Abstract
Abatacept may exert its clinical effect on rheumatoid arthritis (RA) by suppressing anti-cyclic citrullinated peptide (CCP) antibody production. This study was undertaken to test this hypothesis by examining the changes of disease activity of RA and anti-CCP antibody levels over time after starting abatacept. Sixty Japanese RA patients who started abatacept were included in this multicenter, prospective observational study. Simple Disease Activity Index (SDAI) and anti-CCP antibody levels were evaluated at 12, 24, and 52 weeks. The mean SDAI score significantly decreased within 12 weeks after starting abatacept and was maintained thereafter. On the contrary, the mean anti-CCP antibody levels did not change until 52 weeks. At the individual level, there were substantial changes of anti-CCP antibody levels, but these were not correlated with the changes of disease activity at any time points. Thus, abatacept reduces the disease activity of RA independently of modulating anti-CCP antibody production.
Introduction
Anti-citrullinated protein antibody (ACPA) is detected with a high specificity in patients with rheumatoid arthritis (RA) by using cyclic citrullinated peptide (CCP) in daily practice. ACPA is detected even before disease onset and is associated with the progression of joint damage [Citation1], suggesting a role of ACPA in disease development. There have also been lines of experimental evidence supporting pathogenicity of ACPA, such as promotion of osteoclastogenesis [Citation2]. On the contrary, ACPA levels are generally stable and poorly correlated with disease activity [Citation3–5].
Abatacept, a fusion protein consisting of extracellular domain of CTLA4 and Fc portion of IgG, has been used for the treatment of RA. Because abatacept interferes the interaction between the T cell-co-stimulatory molecule, CD28, and its ligand, CD80 or CD86, it might exert an anti-inflammatory effect by suppressing activation of T cells that activates synovial macrophages and fibroblasts. It is also possible that abatacept reduces disease activity of RA by interfering T–B cell interaction required for ACPA production. Interestingly, there have been reports showing higher efficiency of abatacept in ACPA-positive patients with RA, especially in those with high antibody levels, in either clinical trials or clinical practice settings [Citation3,Citation6–9]. It is unknown why the clinical efficiency of abatacept correlates with the ACPA levels and whether it is related to the modulation of antibody production. While some studies reported no significant change of ACPA levels after abatacept administration [Citation10], another study showed that ACPA levels decreased in patients under remission with abatacept [Citation11,Citation12]. Because the relationship between the kinetics of changes in disease activity and ACPA levels has not been analyzed in detail, it is unclear whether abatacept exerts its therapeutic effect by reducing ACPA production.
To elucidate the relationship between the effect of abatacept on disease activity and ACPA production, we conducted a prospective observational study on disease activity and anti-CCP antibody levels in Japanese RA patients who started abatacept.
Materials and methods
Patients
This is a multicenter, prospective observational study of The Fukuoka RA Biologics (FRAB) Registry, a Japanese registry in which 19 medical institutions are involved (UMIN 000007224). This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was collectively approved by the Clinical Research Network Fukuoka Certified Review Board (13-E15). Sixty patients with RA who started abatacept administration from March 2014 to February 2016 were included in this study. The sample size was determined according to a previous study in which 30 patients were included [Citation11]. All patients fulfilled the ACR 1987 criteria for RA, and were more than 20 years old at the time of obtaining informed consent. The history of fulfilling ACR/EULAR 2010 criteria for RA could not be assessed in most patients with long disease duration.
Study design
Abatacept was administered intravenously (500 mg for patients weighing less than 60 kg, and 750 mg for those more than 60 kg per four weeks) or subcutaneously (125 mg weekly). PSL, MTX, and other DMARDs, except for biologics and Jak inhibitors, was prescribed upon the doctors’ decision. The following parameters were evaluated at weeks 0, 12, 26, and 52 after starting abatacept: anti-CCP antibody, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Simple Disease Activity Index (SDAI), DAS28-ESR, and DAS28-CRP. Anti-CCP antibody (the second generation, CCP2) was measured at LSI Medience (Tokyo, Japan) by using a chemiluminescent immunoassay kit (Abbott Japan, Tokyo, Japan).
Statistics
Changes of disease activity or antibody levels from the baseline were analyzed by the linear mixed-effects model. The last observation carried forward (LOCF) imputation method was performed to estimate changes from baseline including missing data. Correlation between the baseline antibody levels and the changes of disease activity or baseline disease activity, between the changes of antibody levels and the changes of disease activity was analyzed by Spearman’s rank correlation coefficient. SAS9.3 was used for the analysis.
