Abstract
Associations between anti-M-type phospholipase A2 receptor (PLA2R) antibodies and disease activity and prognosis have been suggested in primary membranous nephropathy (MN); however, more evidence is needed. We aimed to establish a clinically useful method to measure anti-PLA2R antibodies. We developed a western blot assay and a cell-based enzyme-linked immunosorbent assay (ELISA). Anti-PLA2R antibodies were evaluated retrospectively using these assays and the commercial solid-phase ELISA. Anti-PLA2R antibodies were detected in 12, 6, and 12 out of 23 Japanese patients with biopsy-proven primary MN using the western blot, the cell-based ELISA, and the solid-phase ELISA, respectively. The samples of the lupus MN patients tested negative. The levels of proteinuria correlated moderately with the titres of anti-PLA2R antibodies measured by the three methods (r = 0.39–0.47). Anti-PLA2R antibodies were significantly associated with physicians’ decisions on immunosuppressive treatment without prior knowledge of anti-PLA2R antibody positivity (p < .01). In the longitudinal analysis, the titres of anti-PLA2R antibodies measured by the solid-phase ELISA declined significantly following treatment (p = .03). In conclusion, these results suggest the usefulness of anti-PLA2R antibody as a diagnostic, prognostic, and surrogate biomarker in primary MN. The three methods proved to be reliable for measuring anti-PLA2R antibody titres, but their performances differ.
1. Introduction
Membranous nephropathy (MN) is an antibody-mediated glomerular disease characterised by the subepithelial formation of immune deposits containing antigen, immunoglobulin G (IgG), and complement components [Citation1,Citation2]. Better risk prediction is needed to identify patients with primary (or idiopathic) MN who will benefit from immunosuppressive treatment because of the variability in its natural course, whereby disease-spontaneous remission occurs in 40–50% of patients [Citation1,Citation2].
Since 2009 [Citation3], it has been reported that 70–80% of patients with idiopathic MN, but not patients with secondary MN, have circulating antibodies against the M-type phospholipase A2 receptor (PLA2R), a 185-kDa transmembrane protein located on podocytes in the Bowman's capsule in the kidneys [Citation1,Citation2]. Although measuring anti-PLA2R antibody levels has also been suggested to be a potential method for following and predicting the response to treatment [Citation1,Citation2], there are some discrepancies among studies [Citation4–7]. Thus, we aimed to further assess the usefulness of anti-PLA2R antibodies as a biomarker for purposes other than diagnosis in primary MN.
At the start of this study, the quantitative measuring methods that were widely available were limited to either western blots [Citation3] or a semi-quantitative immunofluorescence test [Citation8], which are semi-quantitative, time-consuming when applied to large numbers of samples, subject to large variation between assay batches, and often run in a mode requiring subjective observer assessment [Citation5]. Thus, we aimed to develop a cell-based enzyme-linked immunosorbent assay (ELISA) for the quantification of anti-PLA2R antibodies and to assess its clinical usefulness in patients with MN. In addition, after the initiation of our project, a commercial solid-phase direct ELISA kit was launched by EUROIMMUN AG (Lübeck, Germany) [Citation9], and we also aimed to validate this novel ELISA kit and compare it with our cell-based ELISA and western blot. Generally, because different methods for measuring autoantibodies often have some different characteristics, it was considered to be worth comparing multiple methods [Citation10].
2. Subjects and methods
2.1. Study subjects and sample collection
Adult patients with primary MN and lupus MN, and healthy controls, were eligible for the present study. In the period from 2001 to 2014, some of the patient serum samples used in this study had been previously collected and stored for unspecified medical studies under general consents, and other samples were prospectively collected specifically for this study under written informed consents at the Tokyo Women’s Medical University. Sera from healthy subjects were used as controls. Primary MN patients, who had already received immunosuppressive treatment before sample collection, were excluded from this study. Patients with secondary MN other than SLE were also excluded from this study. Ultimately, sera from 23 patients with primary MN, 16 patients with lupus MN, and 16 healthy controls were available and used in this study. This study was approved by the ethics committee of Tokyo Women's Medical University (registration number 2993 and 3099) and adhered to the principles of the Declaration of Helsinki throughout the study.
