Abstract
The Hypoxia-Inducible Factor-1 (HIF-1) is a dimeric protein complex that plays a significant role in responding to low oxygen or hypoxia concentrations. Chronic inflammation is one of the immune system responses and can increase HIF expression in involved tissues through lowering the oxygen and hypoxia. The HIF factor has many critical roles in immunity, and thus, we reviewed the crucial roles of this factor in the immune system. The results showed various key roles on the immune system, including physical defenses, innate immune (neutrophils apoptosis, macrophages) and inflammatory responses (pyrexia and local heat, iron access, etc.), upregulation in response to microbial infections, cytokines expression (IL-1, IL-2, IL-6, IL-8, IL-12, IL-18, TNF, etc.), drug targeting, etc. The HIF roles in the acquired immune system include: enhance the adaptation of cells (dendritic cells) to new conditions and triggering the signal pathways. The findings of the present review demonstrated that the HIF has important roles in the immune system, including physical defense, innate immune as well as acquired immunity; therefore, it may be considered as a potent drug targeting several diseases such as cancers, infectious diseases, etc.
1. Introduction
Immunity is resistance to diseases, especially infectious diseases. The immune system includes specific tissues, cells, and molecules, leading to the protection of the body against infections [Citation1].
The immune system exists in all multicellular organism and is divided into three levels include (i) physical defenses; (ii) the innate and adaptive immune system. The immune system is created of lymphoid and myeloid cells, and the pivotal role of the immune system is to protect the body against infectious diseases. The immune system has two strategies invertebrates include the innate and adaptive immune systems [Citation2–4].
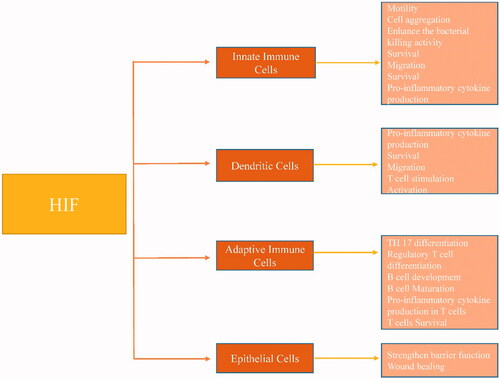
The Hypoxia-Inducible Factor (HIF)-1 is a dimeric protein complex that plays a significant role in responding to low concentrations of oxygen or hypoxia. HIF-1 is a transcriptional and heterodimeric factor and has a constitutively expressed subunit-β and an oxygen-dependent subunit-α. Both the HIF-1α and HIF-1β proteins have helix-loop-helix motifs, which bound to DNA and lead to dimerization of the subunits. HIF-1 is one of the most important genes involved in homeostasis and transcribing factor for thousands of genes and also has an essential role in immune responses [Citation5,Citation6]. Chronic inflammation is one of the immune system responses and can increase HIF expression in involved tissues through lowering the oxygen and hypoxia. Furthermore, it is demonstrated that decreased oxygen levels lead to activation of HIF in immune cells and can play an important role in the survival and activity of immune cells through regulating several genes [Citation7]. HIF plays significant roles in bacterial accumulation, killing and invasiveness bacteria, sensitizing of myeloid cells such as granulocyte, monocyte and macrophage and neutrophils survival in a hypoxic condition. HIF-1α is effective on the survival, function, and activity of dendritic cells linked to innate and adaptive immune systems [Citation8]. It is demonstrated that HIF has an important role in survival and function of B and T cells, neutrophils, macrophages, and dendritic cells. HIF-1α mediates the differentiation of Treg (Regulatory T cell) and T helper 17 cells which are responsible for inflammation and differentiation [Citation9,Citation10]. Suppression of the HIF-1α gene in mouse keratinocytes leads to decreased cathelicidins expression, which shows direct antibacterial activities and has a crucial role in the utilization of immune cells against infections. HIF increases the proinflammatory cytokine production such as 1 L-1β and interferons in the presence of LPS (Lipopolysaccharides) [Citation11,Citation12]. Increasing body temperature nearly 41 °C leads to the accumulation of HIF-1α in the kidney and liver and continuing for hours [Citation13]. Suppression of the HIF-1α gene in myeloid cells of mouses attenuates the Inflammatory responses significantly [Citation14]. In 2002, Mecklenburgh demonstrated that neutrophils apoptosis detained in the hypoxic condition and presence of iron cheaters which can indicate the potential role of HIF. In addition, it is demonstrated that increased survival-depending on HIF-1 in neutrophils probably mediated through NF-KB in the hypoxic conditions [Citation15–17]. HIF-1 induces leukocyte β2 integrin expression, thus can enhance the ability of neutrophils to bind the epithelium. Decreased HIF-1α expression in myeloid cells leads to more sensibility to infectious caused by S.pyogene and potentiates the ability to destroy gram positive and negative bacteria [Citation18,Citation19]. Investigations on the Hela cell line infected by Bartonella henselae demonstrated the upregulation of HIF-1α [Citation20]. Viral infections widely caused induction of stability of HIF-1α in cells and led to local inflammation. Upregulation of HIF-1α was observed in BAL131C mouse infected by Leishmania amazonensis and Toxoplasma gondii [Citation21–23]. HIF factor has many critical roles in immunity, and thus, our aim is to survey these important roles in the immune system.
2. Methods
To identify all relevant literature, a review was conducted on studies found on such online databases as Google Scholar, PubMed, and Scopus by Internet-based search for Hypoxia-Inducible Factor, innate immunity, immunodeficiency, inflammation, infections profiling studies published between 2005 and 2018. Our search strategy aimed to identify all studies published in English that reported results of only immunodeficiency studies associated with Hypoxia-Inducible Factor that presented essential results. The literature search identified 116 potentially relevant articles. After exclusion of irrelevant or duplicate articles by reading titles and abstracts, 87 articles were retrieved for further evaluation.
2.1. HIF and physical defenses
The skin of vertebrates is the first line of physical defenses through producing anti-microbial peptides such as cathelicidins and defensins playing important roles in the immune system and exert their function by direct antibacterial activity and utilization of immune cells, but all of the anti-microbial peptides are not well detected. The control mechanisms of anti-microbial peptides (AMPs) expression are not well understood, but it is suggested that suppression of the HIF-1α gene in mouse keratinocytes leads to decreased cathelicidins expression and increases the necrotic skin lesions caused by inoculation of group A streptococcus. Furthermore, suppression of vhl expression in keratinocytes leads to increased cathelicidin and decreased sensitivity to group A streptococcus [Citation13,Citation24]. HIF activity is essential to induce Intestinal Trefoil Factor gene and Multidrug Resistance Gene-1 in colon epithelial, which are responsible for the physical defense of colon epithelial. HIF-1α deactivation in colon epithelial results in increasing colitis possibility. Epithelial cells of the skin and intestine are in the hypoxic condition in normal physiological conditions; thus, oxygen gradient likely causes physiological signals which can be detected by HIF and result in maximum defense against microorganisms. Moreover, disruption of oxygen transfers such as vascular obstruction leads to hypersensitivity to secondary infections and inflammatory stimulants through increased AMPs [Citation25–27].
2.2. HIF and innate immune and inflammatory responses
2.2.1. Inflammation
Inflammation is a complicated biological response to stimulations such as microbes and chemicals damaging living organisms. Acute inflammation occurs rapidly in response to harmful stimulations. Chronic inflammation happened in long-term tissue damage. It is demonstrated that tissues with chronic inflammation are involved by hypoxia because of increased consumption of oxygen by inflamed cells and infiltration cells [Citation28]. Infiltration of neutrophils in inflammatory bowel disease leads to decreased oxygen levels through the production of hydrogen peroxide by NADPH. By immunohistochemistry, it is elucidated that overexpression of HIF is the consequence of hypoxia in IBD (inflammatory bowel disease) and rheumatoid arthritis. Migration of immune cells to hypoxic inflamed tissue lead to HIF overexpression and result in survival and activation of immune cells. In addition to inhibition of HIF hydroxylase through hypoxia, Pre-inflammatory signals such as IL-1β and LPS can increase the NF-KB-mediated transcription of HIF mRNA in tissues involved with chronic inflammation. NF-KB is overexpressed in inflamed tissues and upregulates HIF-1α expression by binding to HIF-1α promotor. Knockdown of the HIF-1α gene resulted in poor survival and differentiation of B and T cells, neutrophils, macrophages, and dendritic cells [Citation29,Citation30] ().
