Abstract
Adaptive immunity plays central roles in the pathogenesis of rheumatoid arthritis (RA), as it is regarded as an autoimmune disease. Clinical investigations revealed infiltrations of B cells in the synovium, especially those with ectopic lymphoid neogenesis, associate with disease severity. While some B cells in the synovium differentiate into plasma cells producing autoantibodies such as anti-citrullinated protein antibody, others differentiate into effector B cells producing proinflammatory cytokines and expressing RANKL. Synovial B cells might also be important as antigen-presenting cells. Synovial T cells are implicated in the induction of antibody production as well as local inflammation. In the former, a recently identified CD4 T cell subset, peripheral helper T (Tph), which is characterized by the expression of PD-1 and production of CXCL13 and IL-21, is implicated, while the latter might be mediated by Th1-like CD4 T cell subsets that can produce multiple proinflammatory cytokines, including IFN-γ, TNF-α, and GM-CSF, and express cytotoxic molecules, such as perforin, granzymes and granulysin. CD8 T cells in the synovium are able to produce large amount of IFN-γ. However, the involvement of those lymphocytes in the pathogenesis of RA still awaits verification. Their antigen-specificity also needs to be clarified.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects systemic synovial joints. Clinical application of reagents targeting inflammatory cytokines has greatly improved the prognosis of RA, verifying the importance of cells of innate immune system in the pathogenesis. However, the mechanism for the activation of innate immunity in RA is yet to be clarified. Autoimmunity, the adaptive immune response to self-antigens, is implicated here, and there are reasons supporting this hypothesis. The chronic synovitis in multiple joints without the evidence of persistent microbe infection can be simply explained by immune responses against synovial self-antigen(s). The presence of autoantibodies, such as rheumatoid factor (RF) and anti-citrulinated protein antibody (ACPA), indicates the involvement of autoimmunity at least in the disease process. Furthermore, there are various animal models of arthritis induced by autoimmune responses. In fact, synovium of RA patients shows an infiltration of lymphocytes including both T cells and B cells, and significant progress has been made in understanding their functions over the past few years. This review will summarize current knowledge on the cells of adaptive immune system infiltrated in human RA joints.
2. B Cells in RA joint
2.1. Clinical relevance of local B cell infiltration in the pathogenesis of RA
It has been known that the pattern of cellular infiltrate in the synovium varies among RA patients. Recent analysis of biopsy samples of synovial membrane (SM) from a cohort of treatment-naive early RA patients classified the histological feature into three groups (pathotypes): lymph-myeloid, diffuse-myeloid and pauci-immune types. They found that B cell-rich, lymphoid-myeloid group showed the highest disease activity and seropositivity [Citation1]. Response to disease-modifying anti-rheumatic drug (DMARD) therapy correlated with the expression levels of myeloid and lymphoid-associated genes [Citation1], while the pauci-immune pathotype predicts poor response to a TNF-α inhibitor (TNFi) [Citation2]. An analysis on two cohorts of early and established TNFi-inadequate response (IR) RA by semiquantitative B cell scoring revealed that the frequency of B cell-rich pathotype was higher in TNFi-IR established RA than early RA [Citation3]. They also observed significantly higher disease activity in B cell-rich patients in the early RA cohort. Higher levels of cellular infiltrate with B cells were shown in ACPA-positive than negative RA, although another study detected no difference [Citation4,Citation5]. It was also demonstrated that the expression levels of CXCL13, a chemoattractant for B cells, correlated with local disease activity, ACPA level, and erosive disease [Citation6]. Taken together, local infiltration of B cells generally associates with the severity and prognosis of RA.
2.2. Formation of ectopic lymphoid neogenesis in the synovium of RA
In about half cases of RA, T and B cells diffusely infiltrate in the synovium, while in others aggregates consisting of T and B cells are observed, a portion of which forms ectopic lymphoid neogenesis (ELN), an organized aggregation of T and B cells with networks of follicular dendritic cells (FDCs), resembling germinal center (GC) in secondary lymphoid organs [Citation7,Citation8]. ELN formation in RA synovium is associated with the expression of LTβ, CCL21, and CXCL13 [Citation7,Citation9,Citation10]. Gene expression analysis revealed an involvement of IL-7 pathway [Citation11]. The level of IL-23 but not IL-17A, IL-6 nor TNF-α in synovial fluid (SF) was also associated with the presence of ELN [Citation12]. Importantly, Manzo et al. showed that small aggregates formed in perivascular area, while ELN was only detected within large aggregates in the same samples, suggesting sequential appearance of ELN. The expression of CXCL13 and CCL21 might precede ELN formation, as it was also detected in non-organized clusters and small aggregates [Citation9].
It has been a matter of interest whether the presence of ELN discriminates clinical phenotypes of RA, including seropositivity. Thurlings et al. detected ELN in one-third of RA patients, who showed higher level of cellular infiltrate and had higher CRP and ESR, but the presence of ELN and RF or ACPA positivity had no association [Citation13]. Cantaert et al. reported that ELN formation was associated with histological degree of inflammatory infiltrate but was not associated with local production of ACPA and RF [Citation14]. Furthermore, ELN was also formed in the synovium of spondyloarthritis (SpA), including psoriatic arthritis (PsA), and ceased in treatment responders [Citation14,Citation15]. A longitudinal observational study reported that ELN can be detected in any forms of early arthritis and is neither diagnostic for RA nor predict development of persistent and erosive arthritis. The pattern of lymphocyte infiltration can be changed overtime [Citation16]. These all indicate that local disease activity but not seropositivity predicts the presence of ELN.
There are somewhat conflicting data on the relationship between the presence of ELN and treatment response of RA patients. For instance, Canete et al. reported that the presence of ELN is a negative predictor of TNFi response, although reversal of ELN formation occurred in the TNFi responders [Citation17]. On the other hand, Klaasen et al. observed better response to IFX in patients with lymphoid aggregates, although only quarter samples with lymphoid aggregates (one-third of all patients) had ELN formation. Nevertheless, they also found a decrease of lymphocyte aggregate after TNF-α blockade in both RA and PsA [Citation8], again supporting the idea that ELN formation is a consequence of high disease activity.