All of the patients with primary and lupus MN in this study were referred to our university hospital because of active nephritis. MN in all of the cases was diagnosed by kidney biopsy, and all of the initial serum samples were collected before initiation or reinforcement of immunosuppressive treatment, either upon admission, or at the time of kidney biopsy. Sera were collected later again from the available patients with primary MN (n = 9) cross-sectionally in 2014, at our university clinic. The intervals between the time of initial evaluation and re-evaluation ranged from 281 to 2401 days (median, 1330 days). The collected sera were stored at −80 °C before use. Treatment was determined according to physician preference in each individual, based on clinically available information, and without prior knowledge of anti-PLA2R antibody positivity. The clinical data from these patients were analysed retrospectively.
2.2. Diagnosis of primary MN and lupus MN
Diagnoses of primary MN were made on the basis of light microscopy, immunohistochemistry, and electron microscopy findings [Citation8,Citation11]. In addition, all patients underwent screening for secondary causes of MN. Lupus MN was diagnosed according to the revised American College of Rheumatology criteria for SLE [Citation12,Citation13] and concomitant active and biopsy-proven class V lupus nephritis (i.e., MN) according to the 2003 International Society of Nephrology and the Renal Pathology Society classification [Citation14]. Only the cases with pure class V, without the features of proliferative glomerulonephritis (i.e., class III/IV), were included in this study.
2.3. Production of the stable cell line expressing PLA2R
The complementary DNA (cDNA) of PLA2R (accession No. NM_007366.4) was synthesised, and the PLA2R gene was transfected into the HEK293T cells. Stable cell lines, expressing PLA2R, were generated by limiting dilution, and the expression of PLA2R in the cell line was evaluated by flow cytometry and western blotting using standard techniques and rabbit polyclonal antibodies against human PLA2R (Sigma-Aldrich, St. Louis, MO, USA). The cell line with the highest level of expression of PLA2R was used in subsequent experiments.
2.4. Detection of autoantibodies against recombinant PLA2R in serum samples from patients and controls by Western blotting
Western blots using the lysates of the HEK293T cell line, stably expressing PLA2R, were performed with the serum samples as reported [Citation3,Citation11], with some modifications. Briefly, cells of the HEK293T cell line, stably expressing PLA2R, were collected after culture and lysed with radioimmunoprecipitation assay buffer with protease inhibitor cocktail (Nakalai Tesque, Kyoto, Japan). The cell lysates were electrophoresed under nonreducing conditions and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Membranes were blocked and incubated with serum samples at a dilution of 1:100. Subsequently, membranes were probed with goat anti-human IRDye® 800CW IgG secondary antibody (LI-COR Biosciences, Lincoln, NE), and near-infra-red fluorescence (IRDye®) signals on membranes were imaged and quantified using ODYSSEY® CLx Infra-red Imaging System (LI-COR Biosciences). Rabbit polyclonal antibodies against human PLA2R (Sigma-Aldrich) and goat anti-rabbit IRDye® 800CW IgG secondary antibodies (LI-COR Biosciences) were also used to confirm the position of the PLA2R bands and served as intra-assay controls. Quantified band intensity was standardised using these rabbit polyclonal anti-PLA2R antibodies and expressed as arbitrary unit (AU).
2.5. Quantification of anti-PLA2R antibodies by cell-based ELISA
Using the cell line stably expressing PLA2R, we developed a quantitative cell-based ELISA for anti-PLA2R antibodies, as follows: Cells of the HEK293T cell line stably expressing PLA2R or untransfected HEK293T cells, were seeded and cultured in wells of a flat-bottomed 96-well tissue culture plate (Corning, Corning, NY) coated with poly-D-Lysine (Sigma-Aldrich) at a density of 8 × 104 cells/well, for 48 h, until the cells were confluent. After the cells were fixed with 4% paraformaldehyde (Polysciences, Warrington, PA, USA), the wells were gently washed five times with phosphate-buffered saline and blocked with SuperBlock™ Blocking Buffer (Fisher Scientific, Ottawa, Ontario, Canada). Subsequently, diluted serum samples (1:10,000) and standards were added and incubated for 2 h. After six gentle washes with wash buffer (Tris-buffered saline containing 0.05% Tween 20), peroxidase-conjugated F(ab')2 fragment goat anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and 3, 3', 5, 5' tetramethyl benzidine (BioLegend, San Diego, CA, USA) were added in order, and colour development was measured using a microtiter plate reader (Bio-Rad). All the samples were analysed in duplicate, and the difference of mean optical density (OD) values between those with PLA2R-expressing HEK293T cells and those with untransfected HEK293T cells was used as a raw value of each sample. The autoantibody titres were expressed as AU/ml, which were determined by the standard curve method using the sample with the highest raw OD value. Note that ‘AU’ in western blots and ‘AU’ in cell-based ELISA were unrelated in the present study. The arbitrary cut-off value was determined as the mean plus 3 standard deviations of values for healthy controls (n = 16).