2.2.2. Innate immune system
HIF plays important roles in accumulation, invasiveness, the killing of bacteria, mobility of myeloid immune cells, and neutrophils survival in the innate immune system. HIF-1α impresses the survival, function, and activity of dendritic cells, which connected the innate and acquired immune system. Furthermore, overexpression of HIF-1α in immature dendritic cells in a hypoxic condition promotes apoptosis, whereas in mature dendritic cells reduces hypoxia-related cell death [Citation20]. The knockdown of HIF in dendritic cells reduces the potential of promoting allogeneic T-cells and inhibits maturity. Moreover, HIF overexpression by LPS stimulation results in the enhancement of pro-inflammatory cytokines and interferon production. The function of HIF-2α in the immune system is not elucidated up to now. The LPS inhibits HIF hydroxylase through increasing succinate production in macrophages and leads to HIF maintenance. The HIF expression in intestinal epithelial upregulates intestinal trefoil factor and CD55 genes, which are responsible for the improvement of adenosine extracellular signaling pathway, mucin-3, and glycoprotein and results in enhancement of defense ability of intestinal epithelial [Citation1,Citation31].
2.2.3. HIF role in pyrexia and local heat
Local heat is one of the common symptoms of the inflammation process. Invasion by pathogens, especially Gram-positive bacteria, result in pyrexia. LPS impresses the hypothalamus control body temperature and leads to pyrexia through a domino reaction including 1 L-1, TNF-α, 1 L-6, and PGE2. Increasing temperature to 42 °C in HepG2 cells resulted in nonphosphorylated-HIF-1α in a normoxia condition. Phosphorylated HIF-1α is the major HIF-1α which combined with HIF-1β and lead to the expression of target genes, whereas non-phosphorylated-HIF-1α binds to P53 and maintain it [Citation32]. Pyrexia till 41 °C causes a continuous HIF-1α accumulation in the kidney and liver, which remains stable for hours despite the return of body temperature to normal. Induced HIF-1α becomes stable through interaction with HSP10 and HSP90. HSP90 chaperone demonstrated increased levels in a stressful condition. HSP90 binds to HIF-1α thorough PAS domain in the cytosol but cannot transport to the nucleus. Minet demonstrated for the first time that HSP90 is essential for HIF-1α function in hypoxia, which can be inhibited by ansamycin derivatives [Citation33]. Antibiotics, such as geldanamycin, novobiocin, and radicicol, reduce chaperone activity of HSP90 by binding to it and result in downregulation of the HIF-1α expression. Also, treated cells by geldanamycin showed decreased non-phosphorylated-HIF-1α levels, whereas overexpression of HIF-1β result in resistance to geldanamycin because HIF-1β and HSP90 bind to the same domain. HSP90 and hsp70 bind to the PASB domain of HIF-1α, HIF-2α, and HIF-3α. HSP synthesis promoted by PI3K/AKT pathway. If HSP90 does not protect HIF-1α, it will break down through an independent prolyl hydroxylase pathway [Citation34].
2.2.4. Iron access
Iron consumption is almost essential for all of the animals and microorganisms, and the innate immune system utilizes the inhibition of Iron access against infectious. Decreased iron access leads to overexpression of HIF because iron is a prolyl hydroxylase and asparaginyl cofactor. Desferrioxamine as an iron-chelating, iron deficiency anemia results in HIF maintenance in hypoxic tissues [Citation35].
2.2.5. Activation of HIF in innate immune system cells
Suppression of the HIF gene in myeloid cells causes decreased ATP production and results in a significant reduction of inflammatory responses such as reduced invasiveness and mobility of macrophages and disability to kill the engulfed bacteria in macrophages. Differentiation of blood monocytes to tissue macrophages is associated with HIF protein accumulation. Incubation of macrophages with heat-killed Streptococcus and normal oxygen pressure promotes induction of HIF transcriptional activities. Bacteria LPS triggers the independent-oxygen upregulation of HIF-1α through activating TLR-4, leading to activation of NF-KB [Citation36,Citation37].
2.2.6. Neutrophils apoptosis
Neutrophils play significant roles in the innate immune system and are the most abundant white blood cells. Neutrophils have a short life-time and present an 8–20 h half-time. Neutrophils destroy microorganisms via phagocytosis. Neutrophils contain enzymes and reactive oxygen species, which can harm the tissue and, thus, their death must be regulated through apoptotic pathways [Citation38]. Neutrophils phagocytes by macrophages and apoptosis will be delayed in the case of requirement to neutrophils. In 2002, Mecklenburgh demonstrated that hypoxia and iron cheaters reduce neutrophils apoptosis which may introduce the impact of HIF. Dependent-increased survival of HIF-1α in neutrophils probably is mediated by NF-KB in hypoxia. Lacking ascorbate potentiates HIF-1α expression and disturbs the apoptosis pathway because ascorbate is a cofactor for HIF prolyl hydroxylase [Citation17].
2.2.7. Macrophages
Defective HIF-1α reduces mouse macrophage's potential to destroy gram-positive and gram-negative bacteria and results in poor response to skin infections caused by streptococcus pyogenes. HIF-1α is responsible for the production of AMPs such as cathelicidin, cathepsin G, elastase, TNF-α, and nitric oxide (NO) in the innate immune system. In addition to antimicrobial activity, NO can maintain HIF-1α through inhibition of prolyl hydroxylase [Citation39]. Activation of HIF-1α potentiates the ability of macrophages to phagocyte and kill bacteria in hypoxia. Bacteria can promote HIF-1α maintenance stronger than hypoxia conditions. HIF-1α maintenance-induced by bacteria can occur even in normoxic conditions. HIF-1α enhances the binding of neutrophils to epithelium through upregulating of leukocyte β2 integrin [Citation40].
2.2.8. HIF-1α activity and microbial infection
HIF-1α upregulates in response to infection by several kinds of bacteria such as Streptococcus pyogenes, S. agalactiae, S. aureus, Salmonella typhimurium, and Pseudomonas aeruginosa. HIF-1α plays an important role in the immune system; downregulation of HIF-1α leads to the reduced potential of immune cells to destroy gram-positive and gram-negative bacteria such as S. pyogenes. Infected Hela cells by B. henselae showed overexpression of HIF-1α [Citation41]. The chlamydia pneumonia by secreting chlamydial protease–like factor causes ablation of HIF-1α and result in reduced immune system potential. P. aeruginosa can detect and respond to functions and activities of the immune system through upregulation of PA-1lectin/adhesion, which disturb the physical defense of epithelium. HIF-1α is overexpressed in intestinal epithelium in hypoxia and causes Adenosine production, which is associated with protective functions in normal conditions. P. aeruginosa converts adenosine to inosine by adenosine deaminase (ADA) [Citation12,Citation42].
Both adenosine and inosine potentiate the expression of PA-1lectin/adhesion in bacteria leading to lower innate immune system abilities. Reduction of iron access results in downregulation of HIF-1α through bacteria iron consumption. Pathogens such as Yersinia enterocolitica, Salmonella enterica, and Enterobacter aerogenes induce upregulation of HIF-1α in Peyer's particle and can be potentiated by pathogen's siderophores. Pathogens which lose siderophores because of mutations are not able to induce HIF-1α expression. Infected by Y. enterocolitica in mouses lacking HIF-1α demonstrates more intense invasiveness [Citation43,Citation44].