Results
Relationship between the baseline anti-CCP antibody levels and the clinical effectiveness of abatacept
Sixty patients with RA who started abatacept were included in this study. The baseline characteristics of the patients are shown in . In total, 18 patients discontinued abatacept before 52 weeks. Seven patients discontinued by the lack or loss of efficacy, three patients by the occurrence of adverse events, and eight patients by other reasons.
Table 1. Baseline characteristics.
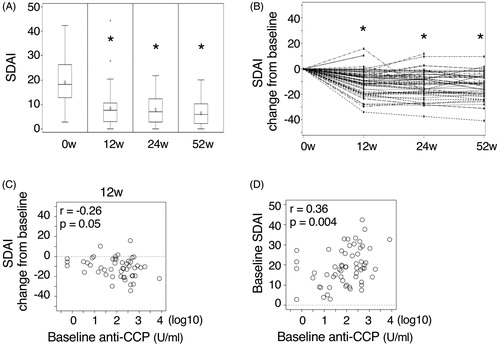
The mean SDAI score decreased within 12 weeks after starting abatacept and was maintained thereafter throughout the observation period (). The mean DAS28-ESR and DAS28-CRP values showed similar kinetics (data not shown). As reported previously, the basal levels of anti-CCP antibody correlated with the changes of SDAI score from the baseline (). There was also a positive correlation between the basal level of disease activity and the basal levels of anti-CCP antibody ().
Figure 1. The changes of disease activity after starting abatacept. (A) The mean SDAI scores at baseline (0w) and 12, 24, and 52 weeks after starting abatacept. (B) The kinetics of the changes of SDAI score from the baseline in each individuals are shown. *p < .001, different from the baseline. (C) The relationship between the baseline anti-CCP antibody levels and the changes of SDAI score from the baseline at 12 weeks after starting abatacept is shown. Similar data were obtained by the analysis at 24 or 52 weeks. (D) The relationship between the baseline anti-CCP antibody levels and SDAI score is shown. Correlation coefficient and p-values are indicated in the figures.
Relationship between the changes of anti-CCP antibody levels and the disease activity in patients with RA treated with abatacept
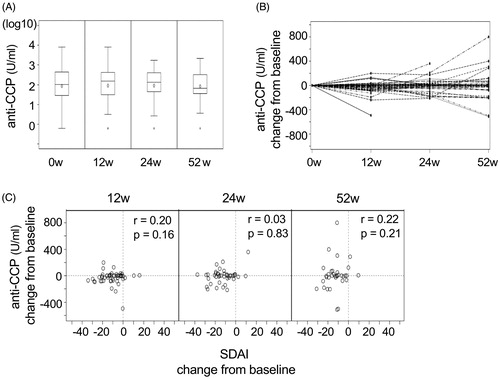
The levels of anti-CCP antibody were measured at 12, 24, and 52 weeks after starting abatacept. No patient showed seroconversion, either from positive to negative or from negative to positive for anti-CCP antibody. Furthermore, the mean antibody levels did not change throughout the observation period (). At the individual level, a portion of patients showed some decrease or increase of anti-CCP antibody (). However, the changes of anti-CCP antibody levels did not correlate with the changes of disease activity at any time points (). We did not detect significant correlation even after excluding anti-CCP antibody-negative or -low (<13.5 U/mL) samples from the analysis (p = .32, .75, and .24, at 12, 24, and 52 weeks, respectively).
Figure 2. The relationship between the changes of anti-CCP antibody levels and clinical effect of abatacept. (A) The mean anti-CCP antibody level at baseline (0w) and 12, 24, and 52 weeks after starting abatacept are shown. (B) The kinetics of the changes of anti-CCP antibody level from the baseline in each individuals are shown. (C) The relationship between the changes of SDAI score and anti-CCP antibody levels from the baseline was analyzed at 12, 24, and 52 weeks after starting abatacept. Correlation coefficient and p-values are indicated in the figures.
Discussion
In this study, we analyzed the relationship between the disease activity and anti-CCP antibody levels after starting abatacept in a cohort of patients with RA. As reported previously [Citation3,Citation6–9], abatacept reduced disease activity more efficiently in those with higher levels of anti-CCP antibody levels. However, in our case, this could be owing to the floor effect, as patients with low levels of anti-CCP antibody levels had lower disease activity at baseline. Similar observation was made by Mochizuki et al. [Citation13]. In contrast to our data, some studies showed no correlation between anti-CCP antibody levels and disease activity [Citation3–5]. This discrepancy could be attributed to the difference in patient characteristics: our cohort includes only patients who need abatacept for the treatment in daily practice, and, in fact, about half of them had history of previous use of biologics. Because joint damage likely progresses only in such ‘difficult-to-treat’ patients with RA, it is possible that, in this subpopulation, disease activity correlates with the progression of joint damage, which is associated with ACPA. A limitation of our analysis is the lack of information about the smoking status of the patients, which might influence the positivity of anti-CCP antibody and response to abatacept therapy.