2.6. Quantification of anti-PLA2R antibodies using commercial solid-phase ELISA kits
Anti-PLA2R IgG antibodies in these samples were also evaluated by commercial solid-phase direct ELISA kits (EUROIMMUN AG, Lübeck, Germany) according to the manufacturer’s instructions [Citation9]. The titres are expressed as relative unit (RU)/ml using calibration sera. All the samples were analysed in duplicate, and the mean values were calculated to obtain the result for each serum sample. EUROIMMUN recommended interpreting results as follows: < 14 RU/ml: negative; > 14 to < 20 RU/ml: borderline; >20 RU/ml: positive. Borderline results were classified as negative in the present study.
2.7. Statistical analyses
Two-group comparisons were analysed using Fisher’s exact test for categorical variables and Welch's t test for continuous variables. Correlations were evaluated by Pearson's correlation coefficient. Concordance between the two methods of anti-PLA2R antibody measurement was evaluated by Cohen's kappa coefficient. The association and correlations between the results of and anti-PLA2R antibody and clinical characteristics of primary MN were evaluated by Welch's t test and Pearson's correlation coefficient. The association and agreement between the results of cell-based ELISA, or solid-phase ELISA, and physicians’ decisions on immunosuppressive treatment were evaluated by Fisher’s exact test and Cohen's kappa coefficient. The difference between the titres of anti-PLA2R antibodies, before and after treatment, was evaluated by the Wilcoxon signed-rank test. p values < .05 were considered to be statistically significant, and all tests were two-tailed. All statistical analyses were performed using JMP® Pro statistical software (version 12.0; SAS Institute, Cary, NC, USA).
3. Results
3.1. Clinical characteristics of patients with primary MN
Of the 23 patients with primary MN who were enrolled in this study, 14 were women and 9 were men. The median age of these patients was 62 years (ranging from 21 to 77 years). The median disease duration of MN was five months (with a range of 1–100 months). The patients were all Japanese. Other clinical characteristics of the patients with primary MN are summarised in .
Table 1. Demographic and clinical characteristics of the patients with primary MN.
3.2. Production of the stable cell line expressing PLA2R
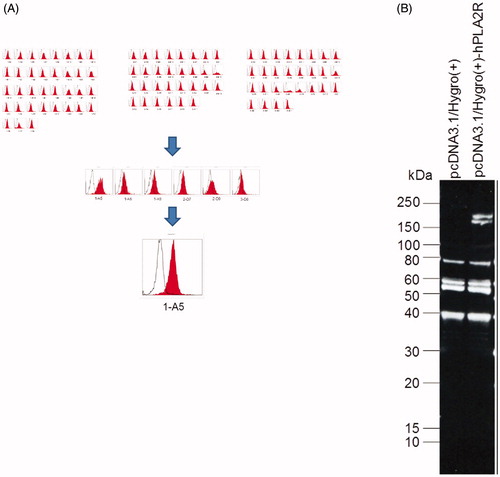
Stable expression of PLA2R was successfully detected in the HEK293T cells by flow cytometry and western blotting ().
Figure 1. Stable expression of PLA2R in the HEK293T cells. (A) Flow cytometry showed the expression of PLA2R on the surface of HEK293T cells. The mean fluorescence intensity of PLA2R (red) and of an isotype control (white) are shown. (B) Western blot showed that the PLA2R transfected HEK293T cells expressed the PLA2R transgene between 150 and 250 kD (right lane), while in the mock-transfected cell line (left lane) no positive signal could be detected.