2.2.9. HIF-1α and viral infections
Viral infections induce HIF-1α maintenance totally in target cells following by local inflammation. Respiratory syncytial virus (RSV) induces HIF-1α through a NO-dependent pathway in bronchial epithelium. Upregulation of HIF-1α promotes VEGF production, which is responsible for respiratory tract edema in RSV infection [Citation45]. HIF-1α reduces cellular damage in viral infections such as vesicular stomatitis virus (VSV), inducing acute cytoplasmic effects. It is demonstrated that hypoxic conditions (14 mmHg and 2% O2) decreased VSV proliferation and pathogenicity. Interferons can upregulate HIF-1α. It was elucidated that proliferation and pathogenicity of VSV increased through suppression of HIF activities by small antagonists such as chemotin and RNA disturbance. Moreover, cell treated by cobalt chloride which is a hypoxic condition mimic leads to increased resistance to infections [Citation46].
2.2.10. HIF and parasite infections
HIF-1α overexpression is observed in cytoplasm and vacuoles of parasitophorous (pv) macrophages which are involved in skin lesions of L. amazonensis in BAL131C mouses. T. gondii, which is obligate intracellular parasites induce transcription of glycolytic enzymes, glucose transporters, and transferrin and VEGF receptors presumably through activation of HIF-1α [Citation45,Citation46]. Investigations demonstrated that T. gondii increases HIF-1α levels rapidly in infected fibroblasts probably because of the need to specific genes for proliferation or as cellular protection against pathogens [Citation47–49].
2.2.11. HIF-1α and sepsis
Sepsis is the reflection of abnormal and inappropriate responses to infections. During sepsis, bacteria and LPS promote the uncontrolled secretion of pro-inflammatory cytokines from immune cells such as monocytes and macrophages. LPS upregulates HIF-1α in macrophages through activating of P42/44MAPK pathway. Furthermore, NFKB induced overexpression of HIF-1α in hepatocytes through activating of JNK and C-JUN pathway [Citation50]. It is recently elucidated that LPS can be detected through TOLL-Like receptor four and leads to HIF-1α overexpression and reduced prolyl hydroxylase mRNA. HIF-1α plays an important role in determining the phenotype of sepsis and can cause high levels of cytokine production such as TNFα, IL-1, IL-6 & IL-12. Knockdown of HIF-1α in macrophages reduced LPS-induced apoptosis and blocked the clinical symptoms of sepsis such as tachycardia, hypotension, and hypothermia [Citation51].
2.3. HIF and cytokines
2.3.1. Lymphocytes
Lymphocytes are the main functional cells in the acquired immune system, which are the potential to detect and remember specific antigens, produce antibody and destroy infected host cells. Lymphocytes are circulating between blood, bone marrow, and secondary lymphocytic organs. Lymphocytes show more activity in hypoxia which can reveal the role of HIF in B and T cells. Occupied receptors of T cells via the AKT/mTOR pathway lead to upregulation of HIF −1α in T cells in a hypoxic condition [Citation52]. Coordination of different signal pathways such as cytokines, cellular interactions, and access to required nutrients resulted in mTOR pathway activation and revealed the potential of this pathway in the regulation of oxygen access. It is demonstrated that activation of T-cell receptors causes increased HIF-1α production [Citation53]. Suppression of vhl gene in the thymus causes HIF hyperactivity and results in reduced thymus size and the number of thymocyte cells CD4/CD8 double-positive and increased apoptosis because of hyperactivity. It is elucidated that the low levels of Ca2+ reduced in response to TCR activation in T cells with suppressed vhl, but not happened in vhl/HIF-1 suppression. Interactions between TCR, HIF, NFkB, Akt/mTOR in T cells demonstrated that oxygen levels are strong regulating signals for the evolution and functioning of T cells. The roles of HIF are not elucidated in B lymphocytes yet [Citation54,Citation55].
2.3.2. Drug-targeting HIF
Activation of HIF induces tumorigenicity through angiogenesis and increases glucose metabolism in hypoxic tumors. Thus suppression of HIF can be used as a cancer treatment. Upregulation of HIF induces erythropoiesis leading to erythropoietin production in anemia. Similarly, activation of HIF-related pathways may be useful in ischemic conditions. Nowadays, different medicines are developed to regulate the expression of HIF in order to diseases treatment [Citation56].
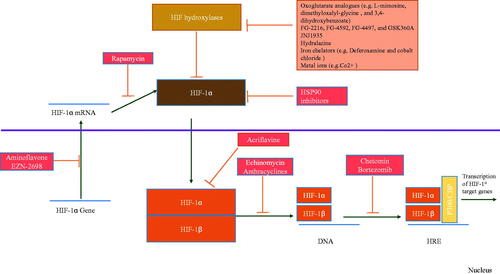
2.3.3. Activators of HIF
Inhibitors of hydroxylase such as Dimethyl oxalyl glycine and FG − 4497, JNJ 1935, which are 2- Oxoglutarate mimics and hydralazine, lead to upregulation of HIF. Desferrioxamine as an iron cheater causes HIF hyperactivity [Citation57] ().
2.3.4. Inhibitors of HIF
Developing inhibitors of HIF is more difficult because of no suppression in hypoxic conditions. The inhibitors of HIF mechanisms are not completely elucidated. The inhibitors of HIF induce their influences through the production of HIF-1α mRNA, HIF −1α protein, HIF-1α protein maintenance, HIF-1α/HIF −1β dimerization, binding of HIF to DNA, and increased transcription of HIF. Anemia is a complication of the inhibitors of HIF, and tumorigenicity and hyperproduction of erythropoietin (EPO) are complications of hydroxylase inhibitors. Since the activators and inhibitors of HIF can result in various complications, there must be more investigations on developing medicines that specifically target HIF [Citation58–60].
2.3.5. Targeting HIF in inflammatory diseases
Most of the investigations about pharmacological interventions in the HIF pathway are related to cancers and ischemic diseases but recently, the utilization of these medicines in inflammatory diseases is surveyed. Primary studies are focused on abdominal inflammatory diseases and the effects of hydroxylase inhibitors on disease progression [Citation61]. Hydroxylase inhibitors such as dimethyloxalylglycine (DMOG), FG4497 demonstrated protective effects in abdominal inflammatory diseases as first investigations. The anti-inflammatory potential of hydroxylase inhibitors is approved by proving their Anti-inflammatory effects on IBD. Hydroxylase inhibitors demonstrated sufficient capability in models suffered from endotoxic shock whereas showed harmful effects in models involved with sepsis which elucidates the diverse functions of hydroxylase inhibitors in vivo. Utilization of hydroxylase inhibitors can’t perform exclusively, for intense 2-Oxoglutarate mimics such as DMOG can impress enzymes using 2-Oxoglutarate as a cofactor and result in undesirable complications [Citation62,Citation63].
2.3.6. Targeting HIF in infectious diseases
HIF maintenance and HIF-related gene expression play important roles in the development of infectious diseases. Few studies are performed to demonstrate the role of HIF pathway targeting in infectious diseases. Epithelium Infecting by P. aeruginosa depends on HIF −2α expression. Hypoxia or treating by DMOG can reduce the infectious burden [Citation64]. The capability of DMGO to reduce the deaths caused by pneumonia is the sign of hydroxylase inhibitor's potential to decrease infectious diseases burden. It is elucidated that medicines that caused HIF maintenance lead to potentiating phagocytes and keratinocytes antibacterial activities, reduction of Staphylococcus aureus proliferation and complications, and high skin innate immune system capacity [Citation65].
