Abstract
Recently, great advancements have been made towards understanding the mechanisms underlying dermatomyositis (DM). Many novel autoantibodies, such as anti-MDA5, anti-TIF1γ, anti-NXP2, and anti-SAE, have been reported to be involved in DM. DM is now classified based on these myositis-specific autoantibodies. Anti-TIF1γ antibodies are closely associated with juvenile DM and adult cancer-associated DM. Anti-TIF1γ antibody-positive DM tends to present severe cutaneous manifestations, mild myositis, and dysphagia. TIF1γ (also known as TRIM33) plays a role in transcriptional elongation, DNA repair, differentiation of cells, embryonic development, and mitosis. Moreover, TIF1γ has been shown to suppress various tumors via the TGF-β/Smad and the Wnt/β-Catenin signaling pathways. In this review, we explore the relationship between TIF1γ, cancer, and DM. We also discuss the pathogenesis of anti-TIF1γ antibody-positive DM.
1. Introduction
Dermatomyositis (DM) has a long history, beginning in 1891 when Dr. Unverricht first coined the term ‘dermatomyositis’ [Citation1]. DM has long been recognized as an autoimmune disease, but the involvement of autoantibodies in this disease was thought to be limited. Anti-ARS antibodies, including the anti-Jo-1 and anti-Mi-2 antibodies, have long since been established to have roles in DM. However, recent studies have revealed more novel autoantibodies involved in DM; among these are anti-MDA5, anti-TIF1, anti-NXP2, and anti-SAE antibodies [Citation2] Moreover, these novel autoantibodies, now known as myositis-specific autoantibodies (MSAs), have been associated with distinct disease phenotypes that have resulted in the classification of DM into subsets [Citation2–6]. For example, anti-MDA5 autoantibodies are associated with clinically amyopathic DM (CADM) and rapidly progressive interstitial lung disease (ILD), while anti-TIF1 antibodies are closely associated with juvenile DM (JDM) and adult cancer-associated DM. Thus, MSAs can be used in the clinical management of DM in order to predict complications and prognosis as well as to help determine the most effective therapeutic strategies. In this review, we explore the relationship between transcription intermediary factor 1 (TIF1) and cancer-associated DM.
2. Transcriptional intermediary factor 1 (TIF1)
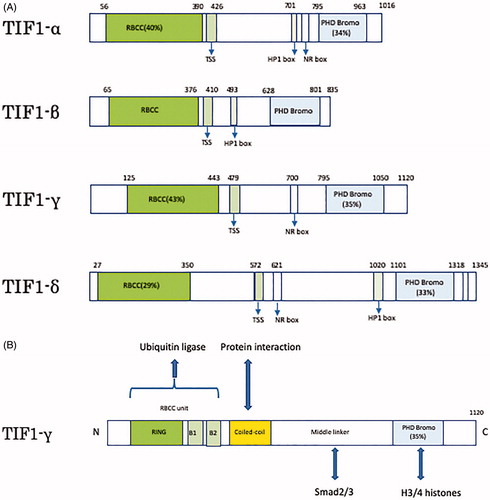
TIF1 is a protein belonging to the tripartite motif (TRIM) super family and is found in four subtypes, known as TIF1-α(TRIM24), TIF1-β(TRIM28), TIF1-γ(TRIM33), and TIF1-δ(TRIM66). All the subtypes have similar structures, which include an N-terminal TRIM which is a protein-protein and oligomerization interface containing an RBCC (Ring-B-box-coiled-coil) domain, a central TIF1 signature sequence (TSS) domain, and a C-terminal combination plant homeodomain (PHD) and bromodomain [Citation7].() Since TRIM is a ubiquitin ligase involved in protein modification and the C-terminal chromatin binding unit performs epigenetic transcriptional regulation, TIF1 can have multifunctional protein properties. TIF1γ has been reported to play a role in transcriptional elongation, DNA repair, differentiation of cells, embryonic development, and mitosis [Citation8,Citation9]. TIF1γ was first reported as a member of the TIF1 gene family in 1999 [Citation10]. TIF1γ is a 123 kDa protein consisting of 1120 amino acids encoded by the trim33 gene. At the N terminus, there is a RBCC unit, containing a RING domain, B boxes, and a coiled-coil domain all of which is involved in the ubiquitination of Smad4. At the C terminus, there is a PHD bromo domain that can interact with histones 3 and 4 while a middle linker region, which interacts with activated Smad2 and Smad3, connects the two terminuses [Citation9] ().