2.3. Antibody production by joint-infiltrating B cells in RA
Although the presence of ELN does not directly relate with serum autoantibody status, it has been known that B cells in RA synovium produce autoantibodies, namely RF and ACPA. Expression of RF by plasma cells (PCs) in RA synovium was reported as early as in 1959 [Citation18]. It was later demonstrated that CD20–CD38+ PCs in SF from seropositive RA patients actively produce RF [Citation19], although the major cellular source of circulating RF might not be synovial cells but bone marrow cells [Citation20]. This might also be the case of ACPA. Although ACPA is detected in SF of RA patients [Citation21], it is detected in the serum before RA onset, when no histological evidence of synovitis is observed [Citation22]. Nevertheless, it is worth noting that ACPA detection in SF is not simply due to passive diffusion from the serum. It was shown by using ELISPOT assay that up to quarter of SF B cells spontaneously produced IgM ACPA in vitro [Citation23]. Intriguingly, they found B cells in peripheral blood (PB) from healthy donors as well as RA patients were able to produce IgM ACPA after stimulation. IgG ACPA production by B cells in PB and SF was reported later by Kerkham et al. [Citation24]. They found that SF cells spontaneously produced higher amount of IgG ACPA and the frequency of IgG ACPA producing cells was higher in SF than PB, which is in line with a study showing relative enrichment of IgG ACPA in SF compared to the serum [Citation25]. They argued that SF mononuclear cells provide an environment for long-term survival of ACPA-secreting cells, irrespective of where they had been generated.
It remains unclear whether the ACPA-producing cells home to the joint or are generated in the joint. In this regard, it is of interest to know whether there is any difference in the antigen-specificity between those in the joint and PB. Sohrabian et al. found somewhat different reactivity of ACPA in the form of immune complex between serum and SF [Citation26]. However, this could either result from local ACPA production by different B cell repertoire or accumulation of different ACPAs from serum according to the expression level of corresponding antigens. Doorenspleet et al. compared B cell receptor (BCR) of B cells from PB and synovium by next-generation sequencing. They found that B cells from synovium showed multiple dominant clones with autoreactive features including the usage of IGHV4-34 and longer CDR3 lengths. There was limited overlap between PB and synovial BCR clones, further suggesting their local expansion [Citation27]. On the other hand, Elliot showed certain extent of clonal relationship in BCR between PB and synovial compartments, suggesting B cell trafficking between PB and synovium, although synovial samples showed greater extent of clonal expansion [Citation28]. Some studies established ACPA-producing B cell clones from the joint, although it is difficult to draw conclusion on the difference from those in PB due to small number of samples. Steen et al. isolated four IgG ACPA-secreting B cell clones from the SF and generate recombinant mAbs. They found those antibodies highly mutated and recognizing diverse array of citrullinated peptides [Citation29]. One of the four ACPA clones showed signs of local expansion. Germar et al. generated immortalized B cell clones by retroviral transduction of Bcl-6 and Bcl-x but were able to obtain only two ACPA-producing clones. They differed from non-ACPA clones in the expression of CD40 and C5a receptor 1 [Citation30]. Lastly, Elliot et al. reported TNF-α production from macrophages stimulated with immune complex that contain recombinant ACPA cloned from RASF [Citation28].
Some studies investigated antibody production from ELN in the synovium of RA. By analyzing locally expressed BCR by microdissection of tissue sections, a clonal relationship between B cells in the follicle and the surrounding plasma cells was demonstrated [Citation31]. In line with this finding, a histological study showed activation-induced cytidine deaminase (AID) expression in ELNs, which is surrounded by ACPA-positive plasma cells. Furthermore, human ACPA was detected in the serum of SCID mice transplanted with the RA synovium expressing AID [Citation32]. B cells within ELN frequently reacted with citrullinated histones in neutrophil extracellular trap (NET), which was shown to form in RA joint [Citation33,Citation34]. Somatic hypermutation and resulting Fab-glycosylation in BCR is important for the reactivity to NET, further suggesting the involvement of GC reaction [Citation35]. Epstein–Barr virus has long been implicated in the pathogenesis of RA and, interestingly, a study showed Epstein–Barr virus infection of ACPA-producing plasma cells surrounding synovial GC [Citation36]. Taken together, ELNs are likely involved in local differentiation of ACPA-producing B cells, even though they are not the primary sites of ACPA production.
2.4. Effector functions of joint infiltrating B cells in RA
So far, the best proof for the importance of B cells in the pathogenesis of RA is the therapeutic effect of B cell-depletion by the anti-CD20 mAb, rituximab. Interestingly, although rituximab induces better response in ACPA-positive RA [Citation37], its efficiency does not necessarily correlate with the decrease of antibody levels [Citation38]. Furthermore, the reduction of ACPA production follows the appearance of clinical effect of rituximab as well as depletion of synovial B cells [Citation39]. In fact, there are lines of evidence that B cells are involved in the pathogenesis of RA other than by autoantibody production.
First, synovial B cells potentially produce proinflammatory cytokines. Yao showed mRNA expression of various cytokines, including IL-1, IL-6, IL-12, and TNF-α, by SF B cells [Citation40]. A single-cell RNA sequence analysis also demonstrated expression of IL-6 and TNF-α in synovial B cells [Citation41]. Regarding the phenotype of these cytokine-producing cells, Cowan et al. showed that IgG + CD27- B cells secreted TNF-α and were enriched in the synovium [Citation42], while Floudas et al. reported PD-1-positive B cells, which express higher levels of TNF-α and IL-6 than PD-1-negative B cells, accumulated in the joint [Citation43]. The relevance of B cell-derived TNF-α and IL-6 in the activation of synovial fibroblast was suggested by an in vitro culture system [Citation44]. Proinflammatory functions of citrulline-specific B cells have been shown. The immortalized B cell clones established by Germar et al. secreted both pro- and anti-inflammatory cytokines, including TNF-α and IL-10, although there was no difference between citrulline-reactive and non-reactive clones [Citation30]. Krystyanto et al. identified ACPA-specific SF B cells by flow cytometry and found them expressing IL-8 that induces neutrophil infiltration in the joint [Citation45], which might explain the link between the clinical efficacy of rituximab and ACPA positivity [Citation37].
