Abstract
Immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1) or PD-1 ligand-1 (PD-L1) have brought paradigm shift in lung cancer treatment. The median overall survival of patients with advanced non-small cell lung cancer treated with former standard platinum doublet cytocidal therapy is less than 1 year; however, patients responding to ICIs have durable antitumor efficacy resulting in survival longer than 5 years. Lung cancer has much gene mutations, which is a characteristic of cancer caused by extrinsic factors, such as cigarette smoking. The heterogeneity underlined by genetic mutations results in the generation of resistant clones against chemotherapy and molecular targeted therapy. On the other hand, gene mutation products generate neoepitopes that are recognized as ‘non-self’ by T cell immune system. This is one of the reasons lung cancer is a good target for ICIs. However, drastic antitumor response is observed in relatively small percentage of patients. The pre-existing T cell immunity required for PD-1 inhibitor to exhibit antitumor efficacy has been elucidated.
Keywords:
1. Introduction
Lung cancer is one of the malignancies with the worst prognosis. Platinum-based doublet chemotherapy, which has been the standard treatment for advanced lung cancer patients, can prolong survivals but merely for months. Epidermal growth factor receptor (EGFR), tyrosine kinase inhibitors (TKI), and anaplastic lymphoma kinase (ALK)-TKI, targeting growth signals to which cancer cells addict by driver mutations, cause marked improvement in response rate; however, acquired resistance underlined by additional mutations and heterogeneity has never been avoidable. Thus, long-term survival of advanced lung cancer patients is hardly achieved with cytocidal agents or TKIs that target cancer cells.
The cancer immune editing theory consisting of elimination phase, equilibrium phase, and escape phase, describes how tumor cells and immunity interact with each other [Citation1–4]. Because every somatic cell is under immunological surveillance, which recognizes and eradicates the cells producing non-self gene products, the transformed cells presenting mutational products can be recognized by the immune system as non-self and be eradicated in the elimination phase. Sporadic cancer cells that somehow survive immune destruction may enter the equilibrium phase, in which cancer cell proliferation is under control by T cell immunity. T cells preferentially eradicate highly immunogenic clones that likely possess more mutations. Thus, during the equilibrium phase, not only number but also immunogenicity and mutational burden of cancer cells are edited. Conversely, T cell immunity is affected by long-lasting cancer cells, which attenuate its ability to control cancer cells. Immunologically sculpted cancer cells finally enter the escape phase, because of T cell immunity attenuation. Programmed cell death-1 (PD-1), which is known to mediate T cell exhaustion phenomenon of chronic viral infection models, plays a critical role in cancer antigen-mediated T cell suppression [Citation5].
It has been demonstrated that PD-1 blockade therapy resulted in survival longer than 5 years in approximately 15% of advanced non-small cell lung cancer (NSCLC) patients [Citation6]. Surprisingly, majority of 5-year survivors received only two year PD-1 blockade therapy without any further treatment in CA209-003 study. Thus, it appears that recovery of the equilibrium phase is possible with PD-1 blockade therapy even in advanced lung cancer patients. To improve the response rate and the probability of long-term survivals, T cell immunity components that are required for durable antitumor efficacy should be elucidated.
2. Efficacy of PD-1 blockade therapy for NSCLC patients
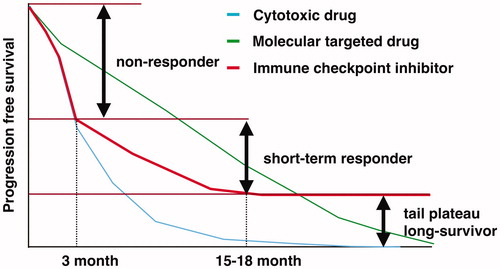
In phase III clinical trials, nivolumab, an anti-PD-1 Ab, significantly improved overall survival (OS) in pretreated NSCLC patients compared to docetaxel [Citation7,Citation8]. The survival curves of nivolumab group showed no decline after a certain time point, and they were referred to as tail-plateau effect. Tail-plateau of PFS curves started 15–18 months after PD-1 blockade therapy. In other words, approximately half of the patients who had partial response (PR) or stable disease (SD) gradually acquired resistance and showed disease progression by 15–18 months after PD-1 blockade therapy (). In contrast, clinical trial results consistently indicated that ∼40% of NSCLC patients exhibited early disease progression in 3 months post-PD-1 blockade therapy. Thus, patients with NSCLC are classified into three distinct subgroups: non-responders showing early disease progression without clinical benefits, long-term survivors achieving durable disease control, and short-term responders. Pembrolizumab and atezolizumab have shown almost the same antitumor efficacy profile [Citation9,Citation10]. It is likely that pre-existing antitumor T cell immunity plays a critical role in determining these distinct outcomes after PD-1 blockade therapy.