Figure 1. Schematics of human TIF1 and TIF1γ. (A) The four subtypes of TIF1-α, β, γ, and δ have two common structures of N-terminal TRIM (RBCC motif) and C-terminal chromatin binding unit (PHD Bromo). (B) Structure of TIF1γ. RBCC unit is involved in ubiquitination. Middle linker can interact with Smad2/3. PHD Bromo domain can interact with histones 3/4.
2.1. TIF1γ and cancer
The transforming growth factor-β(TGF-β) signaling pathway plays an important role in the occurrence and development of cancer as it can regulate cellular proliferation, differentiation, apoptosis, motility, invasion, and immune responses. Smad proteins are phosphorylated by TGF-β causing their translocation to the nucleus, and resulting in the transcriptional activation of downstream genes (). The TGF-β/Smad signaling pathway is involved in tumor growth, metastasis, and epithelial-mesenchymal transition (EMT) [Citation11–14]. TGF-β/Smad signaling acts as a tumor suppressor to inhibit tumor growth and metastasis by regulating downstream genes such as p21, p53, c-myc and snail [Citation9,Citation15,Citation16].
Figure 2. The tumor suppressive function of TIF1γ via the TGF-β/Smad and the Wnt/β-Catenin signaling pathways.
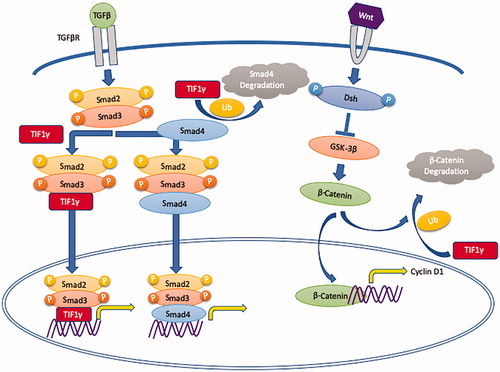
TIF1γ has been reported to be involved in the regulation of the TGF-β signaling pathway [Citation17,Citation18]. TIF1γ regulates the TGF-β/Smad signaling pathway via monoubiquitinating Smad4 and/or acting as a cofactor for the p-Smad2/3 complex, competing with Smad4 and thus inhibiting the formation of the Smad2/3/4 complex (). Many studies have revealed that TIF1γ can inhibit tumor growth, TGF-β-induced EMT and metastasis [Citation9]. In various malignancies, such as non-small-cell lung cancer, breast cancer, glioma, and clear cell renal cell carcinoma [Citation19–23], TIF1γ acts as a tumor suppressor and has decreased expression. However, this is not the case in all malignancies as TIF1γ is shown to be a tumor promotor in B lymphoblastic leukemia, pancreatic cancer and cervical cancer [Citation24–26].
The Wnt/βcatenin signaling pathway regulates stem cell self-renewal as well as cell proliferation, differentiation, adhesion, and migration. This pathway influences tumor growth and metastasis by regulating the expression of certain downstream genes, such as c-myc, cyclin D1, and Snail [Citation27]. In glioblastoma, TIF1γ can regulate the Wnt/β-catenin signaling pathway via β-catenin degradation with ubiquitylation, resulting in inhibition of cell proliferation and tumorigenesis () [Citation21]. Taken together, TIF1γ suppresses tumors via the TGF-β/Smad and the Wnt/β-Catenin signaling pathways in various cancers.