Receptor activator of NF-κB ligand (RANKL) plays a critical role in the development of osteoclasts involved in the joint destruction in RA. Expression of RANKL on fibroblast, osteoblast, and T cells is well known, but a portion of B cells also express RANKL. Yeo et al. reported that the synovial B cells express RANKL in addition to IL-6 and TNF-α [Citation40]. Such RANKL + B cells expressed FcRL4 [Citation46] and, notably, some FcRL4+ B cell clones showed reactivity toward citrullinated proteins [Citation47]. RANKL + B cells localize with RANK + osteoclast precursors and support their differentiation in vitro [Citation48]. We have demonstrated IFN-γ plays an important role in the generation of CXCR3 + RANKL + B cells, which are increased in RA joint [Citation49]. IFN-γ induced expression of T-bet and CXCL9/10, the ligands for CXCR3, in B cells, which might further recruit IFN-γ-producing Th1 cells as well as CXCR3 + RANKL + B cells in the joint [Citation50].
Antigen presentation to T cells is another role of joint-infiltrating B cells, which is presumably involved in the pathogenesis of RA. Except for ELN lesion, B cells and activated T cells usually aggregate together in the synovium. Takemura et al. demonstrated by using SCID mice engrafted with the synovium from RA patients that activation of tissue infiltrating CD4 T cells is B cell-dependent [Citation51]. This suggests a role for B cells as critical APC for CD4 T cells, which might also be the mechanism of action of rituximab in RA.
3. T Cells in RA joint
3.1. Importance of CD4 T cells in the pathogenesis of RA
T cells, especially CD4 T cells, are the critical conductor of adaptive immune responses, and there are lines of evidence suggesting the involvement of CD4 T cells in the pathogenesis of RA. First, there is a massive infiltration of CD4 T cells in synovium of RA, which is not only found in joint synovium but also in tenosynovium [Citation52]. Most CD4 T cells in the synovium show activation markers [Citation53]. Second, RA has a strong genetic association with certain MHC class II alleles, the HLA-DR ‘shared epitope (SE)’, which is prominent in seropositive RA [Citation54]. Again in most animal models, CD4 T cells play essential roles in the development of arthritis [Citation55]. Finally, reagents that inhibit T cell activation, such as abatacept (CTLA4-Ig), exert clinical effect on RA. Although CD8 T cells might also be affected, the therapeutic effect of abatacept associates with ACPA-positivity and the presence of SE [Citation56,Citation57], suggesting that inhibition of CD4 T cells is its main mode of action. CD4 T cells can be involved in the pathogenesis of RA by inducing inflammation and by helping B cell antibody production, details of which will be introduced in the following sections.
3.2. Pro-inflammatory CD4 helper T cell subsets in RA joint
Delayed type hypersensitivity (DTH) reaction is the typical T cell-mediated inflammatory response, which involves activation of macrophages and is a mechanism for host defense against intracellular pathogens. IFN-γ-producing T helper 1 (Th1) cells mediate DTH reaction. Because infiltration of activated macrophages and CD4 T cells is the typical histological feature of RA synovitis, RA was once believed as a Th1-mediated disease. However, in many cases, only low level of IFN-γ was detected in the SF or synovium of RA [Citation58,Citation59], casting a doubt on the importance of T cells in the pathogenesis [Citation60].
On the other hand, a novel T cell-derived cytokine was discovered in 1993, IL17 (IL-17A) [Citation61], which was later detected in the joint of RA [Citation62,Citation63]. IL-17 induces mobilization of neutrophils, activates fibroblasts and induces RANKL expression, all which fit well with the pathological features of RA synovium [Citation63,Citation64]. In fact, animal models of RA demonstrated an importance of IL-17 in the development of arthritis [Citation65,Citation66]. Finally, a distinct CD4 T cell subset that produces IL-17, but neither IFN-γ nor IL4, was identified in mice in 2005 and was named Th17 [Citation67], leading to an idea that RA is a Th17-mediated disease. The presence of Th17 cells in human was reported a couple of years later first in patients with Crohn's disease [Citation68]. At that time, we were examining the frequency of Th17 cells in peripheral blood and joint of RA patients, but we unexpectedly found that Th17 cells neither increased in PB of RA nor correlated with disease activity [Citation69]. Furthermore, the frequency of Th17 cells was rather lower in the joint than peripheral blood, while the frequency of Th1 cells was clearly higher in the joint than PB. Similar observation was made by others [Citation70]. In line with those findings, it was revealed later that targeting IL-17 is not beneficial for RA, although it was highly effective in the treatment of psoriasis [Citation71]. This formally indicates that Th17 cells are not essential in the pathogenesis of RA, at least not through IL-17 production in the established stage.
GM-CSF is a proinflammatory cytokines that is produced by innate immune cells, and clinical trials have proved GM-CSF being a target of RA [Citation72]. Recently, an importance of CD4 T cell-derived GM-CSF in the pathogenesis of RA was reported [Citation73]. Among helper CD4 T cell subsets, Th17 was shown to be the main producer of GM-CSF in mice, but it had been unclear for human. In addition, a distinct subset of helper T cells producing GM-CSF was reported in human [Citation74]. We, therefore, examined which CD4 T cell populations produce GM-CSF in RA and found that most GM-CSF-producing cells in the joint also produced IFN-γ, while those in PB produced GM-CSF alone [Citation75]. Aside from GM-SCF production, a high-dimensional single-cell analysis identified a cluster of IFN-γ-producing CD4 T cells increased in RA PB and enriched in the joint, accounting for up to 10% of CD4 T cells. These are phenotypically CD27- HLA-DR + effector memory CD4 T cells and express cytotoxic molecules, such as PRF1, GZMB, GZMA, and GNLY (genes for Preforin-1, Granzyme B, Granzyme A, and Granulysin, respectively) [Citation76]. Thus, Th1-like cells equipped with multiple pro-inflammatory and cytotoxic functions infiltrate in RA joint, although there has been no formal proof for their involvement in the pathogenesis.