2.3.7. Cytokines and HIF-1α regulation
HIF regulation can be effected through the role of oxygen and changes of oxidation-reduction potential in inflammations. Cytokines participate in molecular responses of different physiological and pathophysiological conditions. It is demonstrated that pro-inflammatory cytokines can activate the oxygen–linked pathway through ROS-related mechanisms [Citation66]. ROS induces pro-inflammatory cytokines, which can be inhibited by the mediation of specific antioxidants. Cytokines can create ROS-dependent pathways causing transcriptional factors activation such as HIF −1α, which is sensitive to reduction conditions. HIF − 1α plays a significant role in cellular responses to oxidative stress [Citation67]. ROS signal pathways controlling HIF − 1α regulation are activated in non-hypoxic conditions, which demonstrates that hypoxia is not the only HIF regulator. Generated ROS in ш complex of mitochondria promotes the activation of HIF protein. Moreover, HIF − 1α mediates the transcription of the VEGF gene resulting in detect a new independent-hypoxic mechanism to regulate the vascular reconstruction. RNS (reactive nitrogen species)-dependent pathways regulate the maintenance and activities of HIF − 1α by the use of this mechanism. For intense, transcription of NO synthase can induce HIF − 1α. Cytokines such as TNF–α and IL − 1β lead to activation of neutrophils and macrophages temporarily during the inflammations, which can result in hyperproduction of O2 through NADPH oxidase activation [Citation68,Citation69]. Oxidative burst releases ROS immediately, which is responsible for defense against pathogens and cancer metastasis. Cytokines can induce their effects by increasing mitochondrial ROS production. Haddad demonstrated the role of cytokines in ROS accumulation. Therefore, the hypothesis that cytokines induce their influences on transcriptional factors such as HIF-1α through ROS-dependent mechanisms can be potentiated. There are at least two sources for ROS production include bound to the membrane NADPH oxidase and mitochondrial respiratory chain, which mitochondrial respiratory chain may be the dominant source because mitochondrial respiratory chain blockage inhibits HIF-1α activation through cytokines. Furthermore, blocking of complex I (nicotinamide adenine dinucleotide phosphate-dependent oxidase) by diphenylene iodonium blocks the converting ubiquinone to ubiquinol and results in inhibition of HIF-1α through the dependent-cytokines pathway, which indicates the essential role of ROS driving from mitochondria in HIF −1α signal pathway [Citation70,Citation71]. It is demonstrated that various kinds of ROS such as H2O2, OH, and O-2 mediate the cytokine effects on maintenance, transporting, and HIF-1α activation. HIF is not only an oxygen sensor but also mediates the immune and inflammatory responses [Citation72].
2.3.8. HIF and inflammation
Activation of HIF-1α is essential for actvation of myeloid cells through independent-VEGF pathway in vivo. Furthermore, VHL downregulates HIF-1α and deficiency of it leads to increased inflammation responses. These results indicate that HIF-1α is essential for regulating of glycolytic capacity in myeloid cells. Suppression of HIF-1α decreases cellular ATP immediately resulting in disturbance to motility and invasion to bacteria by myeloid cells. This role of HIF − 1α demonstrates direct regulation of cellular function and survival during inflammation. TNFα and IL − 1β stimulate binding of HIF to DNA [Citation73,Citation74]. Furthermore. IL-1 promotes HIF − 1 induction in fibroblasts of synovial fluid and gum. iNOS and cyclooxygenase progress the inflammatory responses through immediate and vast production of NO and prostaglandin. NO and TNF α releasing by macrophages promotes HIF-1α maintenance. Overexpression of HIF − 1α by TNF–α in normoxic cells requires NF–kB activation. Moreover, upregulation of HIF-1α by IL − 1β through NF– kB/Cox2 pathway indicates the potential of HIF-1α as a connector between inflammation and ontogenesis [Citation75,Citation76].
2.4. HIF and cytokines
2.4.1. Interleukin-1
IL-1β promotes the binding of HIF–1 to DNA. IL–1 inhibits EPO production independent of HIF-1 which indicate that HIF 1 control gene expression correctly during the inflammations. Hypoxia and IL-1β promote HIF − 1 overexpression of VEGF by binding to hypoxic responsive elements on VEGF promotor and result in monocytes migration and microvascular secretion. IL-1β and insulin induce HIF −1α through PI3-kinase pathway and leads to EPO reduction [Citation6,Citation77]. IL − 1β regulates the maintenance, transferring to the nucleus, and the activity of HIF − 1α through mechanisms sensitizing to ROS/antioxidant, which indicates the dependent-cytokines regulation of HIF − 1α through the mediation of non-hypoxic pathways. IL − 1β induces HIF − 1α expression in normal cytotrophoblast through mitogen-activated protein kinase (MAPK) ERK in normoxic conditions. It is elucidated that IL-1β can also upregulate epidermal growth factor (EGF) and VEGFR receptors. IL-1β plays important roles in angiogenesis by the motivation of endothelial progenitor cells and upregulation of VEGF, VEGFR, and adhesion molecules of endothelial cells in ischemic conditions [Citation78].
2.4.2. Interleukin-2
T-cell antigen receptor (TCR) upregulates lymphocytic HRE (hypoxia response element)-related gene products in hypoxia condition. On the other hand, hypoxia inhibits gene products accumulation lacking of HRE such as IL− 2 and INF- ɤ. These results indicate the role of T cells in secretion of lymphokines and cytokines in hypoxia. Furthermore, it is elucidated that targeting of genes controlling by HIF and hypoxia result in involvement of IL-2 and other cytokines in cancer treatment [Citation79,Citation80].
2.4.3. Interleukin-6
Ureteropelvic junction obstruction with no response to endopyelotomy treatment leads to upregulation of NF–kB and pro-inflammatory cytokines such as IL-6. Patients with NF–kB upregulation indicate IL-6 overexpression. Moreover, HIF promotes NF–kB activation and upregulation of IL-1 and IL-6 in stimulated urothelial cells by hypoxia in comparison with treated cells by NF–kB inhibitors. Investigations on cytokines expression through HIF −1α and NF – kB activation in mast cells stimulating by desferrioxamine indicated that hypoxia could regulate cytokines transcription [Citation81–83].
2.4.4. Interleukin-8
Hypoxia regulates IL-8 expression in human macrophages. Acute hypoxia rapidly increases IL-8, indicating that hypoxia can promote pro-inflammatory cytokines [Citation84].
2.4.5. Interleukin-12
Targeting of HIF-1α and IL-12 is considered as a novel treatment of metastatic renal cancer [Citation85].
2.4.6. Interleukin-15
It is demonstrated that treated IEC 6 cells with IL-15 and EGF lead to a significant reduction of H4 acetylation and P53 levels, whereas induction of HIF-1α was increased. Suppression of ubiquitin-protein can increase the reduction of VEGF through the preservation of HIF-1α [Citation86].
2.4.7. Tumor necrosis factor
TNF–α presumably promotes HIF − 1 binding to DNA like IL-1 function. Furthermore, IL-1 and TNF–α upregulate adhesion molecules in hypoxic conditions, which play important roles in local inflammation. Redox factor − 1 (REF-1) act as an essential cofactor to mediate TNF effects in vascular endothelial cells. Upregulation of TNF receptor type two depends on Nuclear factor interleukin 6 (NF – IL-6) and is independent of HIF −1 and HIF −2 [Citation87]. TNF induces biological effects through TNF receptor type 1 (P 55 TNFR) and TNF receptor type 2 (P75 TNFR). Expression of P 55 TNFR continuously occurs in cells, while transcription of P75 TNFR is controlled by various stimulating factors. Northern blot analyzers indicated that P75 TNFR upregulates in NIH 3T3 cells in hypoxic conditions and re-oxygenation [Citation88]. Co-transfection analysis revealed that transcription of the P75 TNFR gene is independent of HIF − 1 and HIF − 2. TNF–α raises the transfer and activity of HIF − 1α in normoxic conditions. It is suggested that TNF regulates HIF −1α expression through a non-hypoxic and sensitized-ROS pathway. Antioxidants scavenged OH and H2O2 and caused a reduction in HIF −1α activity inducing by TNF–α. Moreover, inhibiting mitochondrial complex 1 suppresses the activation of TNF –α and HIF-1α. NADPH oxidase blocking results in downregulation of HIF-1α through O2- inhibition. (8-Methyl-N-vanillyl-6-nonenamide) Capsaicin regulates VEGF expression independent of IL −1 and TNF –α through HIF-1α [Citation89,Citation90].