3.3. Measurement of autoantibodies against recombinant PLA2R in serum samples from patients and controls by Western blotting, cell-based ELISA, and commercial solid-phase ELISA
By western blotting, anti-PLA2R antibodies were detected in 12 out of 23 (52%) patients with primary MN (). Using samples from healthy controls, the arbitrary cut-off value for the cell-based ELISA was determined as >15 AU/ml. Evaluated on the basis of this cut-off value, anti-PLA2R antibodies were positive in 6 (26%) out of the same 23 samples from patients with primary MN according to testing with cell-based ELISAs. Using the commercial solid-phase ELISAs, anti-PLA2R antibodies were positive in 12 (52%) out of the same 23 samples from the same population. Cohen's κ coefficients of the three tests were 0.32 (western blot vs. cell-based ELISA), 0.65 (western blot vs. solid-phase ELISA), and 0.49 (cell-based ELISA vs. solid-phase ELISA), indicating that there is fair to substantial agreement between these three tests. In addition, there are very strong correlations between these three tests: Pearson's correlation coefficients (r) were 0.94 (quantified western blot vs. cell-based ELISA), 0.84 (quantified western blot vs. solid-phase ELISA), and 0.85 (cell-based ELISA vs. solid-phase ELISA) (, respectively). As shown in , the titres of anti-PLA2R antibodies measured by cell-based ELISA and solid-phase ELISA were significantly higher in western blot-positive samples than those in western blot-negative samples among primary MN patients (p = .048 and .015, respectively). The results for anti-PLA2R antibodies measured in each patient are summarised in .
Figure 2. Western blot of anti-PLA2R in the serum of patients with primary MN. By western blot, anti-PLA2R antibodies were detected in 12 out of 23 (52%) patients with primary MN. ‘M’s represent protein molecular weight markers. ‘P’s represent positive controls (i.e., rabbit polyclonal antibodies against human PLA2R).
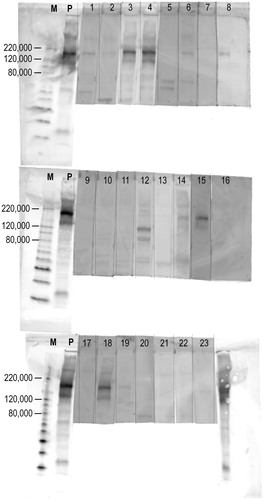
Figure 3. Association between the three measurement methods of anti-PLA2R antibodies. (A) Quantified western blot and cell-based ELISA (Pearson's correlation coefficient, r = 0.94). (B) Quantified western blot and commercial solid-phase ELISA (r = 0.84). (C) Cell-based ELISA and commercial solid-phase ELISA (r = 0.85). (D) The titres of anti-PLA2R antibodies by cell-based ELISAs were significantly higher in western blot-positive samples than those in western blot-negative samples among primary MN patients (p = .048). (E) The titres of anti-PLA2R antibodies by commercial solid-phase ELISAs were significantly higher in western blot-positive samples than those in western blot-negative samples among primary MN patients (p = .015). Correlations were evaluated by Pearson's correlation coefficient. p values were determined by Welch's t-test. Horizontal bars represent mean levels of each group in D and E.
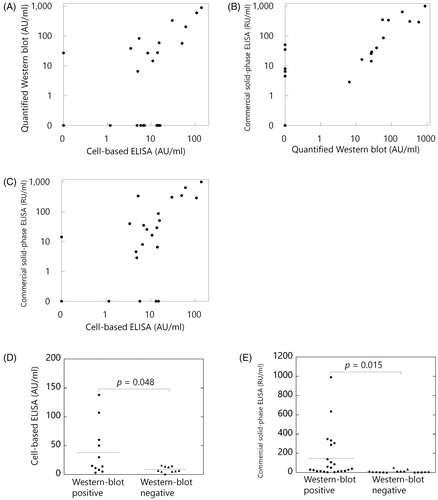
Table 2. Clinical characteristics and the results of anti-PLA2R antibodies in each patient with primary membranous nephropathy.
Conversely, all of the serum samples from the 16 SLE patients with pure MN were negative for anti-PLA2R antibodies using the western blot (data not shown), the cell-based ELISA (titer: 0–13.3 AU/ml), or the solid-phase ELISA (titer: 0–5.3 RU/ml). In addition, all of the samples from the 16 healthy controls were deemed negative using all the three methods (data not shown).