Figure 1. This figure describes the features of progression free survival curves of cytotoxic drug, molecular targeted drug and immune checkpoint inhibitor. The patients treated with immune checkpoint inhibitors are divided into three groups according to antitumor effects, non-responder, short-term responder and long-survivor.
3. Tumor PD-L1 expression and responses
The KEYNOTE024 phase III trial was conducted to compare pembrolizumab and platinum doublet chemotherapy in untreated NSCLC patients who had cancer cells with PD-L1 tumor proportion score (TPS) of 50% or greater [Citation11]. Pembrolizumab significantly improved the overall response rate (ORR), progression free survival (PFS), and OS. The effectiveness of pembrolizumab in TPS of more than 1% was later shown in the KEYNOTE042 trial [Citation12]. These clinical trials provided evidence that PD-L1 expression in tumor cells have predictive significance for responses to pembrolizumab. However, it was demonstrated that cancer cells lacking PD-L1 expression by genome editing technique still responded to PD-1 blockade therapy [Citation13]. In contrast, PD-1 blockade therapy exhibited no antitumor efficacy in PD-L1 knockout mice, although the tumor cells expressed PD-L1. Thus, it is likely that PD-L1 expressed in the immune cells plays roles that are more crucial in the PD-1 dependent T cell suppression mechanism. It is well known that somatic cells including tumor cells express PD-L1 upon IFNγ exposure. The significance of tumor cell PD-L1 expression as biomarker likely reflects that T cell-mediated inflammation, which results in IFNγ production is important for antitumor T cell immunity.
4. Cancer antigen and responses
It has been demonstrated that cancers caused by extrinsic factors, such as lung cancer and melanoma, tend to respond well to ICIs, because they possess much mutational burden that is the source of cancer antigens. Neoepitopes generated from nonsynonymous somatic mutations are recognized by the T cells as non-self, and are capable of evoking antitumor immune responses. However, all synonymous mutations do not equally generate immunogenic neoepitopes. To assess the immunogenicity of neoepitopes, fitness model was demonstrated [Citation14]. In this model, two main factors determined neoantigen fitness: one is the likelihood of neoantigen presentation by the major histocompatibility complex (MHC) and the other is the differences from the original peptide sequence. The relative MHC binding affinity of each neoantigen to its wild type and the nonlinear dependence on sequence similarity of neoantigens to known antigens provide these components. Low-fitness neoantigens predicted anti-PD-1 responses in NSCLC patients. Similar observations were reported in renal cell carcinoma that insertion and deletion type mutations, which induced flame-shift type major sequence alterations and provided 3 times more neoepitopes with high MHC binding affinity, resulted in immunogenic antigens [Citation15].
One more critical factor of somatic mutations as effective neoantigens is clonality. Transformation of a cancer cell is caused by accumulated gene alteration in one somatic cell. The mutations accumulated in the original cancer cell will be clonal mutation shared by all clones of cancer. Meanwhile, during proliferation of cancer cells, they acquire more mutations due to genetic instability. This type of mutation varies among cancer clones and mediates heterogeneity. N. McGranahan et al. [Citation16] demonstrated that sensitivity to PD-1 blockade therapy in NSCLC patients was enhanced in tumors enriched with clonal neoantigens. Interestingly, subclonal neoantigens induced by chemotherapy were rich in certain poor responders. This observation is consistent with cancer immune editing theory. Because the elimination phase is supposed to begin very early when one somatic cell is transformed, T cells that mediate elimination phase likely recognize clonal neoantigens. During the equilibrium phase, the immunogenic cancer clones that possess more mutations are supposed to be preferentially eradicated. Thus, increase in subclonal mutations may reflect attenuation of antitumor T cell immunity. Indeed, melanoma study indicated that tumor mutation burden decreased in patients who responded to nivolumab but increased in PD patients [Citation17].
5. T cell subsets mediating antitumor efficacy
5.1. CD8+ T cell
It has been well established that CD8+ T cells differentiate to acquire a state called cytotoxic T cells (CTL) that recognize MHC class I restricted antigen and induce cancer cell death. Thommen et al. [Citation18] examined lung cancer tissue infiltrating lymphocytes and demonstrated that PD-1high CD8+ T cells showed high clonality compared to PD-1med and PD-1low CD8+ T cells in tumor microenvironment, and secreted IFNγ upon co-culture with cancer cells. Furthermore, PD-1high CD8+ T cells correlated with favorable outcome of PD-1 blockade therapy. Tumor-infiltrating lymphocyte study also indicated that CD8+ T cells, which recognized cancer antigens, appear to be the effector memory phenotype, which lacked CD62L but expressed CD45RO [Citation19].