3.3. Tph cell, a novel helper T cell subset first identified in RA joint
PD-1 is an inhibitory receptor expressed on T cells after activation as a negative feedback mechanism. Blocking PD-1 on T cells continuously expressing PD-1, which are seen during chronic infection or in tumor microenvironment, reverses their exhaustive state [Citation77]. PD-1 might also be expressed on autoreactive T cells chronically stimulated with self-antigens. In fact, an increased expression of PD-1 on CD4 T cells in RA joint was reported in 2003 [Citation78], but it attracted much attention after identification of a unique cluster of CD4 T cells expressing PD-1 in the joint of seropositive RA by a recent comprehensive single-cell analysis [Citation79]. Similar to follicular helper T (Tfh) cells, which help GC B cells in secondary lymphoid organs and express PD-1, the PD-1-positive CD4 T cells in RA joint secrete IL-21 and induce antibody production from B cells in vitro. However, they express neither CXCR5 nor the transcription factor BCL6, unlike Tfh cells. Hence, the PD-1-positive CXCR5-nagetive CD4 T cells are identified as a novel subset of CD4 T cells and called peripheral helper T (Tph) cells. The abundance of Tph cells in RA joint was confirmed by other comprehensive analysis on synovial cells [Citation41,Citation80]. While Tfh cells express BCL6 but not BLIMP1, Tph cells showed opposite expression pattern of these factors, although both Tfh and Tph cells express MAF. There is also difference in the ability of helping naive but not memory B cells between Tfh and Tph cells [Citation81]. In addition to the helper functions on B cells, Tph cells seem to have proinflammatory functions. IFN-γ mRNA was detected in sorted Tph cells [Citation79]. Lebre et al. independently reported the presence of CD4 T cells producing IL-21 and TNF-α in RASF [Citation82]. We have also found that Tph cells can produce TNF-α, IFN-γ, and GM-CSF [Citation83].
CXCL13, a B cell-attractant and the ligand for CXCR5, is produced by FDC in the GC, but is also produced by Tph cells. Actually, CXCL13 production by CD4 T cells in RA joint was reported even before the discovery of Tph cells [Citation84,Citation85]. Manzo et al. observed the lack of CXCR5 and BCL-6 expression in CXCL13-positive cells [Citation84]. Kobayashi et al. identified a subset of CD4 T cells producing CXLC13 and distinct from Th1, Th2, Th17 or Tfh [Citation85], which most likely correspond to Tph cells. Notably, Tph cells spontaneously produce CXCL13 in vitro, suggesting in situ activation in the joint. However, ex vivo re-stimulation is required for producing other cytokines, including IL-21, by the same Tph cells. The molecular mechanism underlying this discrepancy is unclear. Tph cells are found only in seropositive RA [Citation79], while there is no association between seropositivity and ELN formation. Therefore, Tph-derived CXCL13 is not essential for the formation of ELN, which is presumably mediated by FDC-derived CXCL13, but may play primary role in diffuse infiltration of B cells in seropositive RA [Citation4]. In line with this, Tph cells locate diffusely outside the lymphoid follicles [Citation86].
Yoshitomi et al. have analyzed the molecular mechanism of Tph cell differentiation. They found that TCR stimulation in the presence of TGF-β induces in vitro differentiation of the CXCL13-producing CD4 T cells, which are enhanced by blocking IL-2 and is mediated by a transcription factor, Sox4 [Citation86,Citation87]. Notably, Sox4 does not induce CXCL13 production from mouse CD4 T cells, again pointing a difficulty of studying human immune pathogenesis in mice. On the other hand, it was demonstrated that murine CD4 T cells undergone lymphopenia-induced proliferation resemble human Tph cells in that they exhibit PD-1+ CXCR5- phenotype and provide help for antibody production of B cells by producing IL-21 [Citation88]. It is of interest to investigate its relevance to the mechanism of human Tph cell differentiation.
3.4. Functions of CD8 T cells in RA joint
There has been a little information on the role of CD8 T cells in the pathogenesis of RA. Little genetic association is detected between MHC class I and RA, in contrast to the case of MHC class II. However, it has been known that CD8 T cells in the joint express higher levels of activation markers than those in PB, similar to the case of CD4 T cells [Citation53,Citation89]. Moreover, recent comprehensive analysis on the cells in RA joint has attracted attention to CD8 T cells. A single-cell mRNA analysis revealed three CD8 T cell subsets present in RA joint defined by the expression pattern of cytotoxic molecules including GZMK, GZMB, and GNLY, while mass cytometry divided CD8 T cells into four subsets by the expression of HLA-DR and PD-1, among which PD-1-HLA-DR + cells include GZMK + GZMB + effector cells and GNLY + GZMB + cytotoxic T lymphocytes (CTL) [Citation41]. Notably, CD8 T cells of any subsets express higher levels of IFN-γ than any CD4 T cells in RA joint. Regarding PD-1+ CD8 T cells in RA joint, we recently found them producing IL-21 and not expressing CXCR5, similar to Tph cells [Citation90]. An association between ELN formation in the synovium and the presence of CD8 T cells expressing CD40L was also shown [Citation91]. The requirement of CD8 T cells for the formation of ELN in the synovium was demonstrated by depleting CD8 T cells of RA synovial grafts in SCID mice [Citation92]. Thus, CD8 T cells might be involved in the pathogenesis of RA more deeply than previously anticipated.