2.4.8. Mitogen-activated protein kinase and HIF
MAPKs can be activated by growth factors and phosphorylate similar substrates. Their maximum activity is demonstrated when both Tyr and Thr are phosphorylated. MAPKs play a significant role in information transfer. HIF-1 needs to be phosphorylated to activate. Different kinase pathways such as MAPKs target HIF-1 as a substrate [Citation91].
2.4.9. HIF-MAPK p38
Molecular mechanisms of interactions of HIF–MAPK elucidated recently through investigations on phenotype changes in malignancies. Expression and secretion of VEGF upregulate through MAPKp38 and MAPK pathway which influence HIF-1α. Hypoxia induces MAPKp38 activity in head and neck squamous cell carcinoma lines. SB203580 as a MAPKp38 inhibitor suppress HIF −1α binding to DNA. MAPKp38 overexpression is essential for VEGF and HIF-1α expression [Citation92]. Chromium (Vl) is an induced-tumor factor in animals that can promote VEGF and HIF-1α expression in cancerous prostatic DU/45 cell lines through MAPKp38. Moreover, MAPKp38 induces dependent-HIF-1α VEGF in glioma and ovarian cancer cell lines. MAPKp38 regulates chemokines and cytokines expression through NF–kB and AP-1 activation. Besides, the expression of HIF-1-dependent genes leads to protection against ischemia through inflammatory cytokines [Citation93,Citation94].
2.4.10. Hif-mapkp42/44
Primary researches on HIF-MPAKP42/44 interactions revealed the relation between HIF-1 phosphorylation and its transcriptional activities. It is elucidated that MAPK p42/44 can phosphorylate HIF-1α in vitro. Activation of MAPKp42/44 upregulates the VEGF gene depending on HIF-1, while MAPKp42/44 doesn’t mediate the maintenance and decomposition of HIF-1. Long-term stimulation causes MAPK p42/44 deactivation and accumulation in the nucleus; this result indicates that presumably, the nucleus is an important location to terminate the mitogenic signals through the separation of MAPKs from MEK and dephosphorylation [Citation95]. Tyrosine kinase can induce HIF −1α transcriptional activates through MEK −1/MAPKp42/44 pathway. It is demonstrated that transcriptional activities of HIF-1α depend on two amino acid sequences, such as 522-649 and 650-822. They were treated by PD98059 cause both of two amino acid sequence blockages in the C-terminal half which revealed that the MEK − 1/MAPKp42/44 signal pathway could not distinguish between both of the domains. HBX virus can upregulate transcriptional activities of HIF − 1α through activation of MAPKs pathway. Furthermore, HBX promotes HIF − 1α maintenance in hepatic cells. Immunofluorescence assays revealed that virus-induced HIF −1α would be transferred to the nucleus relatively in most of the cells, whereas hypoxia induced by CocCl2 leads to complete transfer of HIF − 1α to the nucleus [Citation96,Citation97]. HBX induces HIF − 1α phosphorylation and MAPKp42/44 activation and can be accelerated by Cocl2 synergic effects. Treating by PD98059 and negative mutations of MAPKs activate induced-transcription by HBX and maintenance and transferring of HIF-1α through MAPKs pathway. HBX reduces the relationship between HIF −1α and VHL, whereas increases the relation of adhesions proteins to CREB, which indicates the molecular mechanisms involving in the maintenance and activation of HIF −1α by HBX. Levels of HIF −1α and VEGF will be increased in the liver of mouses infected by HBX. Therefore, HBX can play important roles in hepatocarcinogenesis through HIF −1α regulation [Citation98,Citation99].
2.4.11. Hif-mapkjnk
The C –Jun involved in the transcription of hypoxia-induced genes and can be controlled by HIF −1 and MAPKJNK. Hypoxia activated C –Jun through Ser phosphorylation of C–Jun sequence in the epithelium. Inhibition of MAPKTNK and mutation of C–Jun disturb the relation between MAPKTNK and C – Jun with HIF −1. AP-1 and HIF −1 can contribute with MAPKs to upregulate genes in hypoxic conditions [Citation100].
2.5. HIF and acquired immune system
HIF roles in the acquired immune system include: enhance the adaptation of cells to new conditions and triggering the signal pathways [Citation101].
2.6. HIF and dendritic cells
Presenting the antigens process relates to the innate and acquired immune system, and monocyte–derived dendritic cells (DCs) play a significant role in this relation by presenting pathogens antigens to the immune cell. Hypoxia suppresses DCs migration and increases cytokine production by DCs. Besides, hypoxia upregulates HIF-1α in mouses DCs. LPS and hypoxia significantly upregulate co-stimulating molecules of DCs, which result in glycolysis hyperactivity and potentiation of DCs to stimulate allogeneic T cells through HIF-dependent pathways. Ischemic conditions lead to hypoxic-dependent differentiation of DCs, which is associated with overexpression of HIF-1α. This differentiation occurred by rapamycin injection, which inhibits mTOR [Citation102,Citation103].
3. Discussion
mTOR–HIF-1α pathway plays important roles in trained immunity because inhibiting its pathway by chemical inhibitors will result in inhibited trained immunity system. IκB kinases (IKK) regulates NF-κB expression and can be activated by hypoxia. Not only HIF-1α result in NF-κB activation, but also NF-κB expression can regulate HIF-1α transcriptionally. It is elucidated by IKK-β knockdown NF-κB can activate HIF-1α transcriptionally and cause accumulation of HIF-1α protein in hypoxic conditions. The nuclear factor-κB (NF-κB) family plays valuable roles in the innate and adaptive immune systems. HIF-1α overexpression in skin epithelial keratinocytes leading to the production of antibacterial peptides such as cathelicidin [Citation104]. Cathelicidin LL-37 is the only family of cathelicidin in humans and upregulate inflammation mediator leading to immune cells differentiation and migration. Deactivation of HIF-1α can impress edema establishment and leukocyte infiltration and results in suppression of inflammatory responses [Citation105].
LPS of bacteria can lead to hyperproduction of cytokines during sepsis through sensitized leukocytes. Inflammation cytokines such as IL-4, IL-12, IL-6, IL-1, and TNF-α increase during sepsis through HIF-1α overexpression. Interleukin-1 beta plays an important role in the immune system. Lipopolysaccharide of bacteria promotes succinate production through innate immune cells. The production of interleukin-1β can be upregulated during the inflammation by succinate generating in the innate immune system through HIF-1α. By analysis of HIF-1α–deficient CD4 T cells, it is elucidated that HIF-1α regulates IL-22 expression [Citation106].
Glycolytic enzymes play important roles in TH17 cells and Treg cell metabolisms and can be regulated by HIF-1α. HIF1-α dependent glycolytic pathway expression was observed in developing TH17 Cells, but lack of HIF1α improves Treg cell differentiation and potentiates mice to be protected against neuroinflammation. Bacterial infection promotes HIF1-α expression in normoxic conditions. It is demonstrated that HIF-1α loss in myeloid cells of mice reduces the ability of the immune system to inhibit infections. Myeloid cells act as the most important cells in innate immunity. HIF-1α regulates the glycolytic capacity of myeloid cells, and knockdown of it results in the disturbance of myeloid cell function [Citation107]. HIF-1α can be upregulated in macrophages when an organ is deprived of oxygen, and overexpression of HIF-1α potentiates macrophages phagocytosis. Th1 cytokines upregulate HIF-1α, while HIF-2α will be upregulated by Th1 cytokines in macrophages, and isoforms of HIF-2α play important roles in NO homeostasis by macrophages. NO causes apoptosis, growth, and proper functioning of immune cells such as macrophages and demonstrated protective activity against inflammation in autoimmunity. HIF-1α can induce IFN-γ by binding to its promoter and improve immune responses such as presenting antigens and cytokines produced by T cells. It is elucidated that HIF-1α regulates neutrophil survival in the hypoxic condition through targeting of NF-κB [Citation108,Citation109].