5.2. CD4+ T cell
As indicated in cancer immune cycle model, priming and expansion of CTL that are specific for cancer antigens occur in lymph nodes draining antigen presenting cells (APC) from cancer tissues [Citation20]. Thus, the potential rate-limiting steps include the priming and expansion of CTL and the trafficking from the lymph nodes to the tumors through the bloodstream. In line with this, the blockade of priming by MEK inhibition resulted in a loss of tumor infiltrating lymphocytes and memory T cells in mice [Citation21].
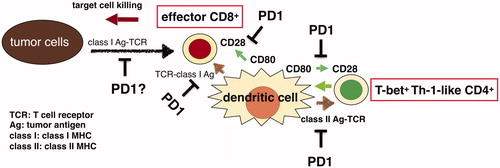
Numerous evidences indicate that CD4+ T cell help is necessary for priming, proliferation, migration and permeation abilities to tumor tissues, killing cell functions, metabolism, and survival of CTL [Citation22]. Spitzer et al. [Citation23] analyzed the tumors, the blood, the spleens, the bone marrow, and the draining lymph nodes to investigate the T cell clusters common in mice that established antitumor immunity sufficient to eradicate tumors, and reported that CD69+CD44+CD27-CD62LlowT-bet+ CD4+ T cell cluster was the most valuable. This CD4+ T cell cluster existed systemically. As a result, Wei et al. [Citation24] analyzed melanoma-infiltrating lymphocytes, not only the exhaustion type PD-1+ TIM-3+ CD8+ T cell but also ICOS+ T-bet+ type 1 helper (Th1) CD4+ T cells were identified as antitumor T cell clusters. The CD4+ T cell cluster in this study showed almost the same phenotype, ICOS+CD69+CD44+CD27-CD62LlowT-bet+ CD4+. We reported that the percentage of CD62Llow CD4+ T cells in the peripheral blood before therapy predicted the outcome of PD-1 blockade therapy and correlated with the duration of responses in advanced NSCLC patients [Citation25].
Summarily, exhausted-type memory effector CD8+ T cells, and T-bet+ Th1-like CD4+ T cells are required for successful PD-1 blockade therapy ().
6. Future directions
PD-1 blockade therapy for NSCLC is proceeding from monotherapy to combined therapy to achieve higher antitumor efficacy.
6.1. PD-1 blockade therapy and radiotherapy
Phase III PACIFIC trial was conducted to examine the efficacy of one-year durvalumab consolidation therapy after standard chemoradiation treatment for locally advanced NSCLC [Citation26]. In comparison with placebo group, durvalumab therapy improved PFS and OS. Notably, the tail plateau of PFS curve was greatly improved with hazard ratio of 0.52; thus, it appears that one-year durvalumab therapy almost doubled the survival of patients requiring other treatment for long-term survival.
After having given radiation therapy, it is reported that the antitumor effect on tumor in the place apart from the irradiation field occurs and is called abscopal effect [Citation27]. Immunogenic cancer cell death induced by irradiation therapy results in the activation of APC that engulf the cancer antigens in the presence of damage associated molecular patterns (DAMPs). The activated APC carrying cancer antigens migrate through the lymphatics to the draining lymph nodes, in which cancer antigen-specific effector T cells are primed and expanded by the APC. Thus, it is likely that radiotherapy can facilitate antitumor T cell priming, resulting in synergistic effects with ICI therapy, which promote antitumor efficacy of primed effector T cells. It was reported that prior radiotherapy improved the efficacy of PD-1 blockade therapy in NSCLC patients [Citation28,Citation29]. Interestingly, in the PACIFIC study, the hazard ratio was better in patients who started durvalumab therapy within 14 days after chemoradiation treatment, suggesting that T cell immunity augmentation by radiotherapy may have time limit.
6.2. Combined immunotherapy with anti-vascular endothelial growth factor (VEGF) antibody
IMpower150 study examined the antitumor efficacy of atezolizumab combined with bevacizumab, an anti-VEGF antibody and cytotoxic anticancer agents for untreated NSCLC patients [Citation30]. OS and PFS were significantly improved by adding atezolizumab to CBDCA, paclitaxel, and bevacizumab. However, improvement in OS and PFS was not achieved by adding atezolizumab to CBDCA and paclitaxel in the absence of bevacizumab. This suggests the possibility that bevacizumab has synergistic antitumor effects with atezolizumab.
VEGF is one of the well-known immunosuppressive mediators. Myeloid derived suppressor cell (MDSC) and regulatory T cell (Treg) are known to express VEGF receptor2. VEGF activates and increases Treg and MDSC resulting in immune suppression. In addition, tumor vessels that are formed when there is high concentration of VEGF inhibit the migration and permeation of effector T cells into tumor tissues.