3.5. Autoreactivity of T cells in RA joint
Despite the longstanding belief that RA is a T cell-mediated autoimmune disease, demonstrating autoreactivity of joint-infiltrating T cells is still a challenging issue. An expansion of TCR clonotype in RA joint has long been observed and is thought to reflect their autoreactivity. Earlier studies examined TCRVβ usage but showed somewhat conflicting results on the clonality of synovial T cells [Citation93,Citation94]. Among those studies, similarly biased TCR Vβ usage by synovial T cells in different joints was reported [Citation95]. Yamamoto has developed an improved method for evaluating TCR clonality, single-strand conformation polymorphism (SSCP) analysis, and demonstrated clonal expansion of T cells, which is shared among different areas of the same joint [Citation96]. Analysis of complementary determining region 3 (CDR3) sequence has later been used to demonstrate the presence of dominant clones in the joint [Citation97]. Interestingly, a study showed higher extent of clonal expansion in ACPA-positive synovium [Citation5]. This might be related with the presence of Tph cells in seropositive RA, as we observed a biased TCR usage by Tph cells (Sakuragi et al., in press). Recent studies utilized non-biased, next-generation sequencing of TCR to investigate T cell expansion in RA joint. Klarenbeek et al. showed highly expanded clones, which are shared between different joints of the same patients [Citation98]. They later showed that the overlap between synovial tissue and SF was limited, indicating these are two separate compartments [Citation99]. However, the phenotype, functions, as well as antigen specificity of the expanded T cell clones identified by these studies are unclear.
Another approach for addressing autoreactivity of joint-infiltrating T cells is examining their reactivity to candidate self-antigens. Most recent studies focused on citrullinated protein antigens, such as tenacin-C [Citation100], alpha-enolase [Citation101], and type II collagen [Citation102]. There have been many studies analyzing PB T cells [Citation103–105]. However, it should be kept in mind that the T cells inducing local inflammation and those helping APCA production, presumably Tfh cells in lymph nodes, do not necessary have the same antigen specificity. Interestingly, in many cases, citrullinated peptide-specific CD4 T cells showed Th1 phenotype. Nevertheless, pathogenicity of these self-antigen specific T cells is unclear. Except for the case of Tph cells that spontaneously produce CXCR13, as described above, production of effector cytokines by any other synovial T cell subsets requires in vitro restimulation. On the other hand, the antigen specificity of Tph cells is yet to be clarified. Although Tph cells have been detected in other inflammatory and tumor conditions [Citation106], their antigen specificity has only been demonstrated in Celliac disease; that is gluten [Citation107]. Identifying antigen(s) recognized by Tph cells in RA joint is an important issue.
There are other concerns on the autoreactivity of T cells in RA joint. Brennan et al. have argued that synovial T cells are more like T cells stimulated with a cocktail of cytokines, including IL-2, IL-6 and TNF-α, than TCR-activated T cells [Citation108]. These cytokine-activated T cells (Tck) and synovial T cells induce contact-dependent TNF-α production from monocytes via the same signaling pathways. Tck cells are induced from memory CD4 T cells and express increased levels of CD25 CD69, and HLA-DR [Citation109]. Tck cells showed chemotaxis toward synovial fibroblast from RA patients [Citation110]. Thus, Tck cells can explain the massive infiltration of activated T cells with diverse TCR repertoire in RA joint. However, now that we know the variety of helper T cell subsets in RA joint, it needs to be addressed which one correspond to Tck. More detailed single-cell analysis on the similarity of Tck and synovial T cells will provide much information. For Tph cells, as far as we have tested, the cocktail of cytokines could not induce them (Sakuragi et al., in press), although it remains possible that a different combination of cytokines with longer culture period induces Tph development.
Antigen non-specific accumulation of memory/activated T cells is another confounder in analyzing specificity of T cells in the joint. There are actually several reports showing an expansion of pathogen-specific T cells, including viral, bacterial and protozoan antigen-specific T cells, in RA joint [Citation111–113]. Interestingly, Fazou et al. showed an oligoclonality of viral antigen specific T cells, which is observed in separate joints of the same patient [Citation114]. Accumulation of bystander T cells to an inflammatory site is inevitable, because cell migration per se is regulated by antigen-nonspecific factors, such as chemokines and adhesion molecules. Although it is also possible that those pathogen-specific T cells actually proliferate locally by recognizing the corresponding antigens, this again indicates that oligoclonality of T cells does not necessarily mean autoreactivity. It is important to develop means to precisely identify synovial T cells that are receiving antigenic stimulation in situ.
4. Conclusions
This review summarized current knowledges on the cells of adaptive immunity in the joint of RA (). The progress in human immunological research has shed light on novel functions of B and T cells infiltrated in the joint and identified previously unacknowledged cell subsets, which have not been found in animal models. These have greatly advanced our understanding on the molecular pathogenesis of RA, especially in the aspect of inflammatory cytokine networks. Nevertheless, the pathogenicity of these lymphocytes still remains elusive. An infiltration of lymphocyte is not necessarily be a cause but a consequence of inflammation. In the case of RA, the infiltrate is actually decreased by effective treatment such as TNFi. The presence of pauci-immune type early RA also needs attention. Identifying the true pathogenic lymphocytes and defining their antigen-specificity is the critical issue to be addressed in the future, provided that RA is an autoimmune disease.
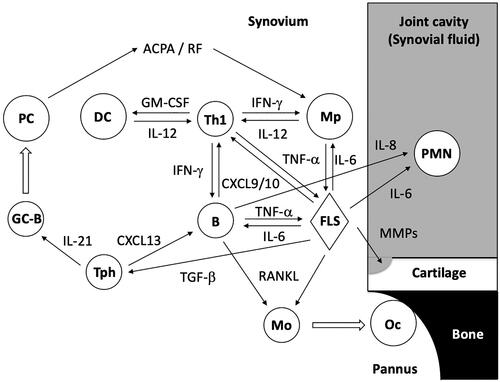
Figure 1. Involvement of cells in adaptive immune system in the pathogenesis of RA. Putative interactions between the cells in RA joint are illustrated. Note that T and B cell subsets are simplified. PC: plasma cell; DC: dendritic cell; Th1: T helper 1 cell; Mp: macrophage, PMN: polymorphonuclear leukocyte; GC-B: germinal center B cells; Tph: peripheral helper T cell; B: B cell; FLS: fibroblast-like synoviocyte; Mo: monocyte; OC: osteoclast.
Disclosure statement
The author reports that they have no conflict of interest.
References
- Humby F, Lewis M, Ramamoorthi N, et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann Rheum Dis. 2019;78(6):761–772.
