary, in previous study by D’arena et al. [Citation29], d-ROMs and BAP test results did not differ among CLL patients positive and negative for ZAP-70 and CD38. However, Musolino et al. [Citation30] have correlated serum levels of carbonyl groups positively with CD38 expression and negatively with ZAP-70 expression in CLL patients.
The current results indicated a negative correlation between the TST and serum MDA level in CLL patients. These results are consistent with the previous study by Labib et al. [Citation31], who illustrated that higher levels of oxidative stress can predict a shorter TST with poor response in CLL patients.
Further negative correlations were observed between RBCs count and PC level (p = .050), serum levels of total bilirubin and MDA (p = .004), serum levels of creatinine and ascorbic acid (p = .023). Likewise, total bilirubin and MDA were negatively correlated in control group (p = .009). Anemia is associated with weak antioxidant system because intracellular enzymes in erythrocytes, such as SOD and catalase, represent an important component of the antioxidant system [Citation32]. It is thus possible to explain the negative correlation, observed here, between PC and RBCs count in CLL. Under conditions of severe oxidative stress, bilirubin has potent anti-oxidant properties [Citation33]; and that can explain elevated MDA level with decreased bilirubin in CLL.
Data from the present study revealed a positive correlation between Hb concentration and serum level of SOD in CLL patients. This can be explained by results obtained by Grune et al. [Citation32], who have detected a weakened antioxidant system in anemia patients. In the present study, a significant positive correlation between platelets count and MDA level (p = .003) was observed in CLL patients. The increase in lipid peroxidation as measured by MDA levels is probably due to the production of ROS by platelets in CLL patients [Citation34].
The MDA was positively correlated with PC, Th9 cells and IL-9 levels, whereas it was negatively correlated with SOD. The positive correlation between MDA and PC has been established in many diseases including coronary heart deisease [Citation35], and Alzheimer disease [Citation36], indicating that MDA and PC levels are early indication of progressive damage of oxidative stress. Niedbala et al. [Citation37] have shown that free radicals potentially induce Th9 cells activation by stimulating expression of p53 gene, which stimulates IL-2 production and activates the signal transducers and activators of transcription (STAT)-5 and the expression of interferon-regulatory factor (IRF)-4; these events leads to activation of IL-9 gene promoter.
The current data showed that serum level of PC was positively correlated with Th9 cells and IL-9 level, and negatively correlated with SOD. Pilette et al. [Citation38] have previously shown that IL-9 inhibits ROS production by human blood monocytes. More recently, Ji and Li have reported that oxidative stress can induce activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) to further enhance the gene expression and synthesis of antioxidant enzymes [Citation39]. But the NFκB activation also upregulates the expression of proinflammatory cytokines, including IL-9 [Citation40], which may be involved in the pathogenesis of CLL. Likewise, current study showed that both Th9 cells (p = .007) and IL-9 levels (p = .008) were negatively correlated with serum levels of SOD in CLL patients.
In accordance with the results, obtained here, from native gel electrophoresis, Oltra et al. [Citation41] have examined the activities of SOD and catalase in lymphocytes of patients with CLL and compared them with controls. They documented decreased activities of SOD and catalase enzymes in CLL. Decreased levels of SOD and catalase cause the accumulation of hydrogen peroxide (H2O2) and superoxide anion (O2•−) in cancer cells that is a cause for the increased plasma levels of MDA and PC in CLL patients when compared to controls [Citation28]. To the best knowledge of the authors, this is the first report to provide a preliminary insight into association of Th9 cells and IL-9 with oxidative stress in the process of CLL.
5. Conclusion
The current study indicated enhanced levels of oxidative stress as measured by lipid and protein peroxidation (MDA and PC) and decreased levels of enzymatic antioxidants (SOD and catalase) in CLL patients. Higher level of oxidative stress was associated with Del(17p), abnormal immunophenotyping and worse clinical presentation in CLL. These findings suggest that an association between oxidative stress and abnormal cytogenetics, immunophenotyping and worse prognosis of CLL and may be used as a future therapeutic target. Also the current study indicated for the first time, according to the best knowledge of the authors, that levels of circulating Th9 cells and serum IL-9 were strongly correlated with oxidative stress indicating that these cells may contribute to CLL by novel mechanisms that could include oxidant injury.
Supplemental Material
Download MS Word (20.9 KB)Acknowledgments
The authors express their gratitude for the Biochemistry Division, Faculty of Science, Mansoura University, Egypt, for supplying part of the required reagents and equipment. Additionally, the authors thank the Oncology Center of Mansoura University, Egypt for providing access for the collection of the CLL cases.
Disclosure statement
The authors declare no competing financial interests.
References
- Al-Sawaf O, Bazeos A, Robrecht S, et al. Mode of progression after first line treatment correlates with outcome of chronic lymphocytic leukemia (CLL). Am J Hematol. 2019.
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815.
- Puiggros A, Blanco G, Espinet B. Genetic abnormalities in chronic lymphocytic leukemia: where we are and where we go. BioMed Res Int. 2014;2014:1.
- Malavasi F, Deaglio S, Damle R, et al. CD38 and chronic lymphocytic leukemia: a decade later. Blood. 2011;118:3470.
- Pflug N, Bahlo J, Shanafelt TD, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124:49.
- Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat Immunol. 2008;9:1341.