Neutrophil apoptosis is regulated by HIF, resulting in increased cell resistance against death in hypoxic conditions. Prolyl hydroxylases (PHD1–3) control HIF expression, which is sensitized to oxygen in neutrophils. Inflammatory stimuli and hypoxic conditions upregulate PHD3 among the PHDs indicating the importance of PHD3 in high neutrophil survival. It is demonstrated that HIF-1α is upregulated in acute cutaneous lesions of leishmaniosis and HIF-1α downregulation in myeloid cells leads to an increased burden of leishmaniasis infection. Moreover, overexpression of HIF-1 caused hyperproduction of vascular endothelial growth factor (VEGF) S100A8 proteins in myeloid cells which resulted in angiogenesis and regulation of inflammatory functions. Myeloid cells with HIF-1α deficiency demonstrated an impaired process of ATP production associating with disturbed myeloid cell motility, invasiveness, and aggregation [Citation110]. It is elucidated by the investigations on a non-differentiated and differentiated monocytes cell line that contact with bacterial lipopolysaccharide results in upregulation of HIF-1α protein and HIF-1α mRNA through activation of NF-κB leading to upregulation of AM (adrenomedullin) which is a hypotensive peptide acting as an immune-modulating agent and regulates different immune system abilities and response to gram-positive and gram-negative bacteria in epithelial cells. Activated HIF-1α makes neutrophils keep watching on the injury site during the resolution phase and manage the PMN actions [Citation111,Citation112].
HIF-2α plays an important role in the regulation of the production of proinflammatory cytokine/chemokine through macrophages. HIF-2α organizes the M-CSFR receptor and the CXCR4 receptor expression resulting in macrophages migration. HIF-2α deficiency in murine neutrophils leads to increased apoptosis and reduced inflammation. The adaptive immune system potentiates through the expression of the hypoxia-inducible factor-1 (HIF-1) and the proto-oncogene MYC in T cells because the overexpression of both of these genes leads to improvement of cell proliferation, differentiation, and apoptosis of T cells [Citation113].
HIF-1α plays a significant role in lymphocyte functions, and deficiency of it leads to disturbed B cell development and autoimmunity. HIF-1α can regulate B lymphocytes cell cycle through control of kinase inhibitor p21 and p27, and loss of it results in increased cell growth. TH17 development can be improved by HIF-1 through activation of IL-17A associated with activation of PORγt transcription and collaboration with PORγt and p300, whereas HIF-1 reduces the Treg ability for development through targeting Foxp3 leading to proteasomal degradation. HIF-1α downregulated by Mir210 increasing in hypoxia in T cells, especially TH17, which results in the inability of immune cells to kill pathogens properly [Citation114].
HIF-1α promotes FoxP3 overexpression in T cells in hypoxia, which playing an important role in T cell metabolism through the improvement of oxidative phosphorylation and downregulation of Myc and glycolysis and result in cell adaptation to low-glucose, lactate-rich environments and regulation of CD25 + CD4+ Treg cells functions. The potential of CD8 + cytotoxic T lymphocytes (CTLs) increases in hypoxia by the regulation of HIFs and Hippel-Lindau tumor suppressor VHL. VHL knockdown downregulates HIFs expression, and loss of VHL leads to improved regulation of persistent viral infection. Regulation of the HIF-1 expression performs by a phosphatidylinositol-3 kinase (PI3K) and Akt through mTORC1 (mammalian target of rapamycin complex 1) mediation in CD8+ T cells resulting in regulation of glucose metabolism and production of chemokines. LPS upregulates Pyruvate Kinase M2 (PKM2), which is a metabolic regulator and inhibits Hif-1α and IL-1β and reduces glycolysis and the Accumulation of Succinate in macrophages [Citation115].
4. Conclusion
The results showed various key roles on the immune system, including physical defenses, innate immune (neutrophils apoptosis, macrophages) and inflammatory responses (pyrexia and local heat, iron access, etc.), upregulation in response to microbial infections, cytokines expression (IL-1, IL-2, IL-6, IL-8, IL-12, IL-18, TNF, etc.), drug targeting, etc. HIF roles in the acquired immune system include: enhance the adaptation of cells (dendritic cells) to new conditions and triggering the signal pathways. The findings of the present review demonstrated that the HIF has important roles in the immune system include: physical defense, innate immune as well as acquired immunity; therefore, it may be considered as a potent drug targeting several diseases such as cancers, infectious diseases, etc.
Disclosure statement
The authors declare no conflict of interest in this study.
References
- Cheng SC, Quintin J, Cramer RA, et al. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684.
- Rius J, Guma M, Schachtrup C, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453(7196):807–811.
- Peyssonnaux C, Boutin AT, Zinkernagel AS, et al. Critical role of HIF-1α in keratinocyte defense against bacterial infection. J Invest Dermatol. 2008;128(8):1964–1968.
- Cramer T, Johnson RS. A novel role for the hypoxia inducible transcription factor HIF-1alpha: critical regulation of inflammatory cell function. Cell Cycle. 2003;2(3):191–192.
- Moorlag SJ, Röring RJ, Joosten LA, et al. The role of the interleukin‐1 family in trained immunity. Immunol Rev. 2018;281(1):28–39.
- Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242.
- Shi LZ, Wang R, Huang G, et al. dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367–1376.
- Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657.
- Acosta-Iborra B, Elorza A, Olazabal IM, et al. Macrophage oxygen sensing modulates antigen presentation and phagocytic functions involving IFN-γ production through the HIF-1α transcription factor. J Immunol. 2009;182(5):3155–3164.
- Walmsley SR, Farahi N, Peyssonnaux C, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α–dependent NF-κB activity. J Exp Med. 2005;201(1):105–115.
- Walmsley SR, Chilvers ER, Thompson AA, et al. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest. 2011;121(3):1053–1063.
- Schatz V, Strüssmann Y, Mahnke A, et al. Myeloid cell–derived HIF-1α promotes control of Leishmania major. J Immunol. 2016;197(10):4034–4041.
- van Dijk A, Hedegaard CJ, Haagsman HP, et al. The potential for immunoglobulins and host defense peptides (HDPs) to reduce the use of antibiotics in animal production. Vet Res. 2018;49(1):1–6.
- Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192(2):553–560.
- Imtiyaz HZ, Williams EP, Hickey MM, et al. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120(8):2699–2714.
- Thompson AR, Elks PM, Marriott HM, et al. Hypoxia-inducible factor 2α regulates key neutrophil functions in humans, mice, and zebrafish. Blood. 2014;123(3):366–376.
- Elks PM, van Eeden FJ, Dixon G, et al. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118(3):712–722.
- Kojima H, Gu H, Nomura S, et al. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1α-deficient chimeric mice. Proc Natl Acad Sci USA. 2002;99(4):2170–2174.
- Goda N, Ryan HE, Khadivi B, et al. Hypoxia-inducible factor 1α is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23(1):359–369.
- Palazon A, Goldrath AW, Nizet V, et al. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528.
- Wang H, Flach H, Onizawa M, et al. Negative regulation of Hif1a expression and TH 17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol. 2014;15(4):393–401.
- Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha–dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109(41):E2784–E2793.
- Angelin A, Gil-de-Gómez L, Dahiya S, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25(66):1282–1293.
- Yoshimura Y. Avian β-defensins expression for the innate immune system in hen reproductive organs. Poult Sci. 2015;94(4):804–809.