2.2. Anti-TIF1γ antibody-positive DM
Anti-TIF1 antibodies are described as anti-155/140 and anti-p155 antibodies that target a 155 kDa nuclear protein with or without 140 kDa protein [Citation28,Citation29]. The 155 kDa nuclear protein and the 140 kDa protein were subsequently identified as TIF1γ and TIF1α, respectively [Citation30].
Anti-TIF1 antibodies are detected in both JDM and adult DM. Anti-TIF1 antibodies were found in 35% of 354 JDM patients in a large USA registry study [Citation31,Citation32], as well as reported in earlier studies with 29% of 103 JDM patients [Citation28] and 23% of 116 JDM patients [Citation33] also having anti-TIF1 antibodies.
A study showed that anti-TIF1γ antibodies were present in 98.7% of patients with anti-TIF1 antibody-positive DM and 0% of these patients were positive for anti-TIF1α antibodies [Citation30]. Conversely, Muro et al. reported that anti-TIF1α antibodies coexist with anti-TIF1γ antibodies or anti-Mi-2 antibodies [Citation34]. In anti-TIF1 antibody-positive DM, myositis tends to be mild. Anti-TIF1γ antibodies are found in 7–31% of adult DM patients and have a positive association with malignancies and dysphagia, but a negative correlation with interstitial lung disease (ILD), Raynaud’s phenomenon and arthritis/arthralgia [Citation4,Citation35,Citation36]. In contrast, none of the anti-TIF1γ antibody-positive DM patients younger than 40 years of age (including JDM) had any malignancies [Citation33]. This suggests that juvenile and/or young adults have low risk of malignancy. However, there are still some reports describing young adult anti-TIF1γ antibody-positive DM patients who had malignancy and therefore this still requires considerable attention [Citation2]. Moreover, a recent study suggested that pregnancy could be one of the triggers for the onset of anti-TIF1γ antibody-positive DM in young adult female patients [Citation37].
2.3. Cutaneous manifestations of anti-TIF1γ antibody-positive DM
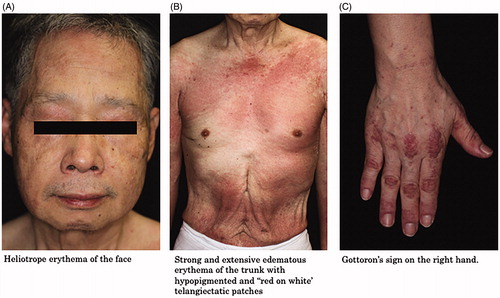
Severe cutaneous manifestations including V-neck sign, shawl sign, heliotrope erythema, Gottoron’s sign/papules, and flagellate erythema are frequently observed in patients with anti-TIF1γ antibody-positive DM () [Citation28,Citation29]. In one study, subcutaneous edema was observed in 17% of the anti-TIF1γ antibody-positive DM patients [Citation38]. Fiorentino et al. described that the characteristic cutaneous manifestations of anti-TIF1 antibody-positive DM patients included palmar hyperkeratotic papules, psoriasis-like lesions, and hypopigmented and ‘red on white’ telangiectatic patches [Citation36]. In contrast, anti-TIF1 antibodies are negatively associated with the presence of calcinosis [Citation36].
Figure 3. Cutaneous manifestations of anti-TIF1γ antibody-positive DM. (A) Heliotrope erythema of the face. (B) Strong and extensive edematous erythema of the trunk with hypopigmented and ‘red on white’ telangiectatic patches. (C) Gottoron’s sign on the right hand.