We measured anti-CCP antibody levels over time after starting abatacept, in order to test the hypothesis that abatacept reduces disease activity of RA by decreasing ACPA production. However, the mean anti-CCP antibody levels did not change until 52 weeks after starting abatacept, despite clear reduction of disease activity in 12 weeks. Although we observed substantial changes in anti-CCP antibody titers at individual levels, the changes of antibody titers did not correlate with the changes of disease activity. Therefore, we conclude that abatacept reduced disease activity of RA independently of ACPA production. It remains possible that ACPA of other specificity decrease before the therapeutic effect, but usually these are less prevalent than anti-CCP antibody. Another limitation of our analysis is the small number of patient samples analyzed for the levels of anti-CCP antibody, although it seems unlikely that this affects the conclusion.
There have been several reports showing a decrease of ACPA levels after abatacept treatment. Scarsi et al. [Citation11] found decreased anti-CCP IgG and IgA levels in RA patients with clinical remission after 6 months of abatacept treatment, while Wunderlich et al. [Citation14] showed a significant reduction of serum anti-CCP2 antibody levels, especially in responders after 12 months. Jansen et al. [Citation12] reported that patients with RA who converted to seronegative after abatacept had a greater probability of achieving sustained remission at 6 months. However, these reports did not clarify whether the reduction of ACPA preceded the therapeutic effect of abatacept. The reduction of ACPA could be the result of decreased disease activity by abatacept. For instance, patients with RA in remission produced reduced levels of inflammatory cytokines, such as IL-6, which is involved in the proliferation and survival of antibody-producing plasma cells. Alternatively, abatacept might directly reduce ACPA production but independently of its therapeutic effect. It was recently reported that changes in RA-autoantibody levels were not associated with disease activity but reflected intensity of immunosuppression, although abatacept was not included in the analysis [Citation15]. In addition, the presence of shared epitope rather than ACPA predicts clinical response to abatacept in RA [Citation16], suggesting that ACPA might be an indicator of T cell-involvement in the pathogenesis. There are also lines of evidence implicating T-cell-independent effect of abatacept on monocytes/macrophages and osteoclasts [Citation17,Citation18]. In fact, abatacept exerted therapeutic effect in animal models without CD4 T cells [Citation19], and in patients with seronegative arthritis as well [Citation20]. Further clinical and basic studies are required to understand the effect of abatacept on the pathogenesis of joint inflammation and ACPA production in RA, which is important for optimizing the use of abatacept for the treatment of RA.
Acknowledgments
The authors thank Clinical Research Support Center Kyushu (CReS, Fukuoka, Japan) for handling and analyzing the data.
Disclosure statement
HY received speaking fees from Chugai Pharmaceutical, Janssen Pharmaceutical, Pfizer, Eisai, Bristol-Myers Squibb, and Astellas. TT received speaking fees from Chugai Pharmaceutical, Takeda Pharmaceutical, Mitsubishi-Tanabe Pharma, Janssen Pharmaceutical, AbbVie, Bristol-Myers Squibb, Eli Lily, Teijin Pharma, Asahi Kasei Pharma, Daiichi-Sankyo, Astellas, and AYUMI. HH received speaking fees from Chugai Pharmaceutical, Takeda Pharmaceutical, Mitsubishi-Tanabe Pharma, Janssen Pharmaceutical, Pfizer, Eisai, AbbVie, Bristol-Myers Squibb, Astellas, AYUMI. TF received speaking fees from Chugai Pharmaceutical, Takeda Pharmaceutical, Mitsubishi-Tanabe Pharma, Eisai, AbbVie, Astellas, AYUMI, Teijin Pharmaceutical, Nihon Kayaku Pharmaceutical, Asahikasei Pharmaceutical. HT received speaking fees from Bristol-Myers Squibb, Chugai Pharmaceutical, Mitsubishi-Tanabe Pharma, Janssen Pharmaceutical, Eisai, AbbVie, Astellas, Nippon Kiyaku, Asahi Kasei Pharma, and Eli Lilly Japan. AM received speaking fees from Mitsubishi-Tanabe Pharma, Eisai, AbbVie, Bristol-Myers Squibb, Taisho Toyama Pharmaceutical. YN received speaking fees from Chugai Pharmaceutical, Mitsubishi-Tanabe Pharma, Janssen Pharmaceutical, Pfizer, Eisai, AbbVie, Bristol-Myers Squibb, Astellas, AYUMI, Hisamitsu, Taisho Toyama Pharmaceutical, and Takeda Pharmaceutical. ES received speaking fees from Chugai Pharmaceutical, Mitsubishi-Tanabe Pharma, Janssen Pharmaceutical, Pfizer, Eisai, AbbVie, Bristol-Myers Squibb, Astellas, AYUMI, Takeda Pharmaceutical, Eli Lilly, Y L Biologics. SY received speaking fees from Bristol-Myers Squibb. MK eceived speaking fees from Chugai Pharmaceutical, Mitsubishi-Tanabe Pharma, Janssen Pharmaceutical, Pfizer, Eisai, AbbVie, Bristol-Myers Squibb, Astellas, and AYUMI.