- Nerviani A, Di CM, Mahto A, et al. A pauci-immune synovial pathotype predicts inadequate response to TNFα-blockade in rheumatoid arthritis patients. Front Immunol. 2020;11:845.
- Rivellese F, Humby F, Bugatti S, et al.; The PEAC‐R4RA Investigators. B cell synovitis and clinical phenotypes in rheumatoid arthritis: relationship to disease stages and drug exposure. Arthritis Rheumatol. 2020;72(5):714–725.
- Orr C, Najm A, Biniecka M, et al. Synovial immunophenotype and anti-citrullinated peptide antibodies in rheumatoid arthritis patients: relationship to treatment response and radiologic prognosis. Arthritis Rheumatol. 2017;69(11):2114–2123.
- Cantaert T, Brouard S, Thurlings RM, et al. Alterations of the synovial T cell repertoire in anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1944–1956.
- Bugatti S, Manzo A, Vitolo B, et al. High expression levels of the B cell chemoattractant CXCL13 in rheumatoid synovium are a marker of severe disease. Rheumatology. 2014;53(10):1886–1895.
- Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(2):1072–1080.
- Klaasen R, Thurlings RM, Wijbrandts CA, et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum. 2009;60(11):3217–3224.
- Manzo A, Paoletti S, Carulli M, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35(5):1347–1359.
- Shi K, Hayashida K, Kaneko M, et al. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166(1):650–655.
- Timmer TCG, Baltus B, Vondenhoff M, et al. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007;56(8):2492–2502.
- Cañete JD, Celis R, Yeremenko N, et al. Ectopic lymphoid neogenesis is strongly associated with activation of the IL-23 pathway in rheumatoid synovitis. Arthritis Res Ther. 2015;17(1):173.
- Thurlings RM, Wijbrandts CA, Mebius RE, et al. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 2008;58(6):1582–1589.
- Cantaert T, Kolln J, Timmer T, et al. B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J Immunol. 2008;181(1):785–794.
- Cañete JD, Santiago B, Cantaert T, et al. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann Rheum Dis. 2007;66(6):720–726.
- van de Sande MGH, Thurlings RM, Boumans MJH, et al. Presence of lymphocyte aggregates in the synovium of patients with early arthritis in relationship to diagnosis and outcome: is it a constant feature over time? Ann Rheum Dis. 2011;70(4):700–703.
- Cañete JD, Celis R, Moll C, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(5):751–756.
- Mellors RC, Heimer R, Corcos J, et al. Cellular origin of rheumatoid factor. J Exp Med. 1959;110(6):875–886.
- Reparon-Schuijt CC, van Esch WJ, van Kooten C, et al. Functional analysis of rheumatoid factor-producing B cells from the synovial fluid of rheumatoid arthritis patients. Arthritis Rheum. 1998;41(12):2211–2220.
- Otten HG, Daha MR, Dolhain RJ, et al. Rheumatoid factor production by mononuclear cells derived from different sites of patients with rheumatoid arthritis. Clin Exp Immunol. 1993;94(2):236–240.
- Caspi D, Anouk M, Golan I, et al. Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis. Arthritis Rheum. 2006;55(1):53–56.
- van de Sande MGH, de Hair MJH, van der Leij C, et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann Rheum Dis. 2011;70(5):772–777.
- Reparon-Schuijt CC, van Esch WJE, van Kooten C, et al. Secretion of anti–citrulline-containing peptide antibody by B lymphocytes in rheumatoid arthritis. Arthritis Rheum. 2001;44(1):41–47.
- Kerkman PF, Kempers AC, van der Voort EIH, et al. Synovial fluid mononuclear cells provide an environment for long-term survival of antibody-secreting cells and promote the spontaneous production of anti-citrullinated protein antibodies. Ann Rheum Dis. 2016;75(12):2201–2207.
- Snir O, Widhe M, Hermansson M, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010;62(1):44–52.
- Sohrabian A, Mathsson-Alm L, Hansson M, et al. Number of individual ACPA reactivities in synovial fluid immune complexes, but not serum anti-CCP2 levels, associate with inflammation and joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2018;77(9):1345–1353.
- Doorenspleet ME, Klarenbeek PL, de Hair MJH, et al. Rheumatoid arthritis synovial tissue harbours dominant B-cell and plasma-cell clones associated with autoreactivity. Ann Rheum Dis. 2014;73(4):756–762.
- Elliott SE, Kongpachith S, Lingampalli N, et al. B cells in rheumatoid arthritis synovial tissues encode focused antibody repertoires that include antibodies that stimulate macrophage TNF-α production. Clin Immunol. 2020;212:108360.
- Steen J, Forsström B, Sahlström P, et al. Recognition of amino acid motifs, rather than specific proteins, by human plasma cell-derived monoclonal antibodies to posttranslationally modified proteins in rheumatoid arthritis. Arthritis Rheumatol. 2019;71(2):196–209.
- Germar K, Fehres CM, Scherer HU, et al. Generation and characterization of anti-citrullinated protein antibody-producing B cell clones from rheumatoid arthritis patients. Arthritis Rheumatol. 2019;71(3):340–350.
- Kim HJ, Krenn V, Steinhauser G, et al. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol. 1999;162(5):3053–3062.
- Humby F, Bombardieri M, Manzo A, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6(1):e1.
- Corsiero E, Bombardieri M, Carlotti E, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis. 2016;75(10):1866–1875.
- Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178): 178ra40.
- Corsiero E, Carlotti E, Jagemann L, et al. H and L chain affinity maturation and/or Fab N-glycosylation influence immunoreactivity toward neutrophil extracellular trap antigens in rheumatoid arthritis synovial B cell clones. J Immunol. 2020;204(9):2374–2379.
- Croia C, Serafini B, Bombardieri M, et al. Epstein-Barr virus persistence and infection of autoreactive plasma cells in synovial lymphoid structures in rheumatoid arthritis. Ann Rheum Dis. 2013;72(9):1559–1568.
- Chatzidionysiou K, Lie E, Nasonov E, et al. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis. 2011;70(9):1575–1580.