3.4. Association between the anti-PLA2R antibodies and clinical characteristics of primary MN
The levels of urine protein were moderately correlated with the titres of anti-PLA2R antibodies using quantified western blot, cell-based ELISA, and solid-phase ELISA among patients with primary MN (r = 0.42, 0.47, and 0.39, respectively). The levels of urine protein were significantly higher among anti-PLA2R positive patients with primary MN according to cell-based ELISAs and solid-phase ELISAs than those in anti-PLA2R negative patients (p = .039 and .048, respectively), whereas the association was not significant when anti-PLA2R antibodies were evaluated using western blots (p = .48). The incidence of nephrotic syndrome was also significantly associated with anti-PLA2R positivity according to cell-based ELISAs (p = .045), whereas such an association was not significant when anti-PLA2R antibodies were evaluated by western blots and solid-phase ELISAs (p = .371 and .069, respectively). Other comparisons with clinical characteristics among the patients with primary MN are summarised in .
Table 3. Comparisons of measurement methods about associations between anti-PLA2R antibodies and clinical characteristics among patients with primary MN.
3.5. Association between the anti-PLA2R antibodies and physicians’ decisions about immunosuppressive treatment
Retrospective analyses revealed that 75–100% of the primary MN patients who were anti-PLA2R positive were treated with immunosuppressive treatment (percentages varied among the detection methods). In contrast, only 9–29% of the primary MN patients with negative results received immunosuppressive treatment. Thus, anti-PLA2R antibodies were significantly associated with physicians’ decision on immunosuppressive treatment without their prior knowledge of anti-PLA2R antibody positivity (p < .01 when anti-PLA2R antibodies were evaluated by cell-based ELISA and solid-phase ELISA, and p = .039 by western blot). Disease-spontaneous remission occurred in at least five of the nine anti-PLA2R-negative patients who did not receive immunosuppressive treatment. Urine protein did not decrease in one of the two anti-PLA2R-positive patients who did not receive immunosuppressive treatment. Renal function did not decline in any of the anti-PLA2R-negative patients.
3.6. Longitudinal analysis in available patients with primary MN
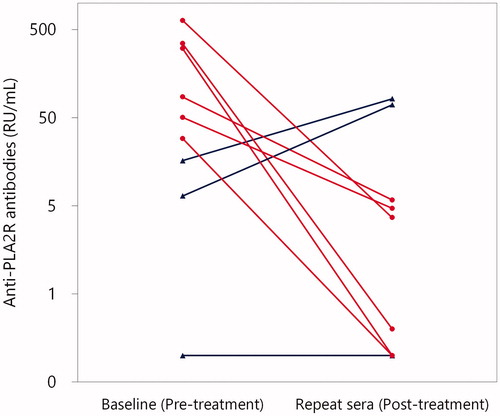
Repeat sera were available from nine patients with primary MN. As shown in , in all of the six patients who were treated with immunosuppressive treatment, the titres of anti-PLA2R antibodies measured by the solid-phase ELISA significantly declined following treatment (p = .03, the six patients were all initially positive for anti-PLA2R antibodies). In addition, the levels of urine protein significantly declined following treatment (p = .03) in these six patients, and all six patients achieved remission defined [Citation15]. In contrast, the titres of anti-PLA2R antibodies increased in two of the three patients in whom immunosuppressive treatment was not administered (). Notably, these two patients were initially negative for anti-PLA2R antibodies; therefore, seroconversion occurred in them. Anti-PLA2R antibodies remained lower than the detection limit in the remaining one patient.
Figure 4. Titres of anti-PLA2R antibodies at baseline and thereafter in patients with primary MN by commercial solid-phase ELISA. Repeat sera were available in nine patients with primary MN. Patients who were treated with and without immunosuppressive treatment were indicated with red circles and blue triangles, respectively. In all of the six patients who were treated with immunosuppressive treatment (red circles), titres of anti-PLA2R antibodies significantly declined (p = .03 by Wilcoxon signed-rank test). By contrast, in two of the three patients in whom immunosuppressive treatment was not administered (blue triangles), the titres of anti-PLA2R antibodies increased.
4. Discussion
The major achievements of this study are the following: (i) the present study suggests the usefulness of anti-PLA2R antibody as a diagnostic, prognostic, and surrogate biomarker in primary MN; (ii) the western blot, the cell-based ELISA, and the solid-phase ELISA are all reliable measurement methods for anti-PLA2R antibodies, but differ in their levels of performance; and (iii) a high prevalence of anti-PLA2R antibodies in patients with primary MN and its absence in patients with lupus MN was confirmed in Japanese subjects.