Okiyama et al. analyzed histological patterns of the finger eruptions in DM patients based on their MSAs including anti-ARS, anti-MDA5, and anti-TIF1γ antibodies [Citation39]. Interface dermatitis was observed in all MSA-positive DM patients while psoriasiform dermatitis and eczematous reaction were not distinctive in the anti-TIF1γ antibody-positive DM patients [Citation39]. Vascular injury was observed in 74% of the anti-MDA5 antibody-positive DM patients and in 44% of the anti-TIF1γ antibody-positive DM patients. Immunohistochemical staining for myxovirus resistance protein A (MxA), whose expression is associated with type I interferon activity, was performed. MxA expression in the epidermis was higher in the MDA5 and TIF1γ groups than in the ARS group [Citation39,Citation40].
2.4. Anti-TIF1γ antibodies and cancer-associated myositis (CAM)
A recent study reported that TIF1 mutations and a loss of heterozygosity (LOH) was detected in tumors of patients with anti-TIF1γ antibody-positive CAM [Citation41]. One somatic mutation and 5 cases of LOH were detected in 7 tumors from patients with anti-TIF1γ antibody-positive CAM. The TIF1γ staining of the tumors from the anti-TIF1γ antibody-positive CAM patients was more intense than the tumors from the anti-TIF1γ antibody negative CAM patients. TIF1 gene mutations and the overexpression of TIF1γ in the tumors may be involved in the etiology and pathogenesis of CAM [Citation41].
Ikeda et al. described the clinical significance of the serum levels of anti-TIF1γ antibodies in patients with DM [Citation42]. They compared a CAM group (n = 16) with a non-CAM group (n = 15) and discovered no significant difference in the anti-TIF1γ antibodies index between these two groups. In the CAM group, 4 patients were completely cured of their malignancies and the anti-TIF1γ antibody levels in 3 patients turned negative with a loss of DM activity. The anti-TIF1γ antibody level tended to be sustained in patients with stage IV malignancies. Changes in the anti-TIF1γ antibody level tended to reflect the activities of the DM and the malignancies [Citation42].
3. Discussion
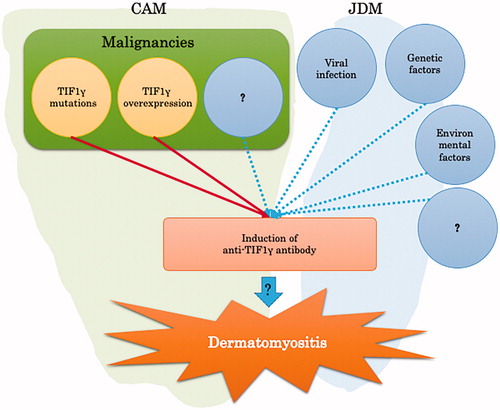
Several factors, including viral infections, genetics, and environment affect the pathogenesis of DM, such as exposure to ultraviolet radiation, vitamin D deficiency, and drugs () [Citation43,Citation44].
So far, we have focused on the relationship between TIF1γ and cancer, and the relationship between anti-TIF1γ antibodies and DM. TIF1γ mutations and/or high expression of TIF1γ in tumors can contribute to the production of anti-TIF1γ antibodies, possibly triggering the onset of CAM. Previous reports support this hypothesis as malignancies were completely cured when the antibody titer returned a negative result and DM was relieved. However, there are many cases of DM that did not improve when just the malignant tumors were treated. This phenomenon is cause for further research as other underlying mechanisms may yet be elucidated.
It has been reported that ferritin is useful as a disease marker in anti-MDA5 antibody-positive DM within interstitial lung disease [Citation45]. In anti-TIF1γ antibody-positive DM, to date no marker has been determined that can reflect the disease activities. A recent study reported that the anti-TIF1γ antibodies titer correlates with DM disease activities [Citation42].
The treatment of anti-TIF1γ antibody-positive DM is basically the same as the treatment for other DMs, such as anti-MDA5 antibody-positive DM, and anti-Mi-2 antibody-positive DM. This treatment mainly involves prednisolone and immunosuppressants, but in anti-TIF1γ antibody-positive DM cases with malignancies, it has been shown that the treatment for malignancies should be prioritized. In cases with dysphagia and/or steroid-resistance, intravenous immunoglobulin (IVIG) therapy has been reported to be effective [Citation46].