- Sabry SA, El-Senduny FF, Abousamra NK, et al. Interaction between Th9 cells, interleukin-9 and oxidative stress in chronic lymphocytic leukemia. Trends Appl Sci Res. 2019;14:56–69.
- Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748.
- Russell S, Johnston J, Noguchi M. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045.
- Prasad S, Gupta SC, Pandey MK, et al. Oxidative stress and cancer: advances and challenges. Oxid Med Cell Longev. 2016;2016:1.
- Sabry SA, El-Senduny FF, Abousamra NK, et al. Prognostic role of T helper 9 cells and interleukin-9 in chronic lymphocytic leukemia. Am J Biochem Mol Biol. 2018;8:23–33.
- Kilo MN, Dorfman DM. The utility of flow cytometric immunophenotypic analysis in the distinction of small lymphocytic lymphoma/chronic lymphocytic leukemia from mantle cell lymphoma. Am J Clin Pathol. 1996;105:451–457.
- Butler AM, Cushman M, MacLachlan E. The determination of ascorbic acid in whole blood and its constituents by means of methylene blue; macro and micromethods. J Biol Chem. 1943;150:453–461.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Levine RL, Garland D, Oliver CN, et al. [49] Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478.
- Mesquita CS, Oliveira R, Bento F, et al. Simplified 2, 4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem. 2014;458:69–71.
- Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854.
- Ornstein L. Disc electrophoresis‐i background and theory. Ann N Y Acad Sci. 2006;121:321–349.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287.
- Lewis A, Du J, Liu J, et al. Metastatic progression of pancreatic cancer: changes in antioxidant enzymes and cell growth. Clin Exp Metastasis. 2005;22:523–532.
- Treadwell FP, Hall WT. Analytical chemistry: based on the German text of F.P. Treadwell … translated and revised by William T. Hall. New York: Wiley; 1948.
- Lv X, Feng L, Fang X, et al. Overexpression of IL-9 receptor in diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2013;6:911–916.
- Klabusay M, Hrabcakova V, Coupek P, et al. Additional value of quantitative expression of ZAP-70 and CD38 for prognostic factors in chronic lymphocytic leukemia. J Clin Oncol. 2017;35:e19006.
- Zelen I, Djurdjevic P, Popovic S, et al. Antioxidant enzymes activities and plasma levels of oxidative stress markers in B-chronic lymphocytic leukemia patients. J Buon. 2010;15:330–336.
- Pujari K, Jadkar S. Superoxide dismutase levels in leukemias. Int J Basic Med Sci. 2011;2:96–100.
- Abd El-Aziz AEAF, Yahya RS, Abd El Messih HM, et al. Activity of some antioxidant enzymes in patients with chronic lymphocytic leukemia. Imp J Interdiscip Res. 2016;2:1213–1224.
- Bakan N, Taysi S, Yilmaz Ö, et al. Glutathione peroxidase, glutathione reductase, Cu–Zn superoxide dismutase activities, glutathione, nitric oxide, and malondialdehyde concentrations in serum of patients with chronic lymphocytic leukemia. Clin Chim Acta. 2003;338:143–149.
- Đurđević P, Zelen I, Ristić P, et al. Oxidative stress markers in chronic lymphocytic leukemia. Medicus. 2006;7:52–56.
- D’arena G, Vitale C, Perbellini O, et al. Prognostic relevance of oxidative stress measurement in chronic lymphocytic leukaemia. Eur J Haematol. 2017;99:306–314.
- Musolino C, Allegra A, Alonci A, et al. Carbonyl group serum levels are associated with CD38 expression in patients with B chronic lymphocytic leukemia. Clin Biochem. 2011;44:1487–1490.
- Labib HA, Hassanein M, Etewa RL. Serum copper is a simple but valuable prognostic marker in B-cell chronic lymphocytic leukemia. Int J Hematol. 2014;100:575–581.
- Grune T, Lietz G, Palou A, et al. β-carotene is an important vitamin a source for humans–3. J Nutr. 2010;140:2268S–2285S.
- Bin-Nun A, Mimouni F, Kasirer Y, et al. Might bilirubin serve as a natural antioxidant in response to neonatal encephalopathy? Am J Perinatol. 2018;35:1107.
- Dzhatdoeva AA, Proskurnina EV, Nesterova AM, et al. Mitochondria as a source of superoxide anion radical in human platelets. Biochem Moscow Suppl Ser A. 2018;12:43–49.
- Pantke U, Volk T, Schmutzler M, et al. Oxidized proteins as a marker of oxidative stress during coronary heart surgery. Free Radic Biol Med. 1999;27:1080–1086.
- Greilberger J, Koidl C, Greilberger M, et al. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer’s disease. Free Radic Res. 2008;42:633–638.
- Niedbala W, Besnard AG, Nascimento DC, et al. Nitric oxide enhances Th9 cell differentiation and airway inflammation. Nat Commun. 2014;5:4575.
- Pilette C, Ouadrhiri Y, Van Snick J, et al. IL-9 inhibits oxidative burst and TNF-α release in lipopolysaccharide-stimulated human monocytes through TGF-β. J Immunol. 2002;168:4103–4111.
- Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med Cell Longev. 2016;2016:1.
- Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158.
- Oltra AM, Carbonell F, Tormos C, et al. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic lerkenia. Free Radic Biol Med. 2001;30:1286–1292.