Anti-PLA2R antibodies were detected in approximately half of the patients with primary MN according to the western blot and the solid-phase ELISA. Conversely, all of the serum samples from the SLE patients with pure MN, and healthy controls were negative for anti-PLA2R antibodies using all three methods. Thus, their usefulness as a diagnostic biomarker in primary MN was confirmed. In addition, the levels of urine protein were moderately correlated with titres of anti-PLA2R antibodies, and the levels of urine protein were significantly higher in anti-PLA2R positive patients with primary MN than those in negative patients. Thus, severity of disease might be associated with the level and presence of anti-PLA2R antibodies in primary MN. However, there are some discrepancies in the correlations between proteinuria and anti-PLA2R antibody levels found in other studies [Citation4–6], and thus the correlation we report here needs to be confirmed in a larger, prospective study.
Interestingly, our retrospective analyses reveal that the presence of anti-PLA2R antibodies is significantly associated with experienced physicians’ decisions on immunosuppressive treatment, without prior knowledge of anti-PLA2R antibody positivity. The physicians’ decision in the present study was probably reasonable because disease-spontaneous remission occurred in at least five of the nine anti-PLA2R-negative patients who did not receive immunosuppressive treatment. Thus, anti-PLA2R antibodies could also serve as a supportive biomarker for the treatment decision in primary MN. Similar to our findings, high anti-PLA2R levels were associated with persistent proteinuria and/or the need for immunosuppressive treatment in a cohort of 73 PLA2R-related MN patients with long-term follow-up [Citation16]. However, in a combined cohort of 117 patients with primary MN, there was no difference in the percentages of patients who were treated with immunosuppressive drugs between anti-PLA2R-positive and negative patients and in the spontaneous remission rates [Citation4].
In the longitudinal analysis of this study, the titres of anti-PLA2R antibodies significantly declined with clinical amelioration following treatment in all of the six patients who were treated with immunosuppressive treatment. In contrast, anti-PLA2R antibodies were initially negative in two patients, and their titres increased while under observation, without immunosuppressive treatment. Thus, anti-PLA2R antibodies are usually reduced by immunosuppressive treatment and can serve as a surrogate biomarker in primary MN. Several other studies have also suggested a temporal relationship between anti-PLA2R antibodies and clinical disease activity [Citation5], and thus the monitoring of anti-PLA2R may be a quicker and more accurate way of assessing spontaneous remission or efficacy of immunosuppression [Citation1]. In addition, seroconversion has also been reported in initially seronegative patients with primary MN, and the development of circulating antibodies may be associated with a poor prognosis [Citation16–18].
We have developed the western blot and the cell-based ELISA using our own stable cell line expressing PLA2R, and compared them to the commercial solid-phase ELISA using a different antigen. They showed similar results and can all be considered reliable measurement methods for anti-PLA2R antibodies. Although it was not our initial intention, the reliability of the EUROIMMUN ELISA of anti-PLA2R antibodies was demonstrated here using different measurement methods by independent investigators. However, they performed somewhat differently than each other. For example, the levels of proteinuria were significantly higher among anti-PLA2R positive patients with primary MN according to the cell-based ELISA and the solid-phase ELISA than those in anti-PLA2R-negative patients, whereas such an association was not significant when anti-PLA2R antibodies were evaluated by the western blot. The degree of association between the anti-PLA2R antibodies and physicians’ decisions on immunosuppressive treatment varied among the three methods, although they were all statistically significant. Similar to our results, a previous study using both indirect immunofluorescence testing (IIFT) and an in-house developed ELISA, found that the performances of these tests were somewhat different; a correlation between baseline proteinuria and anti-PLA2R antibody titres was observed using ELISA but not IIFT [Citation4].