References
- Suwannalai P, Trouw LA, Toes RE, et al. Anti-citrullinated protein antibodies (ACPA) in early rheumatoid arthritis. Mod Rheumatol. 2012;22(1):15–20.
- Schett G. Autoimmunity as a trigger for structural bone damage in rheumatoid arthritis. Mod Rheumatol. 2017;27(2):193–197.
- Sokolove J, Schiff M, Fleischmann R, et al. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis. 2016;75(4):709–714.
- Aletaha D, Alasti F, Smolen JS. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res Ther. 2015;17(1):229.
- Lee DM, Phillips R, Hagan EM, et al. Quantifying anti-cyclic citrullinated peptide titres: clinical utility and association with tobacco exposure in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68(2):201–208.
- Nüßlein HG, Alten R, Galeazzi M, et al. Efficacy and prognostic factors of treatment retention with intravenous abatacept for rheumatoid arthritis: 24-month results from an international, prospective, real-world study. Clin Exp Rheumatol. 2016;34(3):489–499.
- Gottenberg JE, Ravaud P, Cantagrel A, et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the ‘Orencia and Rheumatoid Arthritis’ registry. Ann Rheum Dis. 2012;71(11):1815–1819.
- Alten R, Nüßlein HG, Mariette X, et al. Baseline autoantibodies preferentially impact abatacept efficacy in patients with rheumatoid arthritis who are biologic naïve: 6-month results from a real-world, international, prospective study. RMD Open. 2017;3(1):e000345.
- Harrold LR, Litman HJ, Connolly SE, et al. Effect of anticitrullinated protein antibody status on response to abatacept or antitumor necrosis factor-α therapy in patients with rheumatoid arthritis: a US National Observational Study. J Rheumatol. 2018;45(1):32–39.
- Pieper J, Herrath J, Raghavan S, et al. CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol. 2013;14(1):34.
- Scarsi M, Paolini L, Ricotta D, et al. Abatacept reduces levels of switched memory B cells, autoantibodies, and immunoglobulins in patients with rheumatoid arthritis. J Rheumatol. 2014;41(4):666–672.
- Jansen D, Emery P, Smolen JS, et al. Conversion to seronegative status after abatacept treatment in patients with early and poor prognostic rheumatoid arthritis is associated with better radiographic outcomes and sustained remission: post hoc analysis of the AGREE study. RMD Open. 2018;4(1):e000564.
- Mochizuki T, Yano K, Ikari K, et al. The efficacy of abatacept focusing on anti-CCP antibody in Japanese patients with rheumatoid arthritis for 104 weeks. Mod Rheumatol. 2018;28(3):575–577.
- Wunderlich C, Oliviera I, Figueiredo CP, et al. Effects of DMARDs on citrullinated peptide autoantibody levels in RA patients-A longitudinal analysis. Semin Arthritis Rheum. 2017;46(6):709–714.
- de Moel EC, Derksen V, Thouw LA, et al. In rheumatoid arthritis, changes in autoantibody levels reflect intensity of immunosuppression, not subsequent treatment response. Arthritis Res Ther. 2019;21(1):28.
- Oryoji K, Yoshida K, Kashiwado Y, et al. Shared epitope positivity is related to efficacy of abatacept in rheumatoid arthritis. Ann Rheum Dis. 2018;77(8):1234–1236.
- Bonelli M, Ferner E, Göschl L, et al. Abatacept (CTLA-4IG) treatment reduces the migratory capacity of monocytes in patients with rheumatoid arthritis. Arthritis Rheum. 2013;65(3):599–607.
- Axmann R, Herman S, Zaiss M, et al. CTLA-4 directly inhibits osteoclast formation. Ann Rheum Dis. 2008;67(11):1603–1609.
- Jansen DT, el Bannoudi H, Arens R, et al. Abatacept decreases disease activity in a absence of CD4(+) T cells in a collagen-induced arthritis model. Arthritis Res Ther. 2015;17(1):220.
- Buch MH, Hensor EM, Rakieh C, et al. Abatacept reduces disease activity and ultrasound power Doppler in ACPA-negative undifferentiated arthritis: a proof-of-concept clinical and imaging study. Rheumatology (Oxford). 2017;56(1):58–67.