The recent breakthroughs in DM has led to the discovery of various specific autoantibodies. These autoantibodies have resulted in classification of DM into subsets that are associated with specific phenotypes and malignancies. Anti-TIF1γ antibody-positive DM is one such classification. Thus, the elucidation of the relationship between malignant tumors and TIF1γ along with the relationship between DM and malignancies, which has been a mystery for a long time, is being clarified. So far, only a small part has been elucidated, and it is still unclear how anti-TIF1γ antibodies are involved in the pathogenesis of diseases such as DM. We hope that this will be further researched in order to gain a better understanding of disease pathology and lead to advancements in clinical management and treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Steiner WR. Dermatomyositis, with report of a case which presented a rare muscle anomaly but once described in man. J Exp Med. 1905;6(4–6):407–442.
- Fujimoto M, Watanabe R, Ishitsuka Y, et al. Recent advances in dermatomyositis-specific autoantibodies. Curr Opin Rheumatol. 2016;28(6):636–644.
- Targoff IN, Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985;28(7):796–803.
- Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med. 2016;280(1):8–23.
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1–19.
- Gunawardena H. The clinical features of myositis-associated autoantibodies: a review. Clin Rev Allergy Immunol. 2017;52(1):45–57.
- Iyengar S, Farnham PJ. KAP1 protein: an enigmatic master regulator of the genome. J Biol Chem. 2011;286(30):26267–26276.
- Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11(11):792–804.
- Yu C, Ding Z, Liang H, et al. The roles of TIF1γ in cancer. Front Oncol. 2019;9:979
- Venturini L, You J, Stadler M, et al. TIF1gamma, a novel member of the transcriptional intermediary factor 1 family. Oncogene. 1999;18(5):1209–1217.
- Centurione L, Aiello FB. DNA repair and cytokines: TGF-β, IL-6, and thrombopoietin as different biomarkers of radioresistance. Front Oncol. 2016;6:175.
- Barcellos-Hoff MH, Cucinotta FA. New tricks for an old fox: impact of TGFβ on the DNA damage response and genomic stability. Sci Signal. 2014;7(341):re5.
- Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66.
- Hesling C, Fattet L, Teyre G, et al. Antagonistic regulation of EMT by TIF1γ and Smad4 in mammary epithelial cells. EMBO Rep. 2011;12(7):665–672.
- Samanta D, Datta PK. Alterations in the Smad pathway in human cancers. Front Biosci. 2012;17:1281–1293.
- Seoane J, Gomis RR. TGF-beta family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. 2017;9(12):a022277.
- He W, Dorn DC, Erdjument-Bromage H, et al. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125(5):929–941.
- Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–230.
- Wang L, Yang H, Lei Z, et al. Repression of TIF1γ by SOX2 promotes TGF-β-induced epithelial-mesenchymal transition in non-small-cell lung cancer. Oncogene. 2016;35(7):867–877.
- Kassem L, Deygas M, Fattet L, et al. TIF1gamma interferes with TGFbeta1/SMAD4 signaling to promote poor outcome in operable breast cancer patients. BMC Cancer. 2015;15(1):453.
- Xue J, Chen Y, Wu Y, et al. Tumour suppressor TRIM33 targets nuclear β-catenin degradation. Nat Commun. 2015;6:6156.
- Jingushi K, Ueda Y, Kitae K, et al. miR-629 Targets TRIM33 to Promote TGFβ/Smad Signaling and Metastatic Phenotypes in ccRCC. Mol Cancer Res. 2015;13(3):565–574.
- Xue J, Lin X, Chiu WT, et al. Sustained activation of SMAD3/SMAD4 by FOXM1 promotes TGF-β-dependent cancer metastasis. J Clin Invest. 2014;124(2):564–579.