- Kumar V, Gabrilovich DI. Hypoxia‐inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143(4):512–519.
- Poitz DM, Augstein A, Hesse K, et al. Regulation of the HIF-system in human macrophages–Differential regulation of HIF-α subunits under sustained hypoxia. Mol Immunol. 2014;57(2):226–235.
- Singh SP, Sharma J, Ahmad T, et al. Oxygen stress: impact on innate immune system, antioxidant defence system and expression of HIF-1α and ATPase 6 genes in Catla catla. Fish Physiol Biochem. 2016;42(2):673–688.
- Harris AJ, Thompson AR, Whyte MK, et al. HIF-mediated innate immune responses: cell signaling and therapeutic implications. Hypoxia (Auckl). 2014;2:47–58.
- Thompson AR, Binham J, Plant T, et al. Hypoxia, the HIF pathway and neutrophilic inflammatory responses. Biol Chem. 2013;394(4):471–477.
- Palsson‐McDermott EM, O'neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013;35(11):965–973.
- Hu X, Li YQ, Li QG, et al. Osteoglycin-induced VEGF inhibition enhances T lymphocytes infiltrating in colorectal cancer. EBioMedicine. 2018;34:35–45.
- Sefikogullari M, Kaya A, Aydin H, et al. Increased levels of VEGF-A and HIF-1α in Turkish children with Crimean-Congo hemorrhagic fever. J Arthropod Borne Dis. 2017;11(1):19–26.
- Liu J, Zeng Y, Ma W, et al. Preliminary investigation of the clinical value of vascular endothelial growth factor and hypoxia-inducible factor-1α in pericardial fluid in diagnosing malignant and tuberculous pericardial effusion. Cardiology. 2010;116(1):37–41.
- Wan J, Wu W. Hyperthermia induced HIF-1a expression of lung cancer through AKT and ERK signaling pathways. J Exp Clin Cancer Res. 2016;35(1):119.
- Gupta N, Wish JB. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69(6):815–826.
- Martinez VG, Ontoria-Oviedo I, Ricardo CP, et al. Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):208.
- Burrows N, Maxwell PH. Hypoxia and B cells. Exp Cell Res. 2017;356(2):197–203.
- Berger EA, McClellan SA, Vistisen KS, et al. HIF-1α is essential for effective PMN bacterial killing, antimicrobial peptide production and apoptosis in Pseudomonas aeruginosa keratitis. PLoS Pathog. 2013;9(7):e1003457.
- Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;1(1):65–80.
- Lohninger L, Tomasova L, Praschberger M, et al. Hydrogen sulphide induces HIF-1α and Nrf2 in THP-1 macrophages. Biochimie. 2015;112:187–195.
- Shalova IN, Lim JY, Chittezhath M, et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity. 2015;42(3):484–498.
- Bhandari T, Olson J, Johnson RS, et al. HIF-1α influences myeloid cell antigen presentation and response to subcutaneous OVA vaccination. J Mol Med (Berl). 2013;91(10):1199–1205.
- Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9(9):609–617.
- Zarember KA, Malech HL. HIF-1α: a master regulator of innate host defenses? J Clin Invest. 2005;115(7):1702–1704.
- Morinet F, Casetti L, François JH, et al. Oxygen tension level and human viral infections. Virology. 2013;444(1-2):31–36.
- Gupta-Saraf P, Miller CL. HIF-1α downregulation and apoptosis in hypoxic prostate tumor cells infected with oncolytic mammalian orthoreovirus. Oncotarget. 2014;5(2):561–574.
- Fraga CA, Oliveira MV, Alves LR, et al. Immunohistochemical profile of HIF-1α, VEGF-A, VEGFR2 and MMP9 proteins in tegumentary leishmaniasis. An Bras Dermatol. 2012;87(5):709–713.
- Hammami A, Charpentier T, Smans M, et al. IRF-5-mediated inflammation limits CD8+ T cell expansion by inducing HIF-1α and impairing dendritic cell functions during Leishmania infection. PLoS Pathog. 2015;11(6):e1004938.
- Song T, Li H, Yang L, et al. Expression of hypoxia-inducible factor-1α in the infiltrative belt surrounding hepatic alveolar echinococcosis in rats. J Parasitol. 2015;101(3):369–373.
- Hirota K. Involvement of hypoxia-inducible factors in the dysregulation of oxygen homeostasis in sepsis. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):29–40.
- Schäfer ST, Frede S, Winning S, et al. Hypoxia-inducible factor and target gene expression are decreased in patients with sepsis: prospective observational clinical and cellular studies. Anesthesiology. 2013;118(6):1426–1436.
- Zhang L, Ye SB, Li ZL, et al. Increased HIF-1alpha expression in tumor cells and lymphocytes of tumor microenvironments predicts unfavorable survival in esophageal squamous cell carcinoma patients. Int J Clin Exp Path. 2014;7(7):3887.
- Palazón A, Martínez-Forero I, Teijeira A, et al. The HIF-1α hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discov. 2012;2(7):608–623.
- Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(55):781–790.
- Bollinger T, Bollinger A, Gies S, et al. Transcription regulates HIF‐1α expression in CD4+ T cells. Immun Cell Biol. 94(1):109–113.
- Warfel NA, El-Deiry WS. HIF-1 signaling in drug resistance to chemotherapy. Curr Med Chem. 2014;21(26):3021–3028.
- Kobayashi H, Ohyama T, Kitamura-Miyazaki M, et al. Studies on novel HIF activators, A-503451sII: biological activities of A-503451A. J Antibiot. 2016;69(10):754–758.
- Lee SH, Jee JG, Bae JS, et al. A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol. 2015;230(4):853–862.
- Selvaraju V, Parinandi NL, Adluri RS, et al. Molecular mechanisms of action and therapeutic uses of pharmacological inhibitors of HIF-prolyl 4-hydroxylases for treatment of ischemic diseases. Antioxid Redox Signal. 2014;20(1616):2631–2665.
- Lai FF, Niu F, Yang HZ, et al. Development of a novel screening assay for inhibitors targeting HIF-1alpha and P300 interaction. Yao Xue Xue Bao. 2014;49(6):849–853.
- Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13(11):852–869.
- Ramakrishnan SK, Shah YM. Role of intestinal HIF-2α in health and disease. Annu Rev Physiol. 2016;78:301–325.
- Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. 2013;1;13(4):646–653.
- Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med (Berl). 2007;85(12):1339–1346.
- Feldhoff LM, Rueda CM, Moreno-Fernandez ME, et al. IL-1β induced HIF-1α inhibits the differentiation of human FOXP3+ T cells. Sci Rep. 2017;7(1):1.
- Jang Y, Jeong SH, Park YH, et al. UVB induces HIF-1α-dependent TSLP expression via the JNK and ERK pathways. J Invest Dermatol. 2013;133(11):2601–2608.
- Zhao G, Liu Y, Fang J, et al. Dimethyl fumarate inhibits the expression and function of hypoxia-inducible factor-1α (HIF-1α). Biochem Biophys Res Commun. 2014;6448(3):303–307.
- Wilczynski J, Duechler M, Czyz M. Targeting NF-κB and HIF-1 pathways for the treatment of cancer: part I. Arch Immunol Ther Exp. 2011;59(4):289–299.
- Bobek G, Surmon L, Mirabito KM, et al. Placental regulation of inflammation and hypoxia after TNF-α Infusion in Mice. Am J Reprod Immunol. 2015;74(5):407–418.
- Näthke I, Rocha S. Antagonistic crosstalk between APC and HIF-1α. Cell Cycle. 2011;10(10):1545–1547.
- Robador PA, San Jose G, Rodríguez C, et al. HIF-1-mediated up-regulation of cardiotrophin-1 is involved in the survival response of cardiomyocytes to hypoxia. Cardiovasc Res. 2011;92(2):247–255.
- Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;1;13(3):245–251.
