Abstract
Chimeric antigen receptor (CAR) is generated by fusing a cancer-specific antibody’s antigen recognition site with costimulatory molecules such as CD28 and CD3ζ. T cells transduced with CAR recognizes cancer-specific antigens and kill cancer cells. The effect of CD19-targeted CAR T cells on B-cell hematologic cancer is surprising and has already been approved in many countries including Japan. More targets for several kinds of cancers are being searched now. We have also shown that CAR T cells specific for activated integrin β7 are highly effective for multiple myeloma in pre-clinical tests.
1.CAR T cell therapy
Checkpoint antibodies such as anti-PD1 antibodies have become widely used, and no one doubts that T lymphocytes have the ability to attack cancer. Many of cancer-specific T cells recognize neo-antigen, which is associated with genetic mutations. Therefore, the high efficacy of checkpoint antibody therapy is expected for cancers with many gene mutations such as melanoma and lung cancers. In order to attack cancers with relatively few genetic mutations, such as blood cancers, strategies are needed to make T lymphocytes successfully recognize cancer cells as non-self.
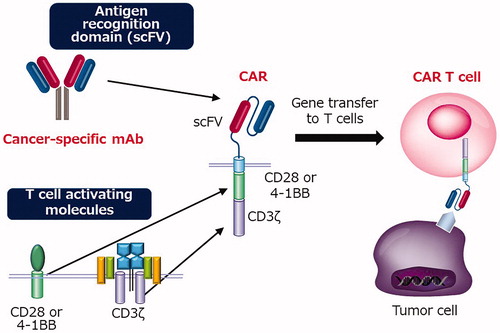
Monoclonal antibody therapy has already been widely applied as a method for allowing the immune system to recognize and eliminate cancer. CAR is constructed by fusing the antigen recognition site (scFv) of the antibody, the signal transduction site of the T cell receptor (CD3ζ), and CD28 or 41BB, a T cell costimulatory molecule. CAR T cells are established by transducing CAR into T cells (). Peripheral blood lymphocytes collected from a patient were transduced with CAR, amplified by in vitro culture, and then infused into the patient. CAR T cells have the advantages of both antibodies and cytotoxic T cells. CAR T cells recognize cancer-specific antigens with high specificity like antibodies and have high cytotoxic activity like cytotoxic T cells [Citation1].
Clinical trials of CAR T cells targeting CD19 against B-cell acute leukemia and malignant lymphoma have reported very high complete remission rates [Citation2]. In addition, durable complete remission was observed in substantial numbers of patients who received CD19 CAR T cell injection. Furthermore, many other institutions reported similar results, which led to the US approval of the CD19 CAR T-cell therapy in 2017 by the FDA.
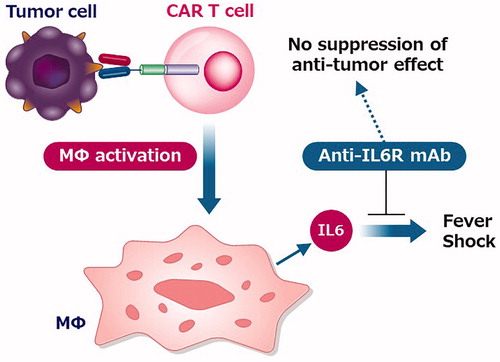
Previously, the high frequency of severe adverse events was a major concern. In early trials, nearly one-third of patients required intensive care unit (ICU) management for cytokine release syndrome (CRS), and some patients died of CRS. CRS is hypercytokinemia caused by ‘too effective’ CAR T cells. Soon, however, it became clear that tocilizumab (an anti-IL6 receptor antibody) had a profound effect on CRS, and CAR T cell therapy became a much safer treatment. In addition, further studies indicate that CRS is associated with hypersecretion of IL6 from activated macrophages following CAR T activation () [Citation3,Citation4]. In addition, blocking of the IL6 receptor by tocilizumab is unlikely to affect antitumor effects.
2. CAR T cell therapy for multiple myeloma
With the great success in B-cell leukemia and lymphoma, the development of CAR T cells for other cancers is now a global competition. A very promising next target is multiple myeloma (MM). MM is a blood cancer in which clonal plasma cells expand. The number of patients in Japan is about 18,000. Although remarkable progress has been made in the treatment of MM in recent years, the cure of MM is still extremely difficult, and the development of a new therapeutic agent has been awaited. CAR T cell therapy is thought to be a promising strategy. One target antigen that is currently considered promising for MM is BCMA, which has no expression except in the blood system and broad expression in MM cells and normal plasma cells. Several clinical trials of BCMA CAR T cell therapy show a high response rate [Citation5–7].