- Toubi E, Kessel A, Slobodin G, et al. Changes in macrophage function after rituximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66(6):818–820.
- Thurlings RM, Vos K, Wijbrandts CA, et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67(7):917–925.
- Yeo L, Toellner K-M, Salmon M, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):2022–2028.
- Zhang F, Wei K, Slowikowski K, Accelerating Medicines Partnership Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019;20(7):928–942.
- Cowan GJM, Miles K, Capitani L, et al. Human autoimmunity, a substantial component of the B cell repertoire consists of polyclonal, barely mutated IgG + ve B cells. Front Immunol. 2020;11:395.
- Floudas A, Neto N, Marzaioli V, et al. Pathogenic, glycolytic PD-1+ B cells accumulate in the hypoxic RA joint. JCI Insight. 2020;5(21):e139032.
- Störch H, Zimmermann B, Resch B, et al. Activated human B cells induce inflammatory fibroblasts with cartilage-destructive properties and become functionally suppressed in return. Ann Rheum Dis. 2016;75(5):924–932.
- Kristyanto H, Blomberg NJ, Slot LM, et al. Persistently activated, proliferative memory autoreactive B cells promote inflammation in rheumatoid arthritis. Sci Transl Med. 2020;12(570):eaaz5327.
- Yeo L, Lom H, Juarez M, et al. Expression of FcRL4 defines a pro-inflammatory, RANKL-producing B cell subset in rheumatoid arthritis. Ann Rheum Dis. 2015;74(5):928–935.
- Amara K, Clay E, Yeo L, et al. B cells expressing the IgA receptor FcRL4 participate in the autoimmune response in patients with rheumatoid arthritis. J Autoimmun. 2017;81:34–43.
- Meednu N, Zhang H, Owen T, et al. Production of RANKL by memory B cells: a link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol. 2016;68(4):805–816.
- Ota Y, Niiro H, Ota S-I, et al. Generation mechanism of RANKL(+) effector memory B cells: relevance to the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2016;18:67.
- Nakayama T, Yoshimura M, Higashioka K, et al. Type 1 helper T cells generate CXCL9/10-producing T-bet + effector B cells potentially involved in the pathogenesis of rheumatoid arthritis. Cell Immunol. 2021;360:104263.
- Takemura S, Klimiuk PA, Braun A, et al. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167(8):4710–4718.
- Kaibara N, Yamada H, Shuto T, et al. Comparative histopathological analysis between tenosynovitis and joint synovitis in rheumatoid arthritis. Histopathology. 2008;52(7):856–864.
- Yamada H, Nakashima Y, Okazaki K, et al. Preferential accumulation of activated Th1 cells not only in rheumatoid arthritis but also in osteoarthritis joints. J Rheumatol. 2011;38(8):1569–1575.
- Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46.
- Kondo Y, Yokosawa M, Kaneko S, et al. Review: transcriptional regulation of CD4+ T cell differentiation in experimentally induced arthritis and rheumatoid arthritis. Arthritis Rheumatol. 2018;70(5):653–661.
- Sokolove J, Schiff M, Fleischmann R, et al. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis. 2016;75(4):709–714.
- Oryoji K, Yoshida K, Kashiwado Y, et al. Shared epitope positivity is related to efficacy of abatacept in rheumatoid arthritis. Ann Rheum Dis. 2018;77(8):1234–1236.
- Firestein GS, Alvaro-Gracia JM, Maki R, et al. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144(9):3347–3353.
- Chen E, Keystone EC, Fish EN. Restricted cytokine expression in rheumatoid arthritis. Arthritis Rheum. 1993;36(7):901–910.
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990;33(6):768–773.
- Rouvier E, Luciani MF, Mattei MG, et al. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–5456.
- Chabaud M, Durand JM, Buchs N, et al. A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42(5):963–970.
- Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352.
- Yamada H. Current perspectives on the role of IL-17 in autoimmune disease. J Inflamm Res. 2010;3:33–44.
- Nakae S, Nambu A, Sudo K, et al. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177.
- Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659.
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132.
- Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–1861.
- Yamada H, Nakashima Y, Okazaki K, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67(9):1299–1304.
- Ito Y, Usui T, Kobayashi S, et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2294–2303.
- Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann Rheum Dis. 2018;77(2):175–187.
- Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor α monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70(5):679–689.
- Reynolds G, Gibbon JR, Pratt AG, et al. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann Rheum Dis. 2016;75(5):899–907.
- Noster R, Riedel R, Mashreghi M-F, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med. 2014;6(241):241ra80.
- Yamada H, Haraguchi A, Sakuraba K, et al. Th1 is the predominant helper T cell subset that produces GM-CSF in the joint of rheumatoid arthritis. RMD Open. 2017;3(1):e000487.
- Fonseka CY, Rao DA, Teslovich NC, et al. Mixed-effects association of single cells identifies an expanded effector CD4+ T cell subset in rheumatoid arthritis. Sci Transl Med. 2018;10(463):eaaq0305.
- Hashimoto M, Kamphorst AO, Im SJ, et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. 2018;69:301–318.
- Hatachi S, Iwai Y, Kawano S, et al. CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol. 2003;30(7):1410–1419.
- Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–114.
- Takeshita M, Suzuki K, Kondo Y, et al. Multi-dimensional analysis identified rheumatoid arthritis-driving pathway in human T cell. Ann Rheum Dis. 2019;78(10):1346–1356.
- Fortea-Gordo P, Nuño L, Villalba A, et al. Two populations of circulating PD-1hiCD4 T cells with distinct B cell helping capacity are elevated in early rheumatoid arthritis. Rheumatology (Oxford). 2019;58(9):1662–1673.
- Lebre MC, Vieira PL, Tang MW, et al. Synovial IL-21/TNF-producing CD4+ T cells induce joint destruction in rheumatoid arthritis by inducing matrix metalloproteinase production by fibroblast-like synoviocytes. J Leukoc Biol. 2017;101(3):775–783.
- Sakuragi T, Yamada H, Haraguchi A, et al. Autoreactivity of peripheral helper T cells in the joints of rheumatoid arthritis. JI. 2021;206(9):2045–2051.