- Choe SS, Shin KC, Ka S, Lee YK, Chun JS, et al. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014;63(10):3359–3371.
- Lee YS, Kim JW, Osborne O, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–1352.
- Liu Z, Xi R, Zhang Z, et al. 4-Hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1α in seawater aspiration-induced lung injury in rats. Int J Mol Sci. 2014;15(7):12861–12884.
- Suzuki A, Osanai T, Tanaka M, et al. Coupling factor 6 attenuates CXCR4 expression through the HIF-1α and c-Src pathways and promotes endothelial apoptosis and inflammation. Hypertens Res. 2014;37(8):708–715.
- Scholz CC, Cavadas MA, Tambuwala MM, Hams E, et al. Regulation of IL-1β–induced NF-κB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci USA. 2013;110(46):18490–18495.
- Filippi I, Carraro F, Naldini A. Interleukin-1β Affects MDAMB231 Breast Cancer Cell Migration under Hypoxia: Role of HIF-1α and NFκB Transcription Factors. Mediators Inflamm. 2015;2015(789414):1–10.
- Bollinger T, Gies S, Naujoks J, et al. HIF‐1α‐and hypoxia‐dependent immune responses in human CD4+ CD25high T cells and T helper 17 cells. J Leukoc Biol. 2014;96(2):305–312.
- D'Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. Febs J. 2016;283(3):413–424.
- Zhu G, Tang Y, Geng N, et al. HIF-α/MIF and NF-κB/IL-6 axes contribute to the recruitment of CD11b + Gr-1+ myeloid cells in hypoxic microenvironment of HNSCC. Neoplasia. 2014;16(2):168–179.
- Vasilopoulos Y, Sourli F, Zafiriou E, et al. High serum levels of HIF-1α in psoriatic patients correlate with an over-expression of IL-6. Cytokine. 2013;62(1):38–39.
- Xing J, Lu J. HIF-1α activation attenuates IL-6 and TNF-α pathways in hippocampus of rats following transient global ischemia. Cell Physiol Biochem. 2016;39(2):511–520.
- Choi SH, Kwon OJ, Park JY, et al. Inhibition of tumour angiogenesis and growth by small hairpin HIF‐1α and IL‐8 in hepatocellular carcinoma. Liver Int. 2014;34(4):632–642.
- Bortolanza S, Bunuales M, Otano I, et al. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol Ther. 2009;17(4):614–622.
- Velásquez SY, Killian D, Schulte J, et al. Short term hypoxia synergizes with interleukin 15 priming in driving glycolytic gene transcription and supports human natural killer cell activities. J Biol Chem. 2016;291(25):12960–12977.
- Remels AH, Gosker HR, Verhees KJ, et al. TNF-α-induced NF-κB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1α. Endocrinology. 2015 May;156(5):1770–1781.
- Lv L, Yuan J, Huang T, et al. Stabilization of Snail by HIF-1α and TNF-α is required for hypoxia-induced invasion in prostate cancer PC3 cells. Mol Biol Rep. 2014;41(7):4573–4582.
- Tang M, Tian Y, Li D, et al. TNF-α mediated increase of HIF-1α inhibits VASP expression, which reduces alveolar-capillary barrier function during acute lung injury (ALI). PLoS One. 2014;9(7):e102967.
- Ghosh S, Gupta P, Sen E. TNFα driven HIF-1α-hexokinase II axis regulates MHC-I cluster stability through actin cytoskeleton. Exp Cell Res. 2016;340(1):116–124.
- Huang CY, Hsieh YL, Ju DT, et al. Attenuation of magnesium sulfate on CoCl2-induced cell death by activating ERK1/2/MAPK and inhibiting HIF-1α via mitochondrial apoptotic signaling suppression in a neuronal cell line. Chin J Physiol. 2015;58(4):244–253.
- Nys K, Van Laethem A, Michiels C, et al. A p38-HIF-1 pathway initiated by UVB irradiation is required to induce Noxa and apoptosis of human keratinocytes. Leuven (Belgium): InOncoforum; 2010.
- Teng M, Jiang XP, Zhang Q, et al. Microtubular stability affects pVHL-mediated regulation of HIF-1alpha via the p38/MAPK pathway in hypoxic cardiomyocytes. PLoS One. 2012;7(4):e35017.
- Lombaert N, Castrucci E, Decordier I, et al. Hard-metal (WC–Co) particles trigger a signaling cascade involving p38 MAPK, HIF-1α, HMOX1, and p53 activation in human PBMC. Arch Toxicol. 2013;87(2):259–268.
- Zhang QL, Cui BR, Li HY, et al. MAPK and PI3K pathways regulate hypoxia-induced atrial natriuretic peptide secretion by controlling HIF-1 alpha expression in beating rabbit atria. Biochem Biophys Res Commun. 2013;438(3):507–512.
- Yan L, Cao X, Zeng S, et al. Associations of proteins relevant to MAPK signaling pathway (p38MAPK-1, HIF-1 and HO-1) with coronary lesion characteristics and prognosis of peri-menopausal women. Lipids Health Dis. 2016;15(1):1–1.
- Guo C, Hao LJ, Yang ZH, Chai R, et al. Deferoxamine-mediated up-regulation of HIF-1α prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp Neurol. 2016;280:13–23.
- Befani CD, Vlachostergios PJ, Hatzidaki E, et al. Bortezomib represses HIF-1α protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J Mol Med (Berl). 2012;90(1):45–54.
- Wang Y, Huang Y, Guan F, et al. Hypoxia-inducible factor-1alpha and MAPK co-regulate activation of hepatic stellate cells upon hypoxia stimulation. PLoS One. 2013;8(9):e74051.
- Cheng YL, Choi Y, Seow WL, et al. Evidence that neuronal Notch-1 promotes JNK/c-Jun activation and cell death following ischemic stress. Brain Res. 2014;1586:193–202.
- Song K, Li M, Xu XJ, et al. HIF-1α and GLUT1 gene expression is associated with chemoresistance of acute myeloid leukemia. Asian Pac J Cancer Prev. 2014;15(4):1823–1829.
- Bosco MC, Varesio L. Dendritic cell reprogramming by the hypoxic environment. Immunobiology. 2012;217(12):1241–1249.
- Filippi I, Morena E, Aldinucci C, et al. Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1α and PI3K/Akt pathway. J Cell Physiol. 2014;229(12):2067–2076.
- Chieosilapatham P, Ikeda S, Ogawa H, et al. Tissue-specific regulation of innate immune responses by human cathelicidin LL-37. Curr Pharm Des. 2018;24(10):1079–1091.
- Poitz DM, Augstein A, Gradehand C, et al. Regulation of the Hif-system by micro-RNA 17 and 20a – role during monocyte-to-macrophage differentiation. Mol Immunol. 2013;56(4):442–451.
- Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42(3):378–386.
- Wan J, Wu W, Che Y, et al. Low dose photodynamic-therapy induce immune escape of tumor cells in a HIF-1α dependent manner through PI3K/Akt pathway. Int Immunopharmacol. 2015;28(1):44–51.
- Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1(8):1397–1406.
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916.
- Zhao T, Ren H, Jia L, et al. Inhibition of HIF-1α by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget. 2015;6(4):2250–2262.
- Zudaire E, Portal‐Núñez S, Cuttitta F. The central role of adrenomedullin in host defense. J Leukoc Biol. 2006;80(2):237–244.
- Patel P, Mishra A, Sheikh AA, et al. Adrenomedullin: a novel peptide hormone. A review. J Pharmacogn Phytochem. 2017;6:2068–2073.
- Koh HS, Chang CY, Jeon SB, et al. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun. 2015;6(1):1–5.
- Tao JH, Barbi J, Pan F. Hypoxia-inducible factors in T lymphocyte differentiation and function. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309(9):C580–C589.
- Doedens AL, Phan AT, Stradner MH, et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat Immunol. 2013;14(11):1173–1182.