Ali et al. performed a phase I dose-escalation study of BCMA CAR T cells using CD28 as a costimulatory molecule [Citation8]. The overall response rate was 81%. A very good partial response (VGPR) or complete response was observed in 63% of patients. The median event-free survival was 31 weeks. Severe CRS occurred in some of the patients who received the highest doses of CAR T cells. A group at the University of Pennsylvania has established BCMA CAR-T cells using 4-1BB as a costimulatory molecule [Citation6]. CRS was observed in 88% of subjects and grade 3 or higher in 32%. Three patients (12%) had grade 3 or higher neurotoxicity. One patient died during the study after developing Grade 4 CRS with candidaemia. Recently, promising results of another multicenter phase I trial of another BCMA-CAR T cell bb2121 were reported [Citation7]. The response rate was 85%, including 15 patients with complete response (45%). Recurrence was observed in 6 of the 15 patients who had a complete response. The median progression-free survival (PFS) was 11.8 months. Severe CRS (grade 3) occurred in 2 patients (6%), but all recovered from CRS. There were no Grade 4 or 5 CRS events. Grade 4 neurotoxicity was observed in one patient (3%). Finally, LCAR-B38M, dual epitope binding CAR T cell therapy was developed by Nanjing Legend Biotech. Recently, the results of the first clinical trial were reported [Citation9]. Among 17 patients enrolled, stringent complete response (sCR) was achieved in 13 patients and a very good partial response (VGPR) was achieved in 2 patients. Eight patients remained under sCR with a median follow-up of 417 days. CRS occurred in 6 patients and was manageable, but one patient died of a severe toxic reaction.
On the other hand, it is considered that the short duration of the effect is a problem, and it is supposed that the cause of the recurrence is currently being elucidated. To cure MM, additional targets should be needed and are extensively searched by many researchers including us. University of Pennsylvania group reports that CD19 CAR is useful as consolidation after autologous transplantation [Citation10]. Expression of CD19 in myeloma plasma cells is extremely low or absent, suggesting that elimination of CD19-positive ‘myeloma progenitor cells’ or ‘myeloma stem cells’ may help prevent a recurrence [Citation11]. The Sloan Kettering group also found that the expression of the molecule GPRCD5 is myeloma-specific [Citation12]. We also have recently developed a new CAR T cell for multiple myeloma [Citation13].
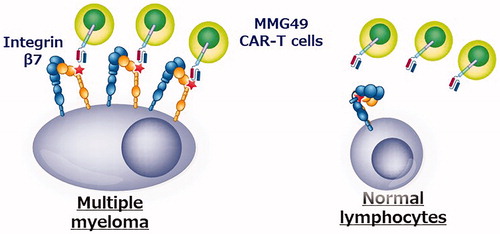
The search for genes and proteins expressed only in myeloma cells has already been extensively performed worldwide, and it is extremely difficult to identify new therapeutic targets. However, if cancer-specific antigens are formed by post-translational changes in proteins (such as glycosylation or conformational changes), they might be missed in the comprehensive analysis. Therefore, we started research by creating various types of monoclonal antibodies that bind to myeloma cells but not to normal hematopoietic cells. As a result, an antibody called MMG49 was identified as an antibody that binds to myeloma cells but does not bind to normal blood cells from among 10,000 or more clones of monoclonal antibodies that bind to myeloma cells. Next, it was revealed that the protein to which MMG49 binds is integrin β7. Curiously, MMG49 did not bind to normal blood cells, even though integrin β7 protein was also expressed in normal blood cells. Further analysis revealed that MMG49 binds only to the activated integrin β7. In addition, we found that in most normal blood cells, integrin β7 exists in an inactive form, whereas in myeloma cells, many integrin β7 molecules constitutively adopt the active conformation. In addition, since integrin β7 is not expressed in tissues other than blood cells, MMG49 that recognizes active integrin β7 binds to myeloma cells very specifically. Thus, the antigen recognition site of MMG49 was cloned and used to generate CAR T cells. In experiments using mice, it was shown that MMG49-derived CAR T cells specifically eradicated only myeloma cells without damaging normal cells. These results indicate that MMG49 CAR T-cell therapy targeting activated integrin β7 is promising novel immunotherapy for myeloma, and is currently being prepared for clinical application (). More importantly, even if the protein itself is not specific to cancer, its three-dimensional structure can be cancer-specific and immunotherapy targeting that ‘cancer-specific three-dimensional structure’ is possible. It is expected that similar ‘cancer-specific three-dimensional structures’ will be identified as therapeutic targets in many other cancer types in the future.
3. Future prospects for CAR T cell therapy
Although CAR T cell therapy is promising immunotherapy, there are still a number of issues that need improvement. Some of them are introduced next.