- Manzo A, Vitolo B, Humby F, et al. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum. 2008;58(11):3377–3387.
- Kobayashi S, Murata K, Shibuya H, et al. A distinct human CD4+ T cell subset that secretes CXCL13 in rheumatoid synovium: CXCL13-producing CD4+ T cells in RA synovium. Arthritis Rheum. 2013;65(12):3063–3072.
- Yoshitomi H, Kobayashi S, Miyagawa-Hayashino A, et al. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nat Commun. 2018;9(1):3762.
- Kobayashi S, Watanabe T, Suzuki R, et al. TGF-β induces the differentiation of human CXCL13-producing CD4(+) T cells. Eur J Immunol. 2016;46(2):360–371.
- Eri T, Kawahata T, Kanzaki T, et al. Intestinal microbiota link lymphopenia to murine autoimmunity via PD-1 + CXCR5-/dim B-helper T cell induction. Sci Rep. 2017;7:46037.
- Carvalheiro H, Duarte C, Silva-Cardoso S, et al. CD8+ T cell profiles in patients with rheumatoid arthritis and their relationship to disease activity. Arthritis Rheumatol. 2015;67(2):363–371.
- Higashioka K, Yoshimura M, Sakuragi T, et al. Human PD-1hiCD8+ T cells are a cellular source of IL-21 in rheumatoid arthritis. Front Immunol. 2021;12:654623.
- Wagner UG, Kurtin PJ, Wahner A, et al. The role of CD8+ CD40L + T cells in the formation of germinal centers in rheumatoid synovitis. J Immunol. 1998;161(11):6390–6397.
- Kang YM, Zhang X, Wagner UG, et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195(10):1325–1336.
- Keystone EC, Minden M, Klock R, et al. Structure of T cell antigen receptor beta chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum. 1988;31(12):1555–1557.
- Jenkins RN, Nikaein A, Zimmermann A, et al. T cell receptor V beta gene bias in rheumatoid arthritis. J Clin Invest. 1993;92(6):2688–2701.
- Alam A, Lulé J, Coppin H, et al. T-cell receptor variable region of the beta-chain gene use in peripheral blood and multiple synovial membranes during rheumatoid arthritis. Hum Immunol. 1995;42(4):331–339.
- Ikeda Y, Masuko K, Nakai Y, et al. High frequencies of identical T cell clonotypes in synovial tissues of rheumatoid arthritis patients suggest the occurrence of common antigen-driven immune responses. Arthritis Rheum. 1996;39(3):446–453.
- Alam A, Lambert N, Lulé J, et al. Persistence of dominant T cell clones in synovial tissues during rheumatoid arthritis. J Immunol. 1996;156(9):3480–3485.
- Klarenbeek PL, de Hair MJH, Doorenspleet ME, et al. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann Rheum Dis. 2012;71(6):1088–1093.
- Musters A, Klarenbeek PL, Doorenspleet ME, et al. In rheumatoid arthritis, synovitis at different inflammatory sites is dominated by shared but patient-specific T cell clones. J Immunol. 2018;201(2):417–422.
- Song J, Schwenzer A, Wong A, et al. Shared recognition of citrullinated tenascin-C peptides by T and B cells in rheumatoid arthritis. JCI Insight. 2021;6(5):145217.
- Pieper J, Dubnovitsky A, Gerstner C, et al. Memory T cells specific to citrullinated α-enolase are enriched in the rheumatic joint. J Autoimmun. 2018;92:47–56.
- Chemin K, Pollastro S, James E, et al. A novel HLA-DRB1*10:01-restricted T cell epitope from citrullinated type II collagen relevant to rheumatoid arthritis. Arthritis Rheumatol. 2016;68(5):1124–1135.
- Rims C, Uchtenhagen H, Kaplan MJ, et al. Citrullinated aggrecan epitopes as targets of autoreactive CD4+ T cells in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71(4):518–528.
- James EA, Rieck M, Pieper J, et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 2014;66(7):1712–1722.
- Snir O, Rieck M, Gebe JA, et al. Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum. 2011;63(10):2873–2883.
- Yoshitomi H. CXCL13-producing PD-1hiCXCR5- helper T cells in chronic inflammation. Immunol Med. 2020;43(4):156–160.
- Christophersen A, Lund EG, Snir O, et al. Distinct phenotype of CD4+ T cells driving celiac disease identified in multiple autoimmune conditions. Nat Med. 2019;25(5):734–737.
- Brennan FM, Hayes AL, Ciesielski CJ, et al. Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells. Arthritis Rheum. 2002;46(1):31–41.
- Brennan FM, Smith NMG, Owen S, et al. Resting CD4+ effector memory T cells are precursors of bystander-activated effectors: a surrogate model of rheumatoid arthritis synovial T-cell function. Arthritis Res Ther. 2008;10(2):R36.
- Bryant J, Ahern DJ, Brennan FM. CXCR4 and vascular cell adhesion molecule 1 are key chemokine/adhesion receptors in the migration of cytokine-activated T cells. Arthritis Rheum. 2012;64(7):2137–2146.
- Shadidi KR, Aarvak T, Jeansson S, et al. T-cell responses to viral, bacterial and protozoan antigens in rheumatoid inflammation. Selective migration of T cells to synovial tissue. Rheumatology (Oxford). 2001;40(10):1120–1125.
- Tan LC, Mowat AG, Fazou C, et al. Specificity of T cells in synovial fluid: high frequencies of CD8+ T cells that are specific for certain viral epitopes. Arthritis Res. 2000;2(2):154.
- Celis L, Vandevyver C, Geusens P, et al. Clonal expansion of mycobacterial heat-shock protein-reactive T lymphocytes in the synovial fluid and blood of rheumatoid arthritis patients. Arthritis Rheum. 1997;40(3):510–519.
- Fazou C, Yang H, Mc Michael AJ, et al. Epitope specificity of clonally expanded populations of CD8+ T cells found within the joints of patients with inflammatory arthritis. Arthritis Rheum. 2001;44(9):2038–2045.