- Sedgwick GG, Townsend K, Martin A, et al. Transcriptional intermediary factor 1γ binds to the anaphase-promoting complex/cyclosome and promotes mitosis. Oncogene. 2013;32(39):4622–4633.
- Ligr M, Wu X, Daniels G, et al. Imbalanced expression of Tif1gamma inhibits pancreatic ductal epithelial cell growth. Am J Cancer Res. 2014;4(3):196–210.
- Pommier RM, Gout J, Vincent DF, et al. TIF1γ suppresses tumor progression by regulating mitotic checkpoints and chromosomal stability. Cancer Res. 2015;75(20):4335–4350.
- Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15(7):873–887.
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54(11):3682–3689.
- Kaji K, Fujimoto M, Hasegawa M, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology. 2007;46(1):25–28.
- Fujimoto M, Hamaguchi Y, Kaji K, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 2012;64(2):513–522.
- Rider LG, Shah M, Mamyrova G, Huber AM, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92(4):223–243.
- Rider LG, Nistala K. The juvenile idiopathic inflammatory myopathies: pathogenesis, clinical and autoantibody phenotypes, and outcomes. J Intern Med. 2016;280(1):24–38.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2007;47(3):324–328.
- Muro Y, Ishikawa A, Sugiura K, et al. Clinical features of anti-TIF1-α antibody-positive dermatomyositis patients are closely associated with coexistent dermatomyositis-specific autoantibodies and anti-TIF1-γ or anti-Mi-2 autoantibodies. Rheumatology. 2012;51(8):1508–1513.
- Mugii N, Hasegawa M, Matsushita T, et al. Oropharyngeal dysphagia in dermatomyositis: associations with clinical and laboratory features including autoantibodies. PLoS One. 2016;11(5):e0154746.
- Fiorentino DF, Kuo K, Chung L, et al. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015;72(3):449–455.
- Oya K, Inoue S, Saito A, et al. Pregnancy triggers the onset of anti-transcriptional intermediary factor 1γ antibody-positive dermatomyositis: a case series. Rheumatology. 2020;59(6):1450–1451.
- Albayda J, Pinal-Fernandez I, Huang W, et al. Antinuclear matrix protein 2 autoantibodies and edema, muscle disease, and malignancy risk in dermatomyositis patients. Arthritis Care Res. 2017;69(11):1771–1776.
- Okiyama N, Yamaguchi Y, Kodera M, et al. Distinct histopathologic patterns of finger eruptions in dermatomyositis based on myositis-specific autoantibody profiles. JAMA Dermatol. 2019;155(9):1080.
- Okiyama N, Fujimoto M. Cutaneous manifestations of dermatomyositis characterized by myositis-specific autoantibodies. F1000Res. 2019;8:1951.
- Pinal-Fernandez I, Ferrer-Fabregas B, Trallero-Araguas E, et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatology. 2018;57(2):388–396.
- Ikeda N, Yamaguchi Y, Kanaoka M, et al. Clinical significance of serum levels of anti-transcriptional intermediary factor 1-gamma antibody in patients with dermatomyositis. J Dermatol. 2020;47(5):490–496.
- Thompson C, Piguet V, Choy E. The pathogenesis of dermatomyositis. Br J Dermatol. 2018;179(6):1256–1262.
- Mainetti C, Terziroli Beretta-Piccoli B, Selmi C. Cutaneous manifestations of dermatomyositis: a comprehensive review. Clin Rev Allergy Immunol. 2017;53(3):337–356.
- Gono T, Kawaguchi Y, Ozeki E, et al. Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol. 2011;21(2):223–227.
- Patwardhan A. The value of intravenous immunoglobulin therapy in idiopathic inflammatory myositis in the current transformed era of biologics. Biologics. Cureus. 2020;12(2):e7049.