3.1. Deficiency of target antigen
There is still no appropriate target for CAR T cell therapy in many cancers, especially solid tumors. CAR T cells eliminate cells expressing their target, regardless of whether they are abnormal or normal. Therefore, it is important to use antigens that are not expressed in vital organs. A disastrous case has been reported in a CAR T cell targeting HER2 [Citation14]. A patient who received Her2 CAR T cell therapy died from CAR T cell infiltration into the lung. This was an ‘on-target, off-tumor effect’ caused by the slight expression of HER2 in the alveolar epithelium. It is necessary to be extremely careful about target antigens that are expressed in normal tissues even at low levels. Comprehensive analysis of cancer-specific genes or proteins has been extensively performed worldwide, and it is unlikely that unknown ones remain unidentified. Attempts have been made to target antigens formed by cancer-specific tertiary structures and glycosylation of proteins [Citation13,Citation15].
3.2. Efficient trafficking of CAR T cells to tumor site
Efficient migration of CAR T cells to tumor sites is critical for successful CAR T cell therapy, especially for the treatment of solid tumors. For this purpose, for example, attempts have been made to introduce a chemokine receptor that controls the migration of T cells into CAR T cells. Of particular note is the ‘Prime CAR T cell’ by Adachi and Tamada et al., which successfully induced high-level infiltration of CAR T cells into solid tumors by expressing IL7 and CCL19 [Citation16]. Interestingly, the effect was not only a direct effect of CAR T cells but also caused by triggering antigen-spreading and inducing T cell response for other cancer antigens. In the future, its efficacy will be examined in clinical trials.
3.3. CAR T cell persistence
The persistence of CAR T cells in the body has been shown to be important for lasting clinical effects in patients with hematological malignancies. Therefore, many researchers in the world are trying to develop CAR T cells that can survive in the body for a longer period of time. One of the notable ones is the new CAR report by Kagoya and Hirano [Citation17]. They added a modified interleukin (IL)-2 receptor β-chain (IL-2Rβ) cytoplasmic domain to the sequence of the CAR in addition to the conventional CD28/CD3ζ, resulting in transducing a cytokine signal in addition to the TCR and costimulatory signals after antigen recognition by CAR. As a result, the in vivo persistence of CAR T cells was enhanced.
3.4. Off the shelf CAR T cell development
The biggest social problem is that CAR T cell therapy is a very expensive treatment. One of the major reasons for the high cost is that CAR T cells must be manufactured for each patient. To solve this problem, the development of off the shelf CAR T cells that can be used for many patients has been attempted. One strategy is CAR T cells from allogeneic donors that have deleted T cell receptors that can cause GVHD using genome editing techniques [Citation18]. Another promising strategy is CAR T cells derived from iPS cells. Functional T cells can be successfully produced from iPS cells [Citation19,Citation20], and they are expected to be applied to CAR T cells.
3.5. Elucidation of the mechanism of resistance to CAR-T cells
Recurrent cases after CD19 CAR T cell therapy have been accumulated, and the cause of the resistance has been elucidated. The causes of recurrence are either loss of the target antigen on the tumor or exhaustion on the CAR-T cell cells. Here we describe the former mechanism. CD19 deficiency is observed in some of the relapsed patients who received CD19 CAR-T cells. The mechanism was first discovered to be a complete deletion of CD19 due to genetic mutations [Citation21,Citation22]. There are also cases in which the disease recurs as myeloid leukemia and CD19 is not expressed [Citation23]. On the other hand, recurrent leukemia cells are not necessarily CD19-negative, and in many cases CD19 is low. Recently it was reported that CAR-T cells take up CD19 from target cells and cause the reduction of CD19 expression on the target cells. This phenomenon is called ‘trogocytosis’ [Citation24].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379(1):64–73.
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517.
- Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–738.
- Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748.
- Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–2280.
- Cohen AD, Garfall AL, Stadtmauer EA, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–2221.
- Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737.
- Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–1700.
- Xu J, Chen L-J, Yang S-S, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA. 2019;116(19):9543–9551.
- Garfall AL, Maus MV, Hwang W-T, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373(11):1040–1047.
- Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68(1):190–197.
- Smith EL, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746.
- Hosen N, Matsunaga Y, Hasegawa K, et al. The activated conformation of integrin β7 is a novel multiple myeloma-specific target for CAR T cell therapy . Nat Med. 2017;23(12):1436–1443.
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851.
- Posey AD, Schwab RD, Boesteanu AC, et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44(6):1444–1454.
- Adachi K, Kano Y, Nagai T, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36(4):346–351.
- Kagoya Y, Tanaka S, Guo T, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24(3):352–359.
- Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374):eaaj2013.
- Minagawa A, Yoshikawa T, Yasukawa M, et al. Enhancing t cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell Stem Cell. 2018;23(6):850–858.
- Maeda T, Nagano S, Ichise H, et al. Regeneration of CD8αβ T cells from T-cell-derived iPSC imparts potent tumor antigen-specific cytotoxicity. Cancer Res. 2016;76(23):6839–6850.
- Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295.
- Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–1506.
- Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406–2410.
- Hamieh M, Dobrin A, Cabriolu A, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112–116.