To the best of our knowledge, this is the third published report in the English language literature that evaluates the prevalence of serum anti-PLA2R antibodies in Japanese patients with primary MN. Moreover, previous reports did not use quantitative methods for measuring anti-PLA2R antibodies. Akiyama et al. reported that anti-PLA2R antibodies were detected in 53 (53%) of 100 patients with primary MN and in 0 (0%) of 31 patients with secondary MN, including 10 patients with lupus MN [Citation19]. However, they only used human glomerular extract as antigen (not truly specific for PLA2R) in their western blot analyses. The prevalence of anti-PLA2R antibodies was similar in our study and in the study by Akiyama et al. and might be somewhat lower than the prevalence in other ethnic groups (approximately 70–80% in most of the studies) [Citation2,Citation19]. However, as shown in this study, because the positivity of anti-PLA2R antibodies is affected by many factors, such as the phase and severity of the disease, and the detection methods, it is not clear whether there is truly a difference in the prevalence of anti-PLA2R antibodies among patients with primary MN based on ethnicity. Interestingly, Hayashi et al. reported that serum anti-PLA2R antibodies were positive according to western blot in 12/22 (55%) of the Japanese patients with primary MN while four out of the 10 primary MN patients with no detectable serum anti-PLA2R antibodies had glomerular PLA2R deposits [Citation20]. Thus, 16 patients (73%) were classified in the PLA2R-related group when combined. Other studies have also demonstrated that renal PLA2R staining increases the sensitivity for the diagnosis of PLA2R-related MN in other ethnic groups [Citation16,Citation21].
There are several limitations in this study. First, the validity of this study could be improved by using a larger sample size and a non-retrospective study design. Second, because the subjects of this study were only of Japanese ethnicity, it is not clear whether anti-PLA2R antibodies have a similar association with MN in patients of different ethnic backgrounds. However, as described above, other studies reported some similar findings in other ethnic groups. Third, we did not examine glomerular PLA2R deposits in kidney biopsies or serum antibody against thrombospondin type-1 domain-containing 7 A (THSD7A) [Citation2]. Last, because the total IgG levels decrease in the patients with proteinuria, the titres of anti-PLA2R antibody might need to be compensated by the corresponding total IgG values, especially when its correlation with proteinuria is analysed. However, previous studies did not perform such analyses [Citation4–6].
In conclusion, despite its limitations, this study suggests that anti-PLA2R antibody can serve as a diagnostic, prognostic, and surrogate biomarker in primary MN. The strength of this study is that we elucidated this issue using three different measurement methods for the first time, to the best of our knowledge. Determining the precise role of anti-PLA2R antibodies in the pathogenesis of MN and their usefulness as biomarkers will require further study.
Acknowledgements
We would like to thank Editage (Cactus Communications) for English language editing.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Beck LH, Jr, Salant DJ. Membranous nephropathy: from models to man. J Clin Invest. 2014;124(6):2307–2314.
- Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet. 2015;385(9981):1983–1992.
- Beck LH, Jr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21.
- Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. JASN. 2012;23(10):1735–1743.
- Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83(5):940–948.
- Hoxha E, Thiele I, Zahner G, et al. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. JASN. 2014;25(6):1357–1366.
- Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. JASN. 2015;26(10):2545–2558.
- Hoxha E, Harendza S, Zahner G, et al. An immunofluorescence test for phospholipase-A-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26(8):2526–2532.
- Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol. 2014;142(1):29–34.
- Hofstra JM, Wetzels JF. Phospholipase A2 receptor antibodies in membranous nephropathy: unresolved issues. JASN. 2014;25(6):1137–1139.
- Qin W, Beck LH, Jr., Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. JASN. 2011;22(6):1137–1143.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725–1725.
- Weening JJ, D'Agati VD, Schwartz MM, et al. ON Behalf of the International Society of Nephrology and Renal Pathology Society Working Group on the Classification Oflupus Nephritis. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521–530.
- Chapter 7: Idiopathic membranous nephropathy. Kidney Int Suppl. 2012;2(2):186–197.
- Timmermans SA, Abdul Hamid MA, Cohen Tervaert JW, et al. on behalf of the Limburg Renal Registry. Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am J Nephrol. 2015;42(1):70–77.
- van de Logt AE, Hofstra JM, Wetzels JF. Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: seroconversion after prolonged follow-up. Kidney Int. 2015;87(6):1263–1264.
- Ramachandran R, Kumar V, Nada R, et al. Serial monitoring of anti-PLA2R in initial PLA2R-negative patients with primary membranous nephropathy. Kidney Int. 2015;88(5):1198–1199.
- Akiyama S, Akiyama M, Imai E, et al. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. 2015;19(4):653–660.
- Hayashi N, Akiyama S, Okuyama H, et al. Clinicopathological characteristics of M-type phospholipase A2 receptor (PLA2R)-related membranous nephropathy in Japanese. Clin Exp Nephrol. 2015;19(5):797–803.
- Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364(7):689–690.