6.3. PD-1 blockade therapy and anti-CTLA-4 therapy
Phase III clinical trial Checkmate227 compared the antitumor efficacy of combination therapy of nivolumab and ipilimumab, an anti-CTLA-4 antibody and chemotherapy in untreated NSCLC patients [Citation31]. OS and PFS were significantly better in the combination therapy group and showed higher tail plateau level indicating long-term survival effect. At first, the synergistic antitumor efficacy was prominent in patients who had high tumor mutational burden, but, later, the antitumor effect was shown in patients who had lower tumor mutational burden. As discussed in the former chapter, the potential rate-limiting steps of PD-1 blockade therapy include the priming and the expansion of T cells. Theoretically, CTLA-4 blockade can promote T cell priming, because CTLA-4 inhibits co-stimulatory signal of CD28, which is required for priming of naïve T cells. Allison et al. reported that ipilimumab but not nivolumab alone caused increase in ICOS+ Th1 CD4+ T cells in the peripheral blood. In addition, Treg constitutively expresses CTLA-4 and some anti-CTLA-4 Ab have Treg removal effect; however, it is still controversial if ipilimumab can eradicate Treg in patients.
6.4. PD-1 blockade therapy and cytocidal drugs
KEYNOTE189 and 407 studies examined the combination therapy effect of pembrolizumab and cytocidal drugs in untreated NSCLC patients [Citation32,Citation33]. OS and PFS were improved by adding pembrolizumab to platinum doublet chemotherapy. The percentage of PD significantly decreased in patients who were treated with the combined therapy. Thus, it appears that combination of pembrolizumab and chemotherapy may eradicate different cancer clones each other, resulting in early additive effects. It has been reported that some cytocidal agents could induce immunogenic cell death of cancer cells [Citation34]. Thus, it is possible that cytocidal agents have synergistic effects to promote antitumor T cell immunity. However, it is still uncertain if immunogenic cell death can promote T cell priming in patients treated with cytocidal agents, because cytocidal agents themselves and corticosteroid, which is used as an antiemetic agent, might damage APC and/or T cell functions. This concern must be further examined.
6.5. PD-1 blockade therapy for surgically resectable patients
Forde and collegues reported that the use of neoadjuvant nivolumab was effective in resectable lung cancer patients [Citation35]. A major pathological response was observed in 9 of 20 resected tumors (45%). Mutation-associated, neoantigen-specific T cell clones rapidly expanded in peripheral blood after nivolumab therapy in complete response patients. This result that early stage lung cancer patients possess antitumor effector T cells sufficient to eradicate cancer cells after nivolumab therapy is not surprising, because early stage patients likely entered the escape phase recently and are supposed to have more antitumor T cells without deep exhaustion than advanced cancer patients. It seems that resectable lung cancer patients can take this advantage with neoadjuvant PD-1 blockade therapy.
7. Conclusion
PD-1 blockade therapy has brought marked improvement in the outcome of lung cancer treatment. It is noteworthy that significant number of advanced lung cancer patients, who were treated with PD-1 blockade therapy, achieved long-term survival without disease progression. T cell clusters including CD8+ and CD4+ cells required for successful ICI therapy have been elucidated. Comprehensive understanding of T cell immunity and precise analysis of factors that prevent the equilibrium phase will provide adequate treatment of lung cancer patients, resulting in immune precision medicine.
Disclosure statement
H.K. has received grant support from Boehringer-Ingelheim and Ono Pharmaceutical. K.K. has no conflict of interest to disclose.
Additional information
Funding
References
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998.
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148.
- Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann NY Acad Sci. 2013;1284(1):1–5.
- Starling S. MHC molecules: immune editing shapes the cancer landscape. Nat Rev Immunol. 2017;17(12):729–729.
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499.
- Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 Study. J Clin Oncol. 2018;36(17):1675–1684.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550.
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833.
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830.
- Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128(2):805–815.
- Luksza M, Riaz N, Makarov V, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551(7681):517–520.
- Turajlic S, Litchfield K, Xu H, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18(8):1009–1021.
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469.
- Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171(4):934–949.e916.
- Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994–1004.
- Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330.
- Borst J, Ahrends T, Bąbała N, et al. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647.
- Spitzer MH, Carmi Y, Reticker-Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168(3):487–502.e415.
- Wei SC, Levine JH, Cogdill AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120–1133.e1117.
- Kagamu H, Yamaguchi O, Shiono A, et al. CD4+ T cells in PBMC to predict the outcome of anti-PD-1 therapy. J Clin Oncol. 2017;35(15_suppl):11525–11525.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929.
- Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–322.
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903.
- Yamaguchi O, Kaira K, Hashimoto K, et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non-small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac Cancer. 2019;10(4):992–1000.
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031.
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092.
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051.
- Galluzzi L, Buque A, Kepp O, et al. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714.
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986.